Clinical outcomes of pancoast tumors treated with trimodality therapy
Introduction
Superior sulcus tumors, or Pancoast tumors, are a rare subset of lung carcinomas, accounting for less than 5% of all non-small-cell lung cancers (NSCLCs) (1). They are locally advanced T3 or T4 tumors (2) that often pose many treatment-related challenges due to their unique anatomical location and potential involvement of adjacent structures such as the apical chest wall, vertebral column, brachial plexus, and subclavian vessels (1).
Over the past several decades, management has drastically improved due to improvement in surgical techniques, incorporation of new drugs, and the quality of radiation delivery. The current standard of care is induction chemoradiotherapy followed by radical surgical resection, which was shown to have markedly improved rates of complete resection and local control of disease (1). In addition to a few prospective trials (3-5), there have also been multiple retrospective series of patients treated with trimodality treatment for Pancoast tumors since the early 1990s (6-11). Reviewing the treatment strategies and outcomes of patients with Pancoast tumors in the province of British Columbia (BC) will add valuable information to this limited knowledge base.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-21-380).
Methods
Patient selection and staging
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the BC Cancer and University of British Columbia Research Ethics Board (No.: H18-01616) and individual consent for this retrospective analysis was waived. We defined Pancoast tumor as NSCLC of the apical segment of the upper lung lobe that involve structures of the apical chest wall (12), including the thoracic inlet, first two ribs, and/or brachial plexus. Our main outcomes were overall survival and disease-free survival. All patients who were treated for NSCLC between January 1, 2000 and December 31, 2015 in BC (population of approximately 4 to 5 million during this time period) were reviewed. Out of 1,373 patients with upper lobe tumors, 329 patients were identified as having primary tumors located in the superior sulcus, but they had various stages and presentations. Eighty-four patients were excluded as they received palliative treatments, and 80 patients were excluded as they had definitive chemoradiotherapy only. Another 108 patients were excluded as they had upfront surgery with adjuvant therapy. Fifty-seven patients were selected for trimodality therapy with curative intent, but 25 were found to be surgically inoperable after induction chemoradiation. The remaining 32 patients with Pancoast tumors completed trimodality treatment.
Patients were assessed by a multidisciplinary team composed of a medical oncologist, radiation oncologist, and thoracic surgeon. Complete staging included CT chest, CT abdomen, brain CT or MRI, PET scan when possible and bone scan if needed. Nineteen (59%) patients underwent either mediastinoscopy or endobronchial ultrasound (EBUS) sampling of nodes for mediastinal staging.
Treatment regimen
Induction chemoradiotherapy included 2 cycles of platinum-etoposide chemotherapy concurrently with radiotherapy. Surgery was planned for 2 months after completion of radiation. Surgical approach was decided by the operating surgeon according to the location and morphology of the tumor.
Pathologic response
The pathologic response to induction chemoradiotherapy was assessed using degree of residual tumor viability. Pathologic complete response was defined as complete tumor necrosis with no residual microscopic disease. Minimal microscopic disease was defined as presence of residual disease in less than 10% of the tumor mass. Macroscopic disease was defined as presence of residual disease in more than 10% of the tumor mass.
Statistical analysis
Overall survival was defined as the date of biopsy to the date of death or last follow-up visit. Disease-free survival was defined as the date of biopsy to the date of relapse, progression, death, or last follow-up visit. Patients were censored at their last follow-up visit. The Kaplan-Meier method was used to estimate the 2-, 5-, and 10-year overall survival and disease-free survival rates for the overall sample and for subgroups based on histology, stage, interval between end of radiotherapy and surgery, pathological response, and margin status. For each estimate, a log-rank test was used to analyze the equality of survivor functions by subgroup for the same variables.
To adjust for the potential of confounding factors when looking at the association between pathological response and survival, Cox proportional hazards modeling was used to account for age, gender, clinical stage, and histology. Due to a small sample size, no more than three variables were entered into each model, including pathological response plus two potential confounding factors. Hazard ratios and 95% confidence intervals were reported.
Results
Patient and tumor characteristics
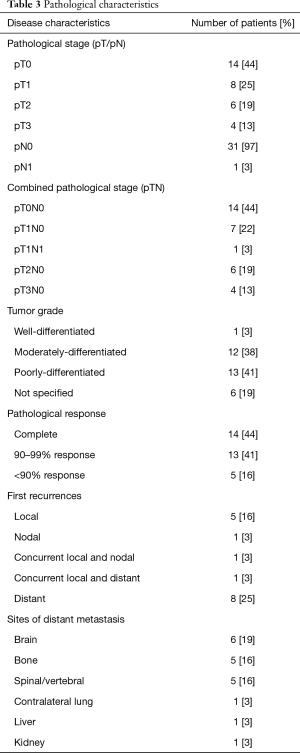
We identified 32 patients with Pancoast tumors who underwent induction chemoradiotherapy and subsequent surgical resection in BC (Table 1). Fourteen (44%) patients were men and 18 (56%) were women, with a median age at consultation of 59 (IQR, 54–68) years. Patients had a median smoking history of 40 (IQR, 30–50) pack years. The tumor primary arose from the right lung in 19 (59%) patients. On initial histology, there were 14 (44%) adenocarcinomas, 12 (38%) squamous cell carcinomas, 2 (6%) large cell carcinomas, 1 (3%) neuroendocrine carcinoma, and 3 (9%) NSCLCs that were not otherwise specified. Most patients had a clinical stage of IIB (T3N0-1) or IIIA (T2N2, T3N2, T4N0-2), with 17 (53%) patients staged T3N0. One patient was staged clinically as IB (T2N0) but they were included in the study despite not being of cT3 or cT4 status as they had a true superior sulcus tumor. Preoperative lymph node stage was cN0 in 24 (75%) patients, cN1 in 2 (6%) patients, and cN2 in 6 (19%) patients.
Full table
Treatment regimen
Thirty (94%) patients were prescribed cisplatin/etoposide, while 2 (6%) patients were prescribed carboplatin/etoposide. Thirty (94%) patients received two or more cycles of chemotherapy concurrently with radiotherapy. Two (6%) patients received only one cycle of chemotherapy due to intolerable neutropenia, and gastrointestinal and renal toxicity. Five (16%) patients received adjuvant chemotherapy after surgery for further risk reduction.
Thirty-one (97%) patients underwent three-dimensional conformal radiation therapy, and 1 (3%) patient underwent intensity-modulated radiation therapy. Thirty-one (97%) completed full radiotherapy. Patients had a median radiation dose of 45 Gy in 25 fractions, with most patients (n=27, 84%) receiving between 45 to 52 Gy in 25 or 26 fractions. Mean interval between chemoradiotherapy and surgery was 52 days (range, 27–145 days).
Thirty (94%) of the primary lung resections were lobectomies and 2 (6%) were wedge resections. Chest wall resection was performed in 20 (63%) patients. Complete resection was achieved in 31 patients (97%). Table 2 shows the summary of treatment regimen.
Full table
Overall survival and disease-free survival
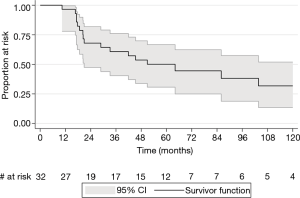
Patients had a median follow-up time of 3.6 years (range, 5 months–18 years). Two (6%) patients were lost to follow-up, and 9 (28%) were discharged from BC Cancer to be followed up with their primary care providers. Two (6%) patients were still on ongoing active follow-up at the time of data extraction. The overall median survival time was 5.3 years. The 2-, 5-, and 10-year overall survival rates were 67.9% (95% CI: 47.4–81.9%), 50.1% (95% CI: 30.7–66.7%), and 31.8% (95% CI: 13.5–51.9%) respectively (Figure 1).
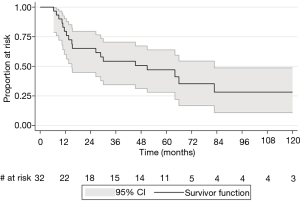
The 2-, 5-, and 10-year disease-free survival rates were 65.1% (95% CI: 44.8–79.5%), 47.1% (95% CI: 28.1–63.9%), and 28.2% (95% CI: 10.9–48.7%) respectively (Figure 2). Two-year disease-free survival rates were similar between clinical stages IIB and IIIA (64.2% vs. 63.6%, P=0.98). There was a trend to suggest that patients with positive nodal status cN1 and cN2 had higher 2-year rates of recurrence than patients staged cN0 (50% and 60% vs. 27.9%, P=0.20), although this was not statistically significant.
Pathological response
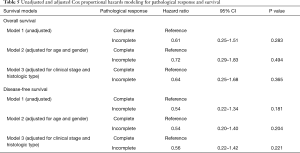
Fourteen (44%) patients obtained pathological complete response after induction chemoradiotherapy, 13 (40%) showed minimal microscopic residual disease, and 5 (16%) showed macroscopic residual disease (Table 3). Downstaging of nodal status after induction chemoradiotherapy was observed in all 8 node-positive patients. Fourteen (44%) patients developed disease recurrence. Nine (28%) cases had distant recurrences, 1 of which had concurrent local spread. The most common site of distant metastasis was the brain (n=6, 19%). We found no statistically significant differences between the 2-, 5-, or 10-year overall survival rates in patients with incomplete pathological response compared to those in patients with pathological complete response (2-year overall survival: 70.6% vs. 64.2%, P=0.599; 5-year overall survival: 58.8% vs. 36.7%, P=0.218; 10-year overall survival: 36.8% vs. 24.4%, P=0.286; Table 4). We also found no statistically significant differences between the 2-, 5-, or 10-year disease-free survival rates in patients with incomplete pathological response compared to those in patients with pathological complete response (2-year disease-free survival: 71.1% vs. 56.3%, P=0.416; 5-year disease-free survival: 59.3% vs. 28.1%, P=0.123; 10-year disease-free survival: 37.0% vs. 14.1%, P=0.118; Table 4). After adjusting for potential confounding variables, pathological response remained a non-significant predictor of survival (Table 5; all P>0.05).
Full table

Full table
Full table
Discussion
Feasibility
The landmark Southwest Oncology Group (SWOG) Trial 9416 was a multi-center phase II trial that demonstrated the feasibility and effectiveness of trimodality treatment (3). It was found that radiation plus surgery yielded a 50% rate of complete resection and a 30% 5-year overall survival rate, which increased to a 76% rate of complete resection and a 44% 5-year overall survival rate with chemoradiotherapy plus surgery. The variability of induction chemoradiotherapy protocols between different institutions makes it difficult to properly compare patient outcomes, but the SWOG offered a reasonable regimen that consisted of (I) concurrent thoracic radiotherapy (45 Gy) and 2 cycles of cisplatin and etoposide, (II) thoracotomy three to five weeks after completion of chemoradiotherapy given no evidence of distant metastases or local progression, and (III) two additional postoperative courses of chemotherapy with a platinum-based doublet. For patients with locally advanced, unresectable disease, as well as those who were medically inoperable, definitive chemoradiotherapy was recommended (3).
In our study, we found the trimodality treatment to be feasible and highly valuable to the population at hand. Only 2 patients were unable to complete their 2 cycles of chemotherapy due to intolerable side effects. Most studies suggest a radiation dose of at least 45 Gy in 25 fractions (biologically equivalent dose of 53.1 Gy, using α/β ratio of 10). We had one patient who received 40 Gy in 15 fractions hypofractionated radiotherapy (biologically equivalent dose of 51.4 Gy, using α/β ratio of 10). The high completion rate of trimodality treatment alludes to its tolerability and beneficial effects.
Overall survival
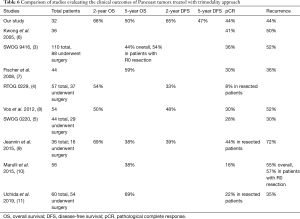
Previous studies reported overall survival and pathological complete response rates that were comparable to our own study results (Table 6). We report a 2-year overall survival rate of 67.9%, which is higher than two other values found in the literature (4,8) despite similar regimens. Our 5-year overall survival rate of 50.1% was also comparable to those of previous studies. Jeannin et al. reported the lowest 5-year overall survival rate of 37.5% (9), likely due to a different chemotherapy regimen consisting of cisplatin/vinorelbine/fluorouracil. This combined with 44 Gy radiotherapy and surgery one month after completion of chemoradiotherapy could have led to their lower rate of pathological complete response of 19.4% as well. Similar results were reported by Marulli et al. with a low 5-year overall survival rate of 38% (10). In this case, while induction therapy consisted of 2–3 cycles of platinum-based chemotherapy, a lower dose of radiotherapy (30–44 Gy) was used. Uchida et al. reported the highest 5-year survival of 69% with either MVP combination chemotherapy or cisplatin/vinorelbine plus radiation dose of 45 Gy in 24 fractions (11).
Full table
Disease-free survival and pattern of recurrence
While long-term survival and local control of disease from trimodality treatment have improved greatly over the last few decades, systemic control remains poor, with distant relapse (usually the brain) being the most common cause of death. Table 6 summarizes and compares disease-free survival and recurrence rates in previous trimodality studies. It has been well-documented that stage III NSCLCs have the highest risk of developing brain metastasis, with an incidence of approximately 30% (13).
We report a 2-year disease-free survival rate of 65.1%, which was higher than previous studies (4,8,9). This could again be explained by differences in chemotherapy regimen and lower radiotherapy doses. Uchida et al. reported the lowest recurrence rate of 35.2% (11); again, we wonder if this is because their MVP chemotherapy regimen was more effective in preoperative downstaging. Fischer et al. reported a low 36% disease relapse rate, which was distant in 25% (7). Jeannin et al. reported a 1- and 2-year disease-free survival rate of 53.6% and 39.1% respectively in all patients including those who were unresectable (9). Recurrences were found in 72% of their patients, which was the highest of the studies reviewed. It should be noted that this study only looked at a very small sample of 16 trimodality patients, and the unresectable patients likely had more advanced disease.
Forty-four percent of our patients experienced disease relapse, which is comparable to the values found in the literature. Our number of recurrences was too small to allow for meaningful comparisons between T3 and T4 tumors. All the studies we reviewed reported that the brain was the most common site of recurrent disease, the only exception being Fischer et al. which reported a tie of highest number of distant metastasis in the contralateral lung (7).
Response to induction chemoradiation and tumor resectability
Our rate of achieving pathological complete response after induction chemoradiotherapy was higher than previously reported values. Moreover, complete resection was achieved in 97% of patients, which compares favorably with R0 resection rates seen in previous studies. Several studies have shown that pathological complete response was associated with improved overall survival (3,8,9,10,14). Studies that did not find statistical differences in survival based on pathological complete response cited small sample sizes (4-6) as well as limited length of follow-up time (11). Similarly, our study did not find pathological complete response to be a statistically significant prognostic indicator for survival, most likely due to small sample size with high variance. Interestingly, there was a trend showing lower overall and disease-free survival rates in the pathological complete response group, for reasons unknown. The one patient that did not complete their intended course of radiotherapy (mainly due to logistical factors) was part of the pathological complete response group. We also wondered whether this trend was due to loss of follow-up, as only 4 patients were followed for the full 10 years. Out of the 14 patients who obtained pathological complete response, 3 were discharged from BC Cancer within 2 years to be followed in the community, and 1 was discharged after 5 years with no evidence of recurrence. However, a similar drop-out rate is found in the incomplete pathological response group. For these reasons, we are unable to draw any conclusions about the prognostic value of pathological response in our study.
Downstaging of nodal status is an important role of induction chemoradiotherapy. Positive N2 lymph nodes occur in approximately 20% of Pancoast tumor cases and are particularly associated with poor prognosis, with many studies reporting outcomes that were clearly inferior to those of patients with N0/N1 only (15). The RTOG 0229 trial found clearance of all nodal disease to significantly improve overall survival as well as disease-free survival (4). Our study showed comparable treatment outcomes for patients of all nodal statuses. Ultimately, we report successful nodal downstaging in all our 8 node-positive patients, all of whom were found to have pN0 status at the time of surgery. Indeed, all our patients were found to be at most pN1 at the time of surgery, which supports the effectiveness of our induction chemoradiotherapy regimen. One cT3N0 patient was found to have a pT1N1 tumor, though in this case the patient had several delays with their chemotherapy regimen because of a complication of periorbital cellulitis that took several weeks to resolve. There is still controversy surrounding the effectiveness of the trimodality approach in patients with existing lymph node metastasis (6,16). Aggressive surgical treatment is still highly recommended even in N2 disease to prevent the onset of therapy-resistant pain due to involvement of the brachial plexus (8).
Strengths and limitations
This study offers the advantage of being based out of a single provincial institution. BC Cancer is a provincial program that consists of multiple cancer centers, and as such our cohort underwent a mostly homogenous therapeutic approach. Due to the scarcity of Pancoast tumors that were eligible for trimodality treatment, we ended up with a relatively small sample size which led to low statistical power. Despite this, our sample size was still comparable to those of previous retrospective studies.
Conclusions
Our study confirms that nodal downstaging and complete surgical resection with negative margins can be achieved after induction chemoradiotherapy, and curative-intent trimodality treatment can lead to long-term survival in some patients. We did not demonstrate any prognostic value of pathological complete response, likely due to small sample size. Distant metastasis continues to be a major downfall after trimodality therapy. Further exploration on long-term prognostic and predictive variables is needed to add to the limited knowledge base of Pancoast tumors. Moreover, an optimal standardized treatment regimen has yet to be established. Advances in imaging, radiotherapy, and surgical techniques will likely improve patient outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-21-380
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-21-380
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-21-380
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-21-380). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the BC Cancer and University of British Columbia Research Ethics Board (
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marulli G, Battistella L, Mammana M, et al. Superior sulcus tumors (Pancoast tumors). Ann Transl Med 2016;4:239. [Crossref] [PubMed]
- Foroulis CN, Zarogoulidis P, Darwiche K, et al. Superior sulcus (Pancoast) tumors: current evidence on diagnosis and radical treatment. J Thorac Dis 2013;5:S342-58. [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for non-small cell lung carcinomas of the superior sulcus: Initial results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Thorac Cardiovasc Surg 2001;121:472-83. [Crossref] [PubMed]
- Suntharalingam M, Paulus R, Edelman MJ, et al. Radiation therapy oncology group protocol 02-29: a phase II trial of neoadjuvant therapy with concurrent chemotherapy and full-dose radiation therapy followed by surgical resection and consolidative therapy for locally advanced non-small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 2012;84:456-63. [Crossref] [PubMed]
- Kernstine KH, Moon J, Kraut MJ, et al. Trimodality therapy for superior sulcus non-small cell lung cancer: Southwest Oncology Group-Intergroup Trial S0220. Ann Thorac Surg 2014;98:402-10. [Crossref] [PubMed]
- Kwong KF, Edelman MJ, Suntharalingam M, et al. High-dose radiotherapy in trimodality treatment of Pancoast tumors results in high pathologic complete response rates and excellent long-term survival. J Thorac Cardiovasc Surg 2005;129:1250-7. [Crossref] [PubMed]
- Fischer S, Darling G, Pierre AF, et al. Induction chemoradiation therapy followed by surgical resection for non-small cell lung cancer (NSCLC) invading the thoracic inlet. Eur J Cardiothorac Surg 2008;33:1129-34. [Crossref] [PubMed]
- Vos CG, Hartemink KJ, Blaauwgeers JL, et al. Trimodality therapy for superior sulcus tumours: evolution and evaluation of a treatment protocol. Eur J Surg Oncol 2013;39:197-203. [Crossref] [PubMed]
- Jeannin G, Merle P, Janicot H, et al. Combined treatment modalities in Pancoast tumor: results of a monocentric retrospective study. Chin Clin Oncol 2015;4:39. [PubMed]
- Marulli G, Battistella L, Perissinotto E, et al. Results of surgical resection after induction chemoradiation for Pancoast tumours †. Interact Cardiovasc Thorac Surg 2015;20:805-11; discussion 811-2. [Crossref] [PubMed]
- Uchida S, Yoshida Y, Ohe Y, et al. Trimodality therapy for superior sulcus tumour: experience of a single institution over 19 years. Eur J Cardiothorac Surg 2019;56:167-73. [Crossref] [PubMed]
- Detterbeck FC. Changes in the treatment of Pancoast tumors. Ann Thorac Surg 2003;75:1990-7. [Crossref] [PubMed]
- Al Feghali KA, Ballout RA, Khamis AM, et al. Prophylactic Cranial Irradiation in Patients With Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Oncol 2018;8:115. [Crossref] [PubMed]
- Truntzer P, Antoni D, Santelmo N, et al. Superior sulcus non small cell lung carcinoma: retrospective analysis of 42 patients. Radiat Onco 2014;9:259. [Crossref] [PubMed]
- Tamura M, Hoda MA, Klepetko W. Current treatment paradigms of superior sulcus tumours. Eur J Cardiothorac Surg 2009;36:747-53. [Crossref] [PubMed]
- Grunenwald DH. The role of surgery for marginally operable tumours (stage IIIBT4). Eur J Cancer 2005;3:21-7. [Crossref]