Sociodemographic disparities in the management of advanced lung cancer: a narrative review
Introduction
Lung cancer is the second most common malignancy in the United States, and the leading cause of cancer death, accounting for nearly 25% of all cancer-related mortality and an estimated 135,720 deaths in 2020 (1,2). While potentially curable if identified at an early stage, only 17% of new cases are localized at presentation, while 57% are metastatic at diagnosis and 22% have regional lymph node involvement, leading to a 5-year survival rate of 20.5% based on the most recent SEER data (2). The treatment landscape has rapidly evolved over the past decade leading to marked improvement in overall survival and quality of life.
Treatment selection based on tumor molecular evaluation and biomarker testing is now the standard of care for advanced lung cancer (3). Numerous oncogenic mutations have been identified, and a growing number of specific, targeted inhibitors of these alterations have been approved and are available for use in clinical practice (4-6). Immune checkpoint inhibitors have drastically altered the treatment landscape and are included in first- and second-line regimens for metastatic disease, as well as maintenance therapy for locally advanced cancer following definitive chemoradiotherapy (7-9). Radiation therapy modalities have grown increasingly precise and effective, through the use of intensity-modulated radiation therapy for definitive thoracic radiation as well as stereotactic radiosurgery (SRS) for intracranial metastatic disease (10-13). Studies have also demonstrated not only quality of life improvement, but survival benefit to the early integration of palliative care for metastatic lung cancer (14). Lastly, the emergence of palliative medicine as a subspecialty has increased access to high-quality end-of-life care, an important element in the continuum of cancer care (15).
These advances in the management of lung cancer define guideline-recommended standards in clinical practice and serve as a metric for high-quality care. However, the rapidly evolving treatment paradigm may exacerbate known racial and socioeconomic disparities in lung cancer care, including inadequate access to care and receipt of low-quality care experienced by Black and low socioeconomic patients with early-stage lung cancer (16-29). Disparities in the management of advanced lung cancer have not been well described in the literature but can undermine the goal of equity in health care, an essential domain of high-quality health care as defined by the Institute of Medicine (30). In this narrative review, we assess sociodemographic disparities—defined as race, ethnicity, insurance status, income, and educational level—in the management of advanced non-small cell lung cancer (NSCLC), encompassing stages III and IV disease. We assess these sociodemographic disparities across four domains as highlighted above: (I) chemoradiation for stage III disease, (II) molecular biomarker testing, (III) systemic therapy including chemotherapy, targeted therapy, and immunotherapy, and (IV) palliative radiation, symptom management, and end of life care. The purpose of this narrative review is to evaluate and synthesize the literature to assess sociodemographic disparities in the management of advanced lung cancer in the targeted therapy and immunotherapy era, to highlight key findings, and identify gaps in the literature that merit future evaluation.
We present the following article in accordance with the Narrative Review Reporting Checklist (available at: http://dx.doi.org/10.21037/jtd-20-3450).
Methods
Research selection
Literature searches were conducted by a medical librarian in MEDLINE® (via PubMed) and Scopus from date of database inception through July 22, 2020. The databases were searched to capture relevant literature using terms for (a) advanced lung cancer and (b) socioeconomic disparities and associated domains of socioeconomic status as defined by Healthy People 2020. We used medical subject headings (MeSH) where available and keywords when applicable. To capture relevant research, no date restrictions were set. Exact search strategies for each database are described in Table S1.
Search results
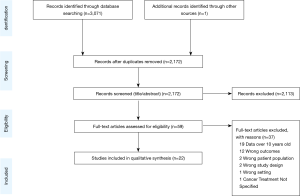
Figure 1 displays the flow diagram of study exclusion. The initial search resulted in 3,071 records, with one additional study identified by a co-author. Deduplication removed 900 records, leaving 2,172 records for title and abstract screening. Articles were excluded if they were on populations outside of the United States; did not evaluate primary lung cancers; had outcomes other than those described above. Studies were also excluded if patients were only treated on clinical trials, lacked specific analyses on advanced lung cancer, or data collection ended before the year 2010. Data before 2010 were excluded given recent advances in the treatment paradigm as detailed above. We identified 22 studies for inclusion. All data were from retrospective cohort studies using large administrative databases. Detailed characteristics and outcomes of each study are summarized in Tables 1-4, grouped by topic area. We describe study design, data source, sample size, description of the population by age and cancer types included, specific disparities assessed, outcomes evaluated, and an aggregate quality score, as well as outcomes assessed in each study including odds ratios and 95% confidence intervals.

Full table
Full table

Full table
Full table
Quality assessment
Each study was rated by two reviewers (JS, MC) on eleven quality criteria adapted from an official American Thoracic Society systematic review (24). The criteria were: inclusion of appropriate statistical testing, adjustment for important confounders, sufficient follow-up period, use of valid and reliable outcome measures, clearly defined outcome measures, reporting of power calculations, use of an appropriate comparison group, generalizability, use of appropriate exclusion criteria, explicitly defined cohort, and full disclosure of conflicts of interest and funding. Any disagreements were resolved by consensus. The total quality rating by individual criteria is presented in Figure S1.
Discussion
Chemoradiation for stage III
Guideline concordant care (GCC) in locally advanced lung cancer consists of complex multi-modality treatment including chemoradiotherapy (CRT) or, less frequently, trimodality therapy (chemotherapy and surgery ± radiotherapy) for resectable stage IIIA disease or CRT in stage IIIB disease. In contrast, non-GCC includes no cancer-directed therapy, radiotherapy alone, CRT with radiation doses less than 60 Gray, or surgery without additional therapy. While the nature of multi-modality therapy greatly improves the efficacy of treatment and survival outcomes, the complexity of care coordination and management of treatment-related toxicities can result in disparities in treatment recommendations.
Only three studies effectively addressed the question of sociodemographic disparities in cancer care delivery in stage III lung cancer (Table 1). All studies were retrospective cohort analyses utilizing the National Cancer Database (NCDB), evaluating outcomes of patients with stage III lung cancer over roughly one decade (2004–2014). All studies were highly powered, with an aggregate total of 172,411 patients captured. Ahmed et al. (31) found that only 23% of Stage III NSCLC patients received GCC with CRT and evaluated factors predicting receipt of CRT. They found that in comparison with White patients, Black (OR 1.13), Hispanic (OR 1.30), and “other” race (OR 1.24) patients were more likely to receive non-GCC, as were the uninsured (OR 1.54 compared with privately insured). Cassidy et al. (32) were specifically interested in the care of patients over age 80, and they found that a large majority of these patients received no cancer-directed therapy (62.7%). In this population, certain socioeconomic factors were associated with receiving no therapy, including Black race, any non-White race, and residence in a census tract with lower educational achievement. Patients who underwent evaluation at an academic medical center were more likely to receive treatment. In their analysis, patients who were treated with combined chemoradiation (cCRT) had improved OS, but receipt of cCRT was associated with socioeconomic disparities. Residence in an urban region was associated with treatment with cCRT, while Black race and residence in a lower educated region were less likely to receive cCRT. Vyfhuis et al. (33) also evaluated patterns of care in stage III NSCLC and had the largest sample size with 113,945 patients assessed. Unlike the previous two studies, this analysis included trimodality therapy for stage IIIA disease as GCC in addition to CRT. They found patients with government insurance or uninsured status were less likely to receive GCC in stage IIIA disease (OR 0.49 and 0.64 respectively), Black race (OR 0.89) and residence in an area with a low median income (OR 0.83) were also both associated with decreased receipt of GCC. For stage IIIB disease, GCC was less likely in regions with low educational achievement (OR 0.86) although they did not see disparities by race or insurance status.
Taken in aggregate, these findings demonstrate the limited data available about sociodemographic disparities in stage III disease, perhaps in part due to the complexity of the multi-modal treatment approach. However, the studies are consistent in demonstrating disparities in the delivery of appropriate GCC for stage III disease for Black patients and the uninsured and they suggest that patients from regions with lower education attainment are also undertreated. Over the past several decades, the treatment of stage III lung cancer has increased in both complexity and efficacy from the addition of sequential chemotherapy to definitive radiation (53), transition from sequential to concurrent chemoradiotherapy (54,55) and more recently the addition of adjuvant immunotherapy following CRT (8). While these advances have dramatically improved overall survival in patients with stage III lung cancer, they also increase the importance of overcoming the disparities in the delivery of guideline-concordant care that have been identified.
Molecular biomarker testing
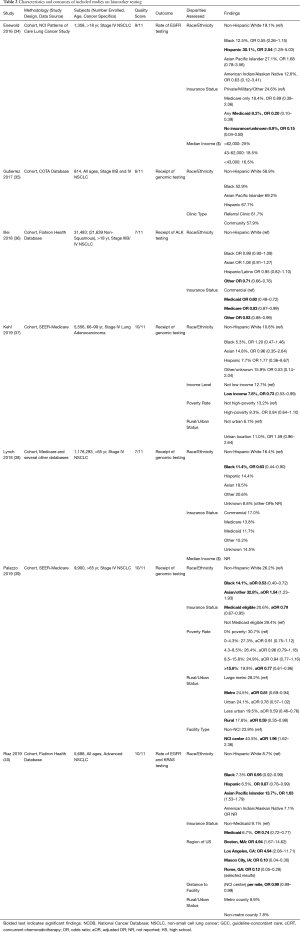
The National Comprehensive Cancer Network (NCCN) and the American Society for Clinical Oncology (ASCO) first recommended inclusion of EGFR testing in patients with advanced lung cancer in 2011 (56), and a complete set of guidelines on molecular testing for lung cancer was subsequently issued in 2013 by several professional societies (57,58). The 2013 guidelines recommended testing for EGFR and ALK mutations in all patients with advanced-stage adenocarcinoma, regardless of sex, race, smoking history, or other clinical risk factors. An updated guideline in 2018 added several other genes to the recommended panel of testing, including ROS1 for all adenocarcinomas, and ERBB2, MET, BRAF, KRAS, and RET if next-generation sequencing is available (59). The increasing discovery of targetable molecular drivers has further enhanced precision oncology by providing patients with effective treatments with minimal toxicity. To assess for variation in patterns of biomarker testing by sociodemographic factors, we identified seven relevant articles (Table 2).
Two studies specifically assessed EGFR testing. The Enewold and Thomas (34) study published in 2016 analyzed a random sample of 1,358 patients diagnosed with stage I-IV NSCLC in 2010 using SEER data to evaluate the frequency of EGFR testing and treatment with erlotinib. Overall, EGFR testing rates for patients with stage IV adenocarcinoma were 23%. Patients with Medicaid or no insurance were less likely to receive testing compared with privately insured (OR 0.20, 0.15), and Hispanic patients were more likely to be tested compared with non-Hispanic Whites (NHW) (OR 2.54). Non-significant differences were noted for Black (OR 0.55, CI 0.26–1.15), American Indian/Alaska Native (AI/AN) (OR 0.63, 0.12–3.41), and Asian Pacific Islander (API) patients (OR 1.68, CI 0.78–3.56). While these results are interesting, the timing of the study greatly limits applicability. As noted above, guidelines recommending molecular biomarker testing were first released in 2011, thus findings derived from 2010 data are limited within the current landscape of practice surrounding biomarker testing. Lynch et al. (38) also evaluated EGFR in addition to KRAS testing using Medicare claims data from 2011–2013 across nearly 1.2 million patients. Notable findings were that fewer Black and Hispanic patients underwent biopsy for suspected lung cancer, which in turn decreased the chance of molecular testing. In the total population, Black (OR 0.95) and Hispanic (OR 0.87) patients were less likely to have EGFR and KRAS testing performed, as compared with NHW. Having Medicaid was the strongest negative predictor for molecular testing (OR 0.74), compared with non-Medicaid. Living in a metropolitan area or closer to an NCI-designated cancer center was associated with an increased rate of testing. In the one study evaluating ALK testing, Illei et al. (36) used the Flatiron Health Database to assess 31,484 patients treated in community practices from 2011–2017. This study found no difference in testing rates between NHW, Black, or Hispanic patients. ALK testing rates improved over time in all patients and patients with nonsquamous NSCLC, although 25% of nonsquamous NSCLC patients in 2017 were not tested. Testing rates were lower in Medicaid (OR 0.60) and Medicare (0.93) recipients, compared to those with commercial insurance.
Gutierrez et al. (35) performed a retrospective analysis of 814 patients with stage IIIB (11%) and stage IV (89%) nonsquamous NSCLC treated by 89 oncologists at 15 sites throughout New Jersey and Maryland from 2013 to 2015 to assess patterns of EGFR and ALK testing and broad genomic testing by race and site of care (referral or community-based clinic). They found that only 59% of patients underwent EGFR and ALK testing and 8% underwent broad genomic testing (BGS). They did not find a significant difference in testing frequency by race nor site of care. The authors do highlight that insufficient tissue and lack of integration of biomarker testing into routine pathology were the main barriers to testing. The study by Palazzo et al. (39) published in 2019, also studied BGS. They analyzed SEER data from 9,900 patients over 65 diagnosed with NSCLC between 2007–2011. After adjusting for demographic variables, low-income status had the strongest association with low testing rates (OR 0.73). Differences in testing were not statistically significant for living in a high-poverty area nor Black or AI/AN patients. As with other studies, time frame of the analysis limits applicability of the findings as they preceded guideline release. Kehl et al. (37) analyzed SEER-Medicare data in 5,556 patients with stage IV lung adenocarcinoma diagnosed between 2008–2013. Only 25.9% of patients had molecular testing within 60 days of diagnosis. Among Medicare recipients, molecular testing rate was significantly lower in Black patients (OR 0.53) and higher in Asian patients (OR 1.54) compared with NHW. Testing rates were lower in Medicaid eligible patients (OR 0.79) and individuals from high poverty areas (OR 0.77). Care at an NCI center (OR 1.96) or residence in a large metro area were associated with increased rates of testing, compared with rural (OR 0.59), less urban (OR 0.59), or metro (OR 0.81) regions. Finally, a 2019 research letter by Riaz et al. (40) described an analysis of 5,688 patients in the Flatiron Health Database with advanced nonsquamous NSCLC treated at 233 community and academic oncology practices between 2011–2016. The primary outcome was the rate of BGS, which was received by only 15.4% of patients. Testing rates were low for all groups; notably lower for Medicaid (11.7%) or Medicare (13.8%) compared with commercial insurance (17.0%). On analysis by race, Black patients were significantly less likely to undergo BGS (OR 0.63) compared with Whites.
In summary, five of the seven studies detected disparities in molecular testing by race, with more frequent testing for patients of Hispanic or Asian descent, and less frequent for Black patients. Importantly, although Asian and Hispanic patients have been shown to have higher rates of certain driver mutations, such as EGFR (60), guidelines specifically recommend testing all patients with advanced lung cancer, regardless of race or ethnicity. All five studies that evaluated insurance status noted disparities for Medicaid patients, which may be explained by the fact that molecular testing was not covered by Centers for Medicare and Medicaid Services (CMS) until 2015 and next generation sequencing was not approved until 2018 (61,62). However, most of these studies concluded their data analysis in 2014 or earlier, with only Illei, Riaz, and Gutierrez including data from the past five years. As such, these findings are significantly limited in their applicability to the rapidly evolving practice of precision oncology. Updated research including real-world prospective data may provide a better understanding of the current practice of molecular testing in advanced lung cancer across sociodemographic groups.
Systemic therapy
Upfront platinum-based chemotherapy doublets followed by single-agent chemotherapy has long been the standard of care for first- and second-line treatment for metastatic lung cancer (63). Advances in precision oncology with targeted therapies for identified oncogenic driver mutations in addition to immune checkpoint inhibitors have led to significant population-level improvements in lung cancer survival (64). We identified eight studies that assessed variation in systemic therapy use for NSCLC by sociodemographic factors (Table 3). Two studies evaluated trends in the use of palliative chemotherapy, three assessed immunotherapy, and one principally assessed the use of tyrosine kinase inhibitors (TKIs). There was overlap in the systemic therapies evaluated across studies. Additionally, two of the studies reviewed for disparities in EGFR biomarker testing described above also assessed patterns in erlotinib treatment use and are included below.
Chemotherapy and biologics
Chemotherapy doublets with and without biologic therapy such as bevacizumab remain the standard of care in the first-line treatment for patients with metastatic NSCLC without an identifiable molecular biomarker and with a contraindication to the use of immunotherapy. The study by Duma et al. (42) was unique in assessing the influence of sociodemographic factors on treatment refusal for palliative chemotherapy and radiation among patients with stage IV NSCLC identified in the NCDB. Of those with provider recommendations for chemotherapy, 10.3% refused therapy, which increased over time. In multivariate analyses, chemotherapy refusal was associated with low neighborhood income, no insurance (OR 2.24), Medicaid (OR 2.17), Medicare (1.17), and other governmental insurance (OR 1.74) in comparison to the privately insured. Compared with NHW, Asians had lower rates of chemotherapy refusal (OR 0.54), Black patients had no significant differences, while those classified as “other” race were over twice as likely to refuse. There were also significant interactions between race and year of diagnosis and between race and gender. Overall, insurance status, rather than race/ethnicity, seems to have a greater influence on refusal of chemotherapy in patients with stage IV NSCLC.
Maguire (44) and Kehl (43) and colleagues also assessed the rates of systemic therapy use in advanced NSCLC. Maguire used the California Cancer Registry and found lower rates of any systemic treatment (chemotherapy, bevacizumab, and TKIs) among Medicaid and uninsured patients compared with privately insured (RR 0.78 and 0.68, respectively). In patients with nonsquamous NSCLC, the uninsured, Medicaid, low neighborhood SES, Black, Asian, and Hispanic race/ethnicity were less likely to receive chemotherapy with bevacizumab. Kehl et al. (43) used SEER-Medicare and found a similar disparity in first-line systemic therapy use. Unlike Maguire, Kehl found variable findings based on race with lower rates of systemic therapy use among Black patients (OR 0.82) and higher among Asian (OR 2.02) and Hispanic patients (OR 1.36) compared to NHW. The differences between their findings may be attributed to variation in the systemic therapies assessed and use of a state cancer registry compared with a more generalizable database in SEER-Medicare. Overall, these studies consistently found that patients with Medicaid or without insurance were less likely to receive chemotherapy and more likely to refuse recommended chemotherapy. Black patients also appeared to be undertreated with first-line chemotherapy compared to other races and ethnicities.
Immunotherapy
The use of immune checkpoint inhibitors (immunotherapy) for the treatment of advanced lung cancer first gained FDA approval in 2015 and was introduced into clinical practice guidelines for second-line therapy regardless of PD-L1 status in 2017 (65,66). Single-agent immunotherapy was recommended for the first-line treatment of metastatic lung cancer with high PD-L1 expression (>50%) in 2017 (66). Most recently, combination chemo-immunotherapy was approved with survival benefits noted irrespective of PD-L1 status (7). However, literature on prevalence of PD-L1 testing is scarce, limiting the evaluation of appropriate immunotherapy use. Adoption of PD-L1 testing is limited in clinical practice, with up to 87% of patients lacking PD-L1 testing (67). Data on sociodemographic variation in PD-L1 testing are also limited and were therefore not included in this narrative review.
Three studies assessed disparities in the use of immunotherapy. Verma et al. (46) assessed racial and insurance disparities in the use of all immunotherapy compounds for metastatic NSCLC. They found lower likelihood of treatment in Black patients (OR 0.86), the uninsured (OR 0.84), and Medicaid recipients. They notably found underutilization of immunotherapy for Black patients even among those with Medicare or Medicaid (OR 0.88 and 0.83), suggesting this racial disparity extends beyond insurance coverage. They also found increased immunotherapy use among patients from regions with higher education attainment (OR 1.14). O’Connor et al. (45) evaluated disparities in programmed death 1 (PD1) checkpoint inhibitors by race and gender and found no significant difference by race for Black, White, or Asian patients, but lower rates of treatment were noted for “other” race (OR 0.76). There was no data on socioeconomic status such as income, education, or insurance type. Kehl et al. (43) looked at first and second-line infusional systemic therapy and also evaluated immunotherapy use in the second-line. They found no disparities by race, Medicaid status, education, or area-level poverty. Notably, confidence intervals for the estimates regarding second-line immunotherapy use were wide, indicating the study was likely underpowered to assess disparities in immunotherapy use. Additionally, two studies only evaluated data through 2015 and one through 2016, which precedes guideline recommendations for use, limiting the applicability of these findings to the current treatment paradigm for immunotherapy.
Tyrosine kinase inhibitors
Tyrosine kinase inhibitor (TKI) administration was studied in four studies with most focusing on first-generation TKIs targeting EGFR mutations. Several studies were limited in the evaluation of appropriate use of targeted therapies, given biomarker testing results were unknown in most (Palazzo, Enewold) or all patients (Chou, Maguire). Chou et al. (41) evaluated the association between the low-income subsidy (LIS) for Medicare part D and oral anticancer drugs (gefitinib, erlotinib, crizotinib, ceritinib, and afatinib) using SEER-Medicare. They postulated that the LIS is a surrogate for poverty but would defray much of the cost associated with anticancer treatment, allowing for greater uptake of therapy. Their findings were consistent with this hypothesis, as patients receiving the full LIS were more likely than those without or partial LIS to receive anticancer therapies and had a shorter time to initiation of treatment. Notably, those with a partial LIS had the lowest uptake of oral anticancer therapies and the longest time to treatment initiation. Partial LIS indicated lower economic status but not low enough to receive full subsidy support, thus lacking coverage to offset treatment costs. These findings highlight that out-of-pocket costs remain a significant barrier to TKI use among lower-income patients. Findings from the remaining three studies also support lower uptake of erlotinib therapy among patients with low-income status. Enewold and Thomas demonstrated that patients from lower-income census tracts were less likely to be treated with erlotinib compared to patients from higher-income census tracts. Differences in erlotinib treatment by race/ethnicity were also noted in multivariate analyses that included all NSCLC histologies, with patients of non-White race and Hispanic ethnicity more likely to receive erlotinib. However, in analyses limited to adenocarcinoma, there were no statistically significant differences by race/ethnicity or insurance status. The interpretation of these findings in light of the current treatment paradigm is limited, given most patients (80%) had unknown EGFR status, and guidelines are based on known molecular marker status before initiation of targeted therapy. In Palazzo’s study (39), low-income status was also associated with low likelihood of erlotinib therapy in multivariate analyses (OR 0.78) but there was no association with race or urban/rural location. Maguire et al. (44) used the California Cancer Registry and found lower likelihood of treatment with TKIs among Medicaid (OR 0.70) and military-issued insurance (OR 0.51) in comparison with private insurance, but no difference in erlotinib therapy for Medicare or dual-eligible patients. Compared to highest neighborhood SES status, all other quintiles of SES were less likely to be treated. Notably, API (OR 3.37) and Hispanic patients (OR 1.75) were more likely to be treated with TKIs compared to NHW. Again, the findings were limited as biomarker testing results were unknown for all patients.
Overall, the findings on disparities in systemic therapy use are heterogeneous given the variability in the sociodemographic factors evaluated and range of systemic therapies assessed across studies. The uninsured, Medicaid recipients and Black patients were less likely to receive chemotherapy. Sociodemographic disparities in immunotherapy were not consistently seen, but those studies preceded current treatment guidelines. Essentially all studies found that patients with low income, the uninsured, or those with Medicaid were less likely to receive TKIs. For most studies, there were no racial disparities in receipt of TKI therapy. However, all included studies were limited given unknown biomarker status and inability to assess appropriateness of targeted therapy use. Further research is needed to assess the appropriate use of targeted therapies and immunotherapy by sociodemographic status in the era of precision oncology and immunotherapy. Additional research is also needed to better understand refusal patterns among patients with low socioeconomic status.
Palliative and end of life care
Palliative care is essential and recommended for all patients with metastatic cancer by NCCN guidelines given the symptom burden and poor quality of life experienced by patients with advanced malignancy (58). Palliative care encompasses a broad range of interventions, including management of cancer-related symptoms, patient-centered communication about goals of care and prognosis, and/or cancer-directed treatments such as radiation, surgery, or chemotherapy with an explicit aim to relieve suffering rather than to prolong life (68). Impressively, early palliative care was shown to prolong survival as well as improve quality of life in a landmark 2010 study (14). Correspondingly, high-quality end-of-life care is also essential given the vast majority of patients do not have prolonged survival after a lung cancer diagnosis. Existing research has demonstrated racial and ethnic disparities in end-of-life care in non-cancer settings (69). We assessed the literature to evaluate if these disparities extend to lung cancer.
Palliative radiation and supportive care
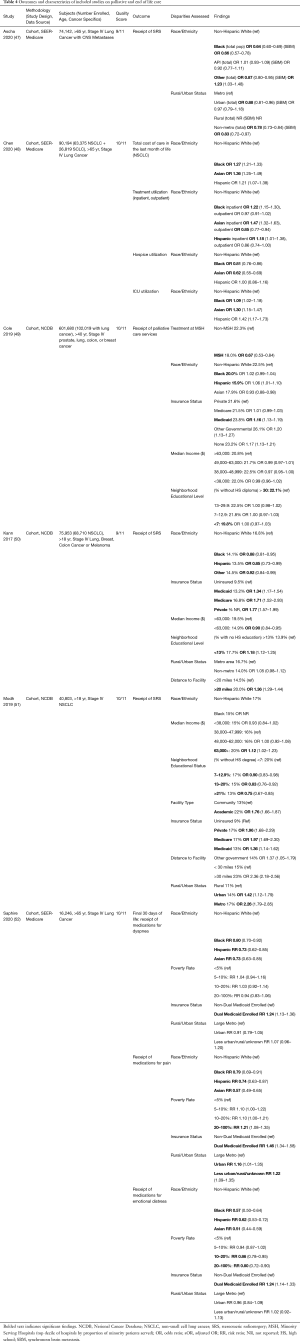
As systemic therapy is discussed separately, this section describes other palliative interventions for advanced lung cancer. Of the four included studies on palliative care, three focused on delivery of radiation for brain metastases from NSCLC, and one evaluated inpatient palliative care delivery. The three studies assessing receipt of radiation for brain metastases were secondary analyses of administrative data sets that sought to evaluate the predictors of delivery of stereotactic radiosurgery (SRS) as opposed to whole brain radiation (WBRT). Kann et al. (50) analyzed 75,953 patients, 68,710 of whom had NSCLC in the NCDB. They reported increasing use of SRS over time, but numerous socioeconomic disparities in its delivery. SRS was less likely to be delivered to non-White populations (OR 0.88 for Black, OR 0.85 for Hispanic, 0.92 for patients of unknown race). Racial disparities were also seen in Ascha et al. (47) (SEER-Medicare) with Black patients having 0.69 the odds of receiving SRS compared with White patients. Modh et al. (51) (NCDB) also found lower rates of SRS in Black patients (15% versus 17% in White patients), although this was not reported in their regression analysis. Kann and Modh identified very similar point estimates of disparities by insurance status, with lower rates for the uninsured (referent) compared with Medicaid (OR 1.34, 1.36 in the two studies), Medicare (OR 1.71, 1.97), or private insurance (OR 1.77, 1.96). Modh also identified disparities by geography and treating facility, with patients in metro and urban regions more likely to receive SRS than rural (OR 2.26 and 1.42 respectively), although Kann found no difference between metro and non-metro regions. SRS was more likely at academic centers in both analyses, with Modh finding an OR 1.76 for academic centers, and Kann calculating the inverse value (OR of 0.52 for non-academic centers). Both studies also found higher rates of SRS in patients from higher-income regions (Modh OR 1.12 for median income >$63,000, Kann OR 0.90 if <$63,000). Importantly, prolonged survival was associated with receipt of SRS in Kann’s analyses, while survival was worse for Black patients in Ascha’s study.
Cole (49) and colleagues evaluated how the site of care influences disparities in palliative lung cancer care delivery. They ranked hospitals by the proportion of minority patients served and evaluated the patterns of palliative care use (surgical treatment, radiation therapy, and palliative chemotherapy) between the highest decile and all others. Among patients with metastatic lung cancer in the NCDB, only 25.4% received palliative care. They identified lower rates of palliative care for racial minorities across the entire combined cohort (metastatic breast, colon, prostate, and lung) over 12 years, which included 601,680 patients, finding that 22.5% of NHW received palliative care, while only 20.0% of Black patients and 15.9% of Hispanic patients received palliative care (P<0.001). They also found that patients were less likely to receive palliative care at the top decile of “minority-serving hospitals,” (MSH) by a margin of 18.0% vs. 22.3% (P=0.002) regardless of ethnicity. On multiple logistic regression, they found treatment at an MSH conferred an OR of 0.67 of receiving palliative care, but racial disparities did not retain significance in adjusted analyses. Thus, they concluded disparities in palliative care delivery are not due to individual racial biases, but rather due to lower rates of palliative care at the facilities where minorities are more likely to seek care. These facilities care for a population with lower economic and educational achievement, and more likely to have public insurance. Further, Medicaid and uninsured patients were more likely to receive palliative care (OR 1.16) in their analysis, raising the possibility that they were not offered as much cancer-directed therapy either due to comorbidities, expense, or intrinsic biases.
These studies highlight that evaluation of disparities in the delivery of palliative care is limited in the literature and remains an important area of study. The studies on the delivery of SRS paint a clear picture of rising but disparate uptake in this important new treatment modality, with less accessibility to Black and Hispanic patients, those of lower-income status, or without access to academic centers. Data is lacking on disparities in other palliative interventions for metastatic lung cancer, including surgical management of pleural effusions, SVC syndrome, cord compression, and other complications.
End of life care
We identified only two studies that specifically evaluated sociodemographic disparities at the end of life (EOL) in advanced lung cancer. Both were large, retrospective cohort studies utilizing the SEER-Medicare database, both concluding data collection in 2013 and assessing a total of 106,440 patients. Chen et al. (48) evaluated the care utilized by various racial and ethnic groups with lung cancer at the EOL and the associated costs. They found higher EOL costs for all minority racial and ethnic groups, with OR of 1.27 for Black, 1.21 for Hispanic, and 1.36 for Asian patients. This was partly driven by higher hospital admission rates in the final month of life, with OR for hospitalization at 1.22 for Black, 1.18 for Hispanic, and 1.47 for Asian patients. ICU admission was also higher for racial and ethnic minorities, with Black patients having 1.09 times the odds, Asians 1.30, and Hispanic patients 1.42 compared with NHW. Hospice enrollment was correspondingly lower in Black (OR 0.81) and Asian patients (OR 0.62). Saphire et al. (52) evaluated the patterns of receipt of symptomatic medications at the EOL. They found that all minority racial and ethnic groups were less likely to receive medications for symptom control, with Black patients receiving medications for dyspnea at 0.80 times the rate of NHW, aRR 0.79 for pain medication, and aRR 0.57 for medications for emotional distress. Similar trends were seen in Hispanic (aRR 0.73 for dyspnea, 0.74 for pain, 0.62 for emotional distress) and Asian patients (aRR 0.73 for dyspnea, 0.57 for pain, and 0.51 for emotional distress). Previous studies outside of the EOL setting have also shown that minority patients, particularly Black Americans, are less likely to be prescribed pain medications while receiving cancer treatment, an issue that has been reported for over 20 years (70-72). Other interesting patterns emerged, as patients from high poverty regions were more likely to get medications for pain (aRR 1.21) but less likely for emotional distress (aRR 0.80). Dual Medicaid and Medicare enrollees had increased likelihood to receive all symptomatic medications (aRR 1.24 for dyspnea, 1.46 for pain, 1.23 for emotional distress). While the data are limited to SEER-Medicare reporting sites, they are of high quality, with large sample sizes, and rigorous methodology to claims analysis.
The findings of these two studies are congruent with previous observations in patients with metastatic lung cancer. They highlight several concerning trends in EOL care by socioeconomic status and racial/ethnic background. It is possible that the more aggressive care at the EOL is partially driven by cultural perceptions and religious beliefs around sickness and death in those communities, and may well be consistent with patients’ and family wishes (73). In the case of Hispanic patients, language barriers remain a significant obstacle for care at the EOL. Utilization of interpreters and incorporation of Hispanic/Latinx healthcare providers into hospice care could potentially improve current practices (74).
However, on a population basis, highly aggressive care at the end of life is not considered high-quality care, and thus raises the concern that the medical system is causing harm and inflicting suffering on patients of racial and ethnic minority backgrounds. Chen’s analysis highlights the ramifications for the health care system, as aggressive care drives up health-related expenditures, while cost-saving programs such as hospice are underutilized in these populations. The benefits of hospice care extend well beyond financial aspects, as patients with lung cancer enrolled in hospice report better quality of life and pain control. The finding that minority populations are less likely to receive symptomatic medications is highly troubling. These are not solely grounded in income or insurance-based disparities, as those populations had increased likelihood of receiving medications for pain. Thus, while the literature remains limited, these studies highlight the significant shortcomings in EOL care for racial and ethnic minority populations with NSCLC.
Conclusion
The treatment paradigm in lung cancer continues to evolve rapidly, and the inclusion of precision oncology and immunotherapy has offered new optimism in a disease that has long been challenging to treat. Yet disparities continue to hamper the delivery of modern cancer care for many groups, including racial and ethnic minorities, uninsured individuals, those with Medicaid, and patients from rural, less educated, and impoverished communities. Across the 22 studies we identified, while individual results varied, there was a consistent pattern of disparities by sociodemographic factors. Black patients and the uninsured are less likely to receive appropriate chemoradiotherapy for stage III NSCLC, a potentially curative disease. Molecular testing does not seem to be equitably distributed, with increased testing for Asian and Hispanic patients and decreased testing for Black patients, the uninsured, or those with Medicaid. Systemic therapies are also less frequently offered to Black patients and those with Medicaid or without insurance. Patients with no insurance, Medicaid, or living in low-income areas are more likely to refuse chemotherapy, and TKIs are less frequently prescribed to patients from lower-income regions. Palliative radiation, specifically SRS, is less available to racial and ethnic minority populations, those of lower-income status or without access to academic medical centers. Black patients receive fewer medications for symptoms and experience increased rates of hospitalization and ICU care at the end of life, and less inpatient palliative care is partly driven by site of care.
This narrative review highlights notable sociodemographic disparities in the treatment of advanced lung cancer. Assessing social determinants of health should be an essential part of the patient evaluation when discussing treatment options, as the literature shows that this can have significant effects on receipt of appropriate therapy. Out of pocket costs remain a substantial barrier to cancer therapy for lower-income patients, especially for novel therapies and TKIs, and future health policy efforts should address the challenge of high-cost treatments that are now standard of care. The current literature on disparities in biomarker testing, targeted therapies, and immunotherapy is outdated in the context of current practice guidelines and needs to be updated. Given the wide availability of electronic health record systems, there is an opportunity to leverage health information technology to identify gaps in care across sociodemographic domains in real-time. Health information technology also provides the opportunity to develop and study multi-level system-based interventions to address these disparities and broaden the reach of modern cancer care to all patients with advanced lung cancer.
Acknowledgments
We would like to thank the University of North Carolina at Chapel Hill Health Sciences librarian, Jennifer Walker Bissram, M.S.I.S for assistance with conducting the literature search, and Samuel Cykert, MD for review of earlier iteration of the manuscript.
Funding: Jacob Stein is supported by the National Cancer Institute funded Cancer Health Disparities Training Grant (T32CA128582). The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by NCI. The funding source had no role in the review of the literature, preparation of the manuscript, or decision to submit the manuscript for publication.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Virginia Litle and Kei Suzuki) for the series “Socioeconomic Disparities in the Treatment of Thoracic Malignancies” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review Reporting Checklist. Available at: http://dx.doi.org/10.21037/jtd-20-3450
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-3450
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-3450). The series “Socioeconomic Disparities in the Treatment of Thoracic Malignancies” was commissioned by the editorial office without any funding or sponsorship. M. Patricia Rivera serves on advisory committees for Biodesix, BioAffinity Technologies, and Johnson & Johnson. Narjust Duma has performed advising and/or consulting for Neogenomics/Inivata, Inc., Pfizer, and AstraZeneca. She has been a speaker for Physician Education Resources (PER) and Clinical Care Options. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lung Cancer Statistics | How Common is Lung Cancer. Available online: https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html. Accessed August 25, 2020.
- Lung and Bronchus Cancer — Cancer Stat Facts. Available online: Available online: https://seer.cancer.gov/statfacts/html/lungb.html. Accessed November 30, 2020.
- Yang SR, Schultheis AM, Yu H, et al. Precision medicine in non-small cell lung cancer: Current applications and future directions. Semin Cancer Biol 2020; Epub ahead of print. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:813-24. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Baumann M, Krause M, Overgaard J, et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer 2016;16:234-49. [Crossref] [PubMed]
- Badiyan SN, Regine WF, Mehta M. Stereotactic Radiosurgery for Treatment of Brain Metastases. J Oncol Pract 2016;12:703-12. [Crossref] [PubMed]
- Jegadeesh N, Liu Y, Gillespie T, et al. Evaluating Intensity-Modulated Radiation Therapy in Locally Advanced Non-Small-Cell Lung Cancer: Results From the National Cancer Data Base. Clin Lung Cancer 2016;17:398-405. [Crossref] [PubMed]
- Koshy M, Malik R, Spiotto M, et al. Association between intensity modulated radiotherapy and survival in patients with stage III non-small cell lung cancer treated with chemoradiotherapy. Lung Cancer 2017;108:222-7. [Crossref] [PubMed]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [Crossref] [PubMed]
- Cruz-Oliver DM. Palliative Care: An Update. Mo Med 2017;114:110-5. [PubMed]
- Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med 1999;341:1198-205. [Crossref] [PubMed]
- Wisnivesky JP, McGinn T, Henschke C, et al. Ethnic disparities in the treatment of stage I non-small cell lung cancer. Am J Respir Crit Care Med 2005;171:1158-63. [Crossref] [PubMed]
- Meza R, Meernik C, Jeon J, et al. Lung cancer incidence trends by gender, race and histology in the United States, 1973-2010. PLoS One 2015;10:e0121323 [Crossref] [PubMed]
- Wolf A, Alpert N, Tran BV, et al. Persistence of racial disparities in early-stage lung cancer treatment. J Thorac Cardiovasc Surg 2019;157:1670-1679.e4. [Crossref] [PubMed]
- Dalwadi SM, Lewis GD, Bernicker EH, et al. Disparities in the Treatment and Outcome of Stage I Non-Small-Cell Lung Cancer in the 21st Century. Clin Lung Cancer 2019;20:194-200. [Crossref] [PubMed]
- Cykert S, Dilworth-Anderson P, Monroe MH, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA 2010;303:2368-76. [Crossref] [PubMed]
- Ellis L, Canchola AJ, Spiegel D, et al. Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. J Clin Oncol 2018;36:25-33. [Crossref] [PubMed]
- Niu X, Roche LM, Pawlish KS, et al. Cancer survival disparities by health insurance status. Cancer Med 2013;2:403-11. [Crossref] [PubMed]
- Slatore CG, Au DH, Gould MK, et al. An official American Thoracic Society systematic review: insurance status and disparities in lung cancer practices and outcomes. Am J Respir Crit Care Med 2010;182:1195-205. [Crossref] [PubMed]
- Nicoli CD, Sprague BL, Anker CJ, et al. Association of Rurality With Survival and Guidelines-Concordant Management in Early-stage Non-Small Cell Lung Cancer. Am J Clin Oncol 2019;42:607-14. [Crossref] [PubMed]
- Atkins GT, Kim T, Munson J. Residence in Rural Areas of the United States and Lung Cancer Mortality. Disease Incidence, Treatment Disparities, and Stage-Specific Survival. Ann Am Thorac Soc 2017;14:403-11. [Crossref] [PubMed]
- Paquette I, Finlayson SR. Rural versus urban colorectal and lung cancer patients: differences in stage at presentation. J Am Coll Surg 2007;205:636-41. [Crossref] [PubMed]
- Guidry JJ, Aday LA, Zhang D, et al. Cost considerations as potential barriers to cancer treatment. Cancer Pract 1998;6:182-7. [Crossref] [PubMed]
- Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950-2014: Over Six Decades of Changing Patterns and Widening Inequalities. J Environ Public Health 2017;2017:2819372 [Crossref] [PubMed]
- Institute of Medicine (US) Committee. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001:1-364.
- Ahmed HZ, Liu Y, O'Connell K, et al. Guideline-concordant Care Improves Overall Survival for Locally Advanced Non-Small-cell Lung Carcinoma Patients: A National Cancer Database Analysis. Clin Lung Cancer 2017;18:706-18. [Crossref] [PubMed]
- Cassidy RJ, Zhang X, Switchenko JM, et al. Health care disparities among octogenarians and nonagenarians with stage III lung cancer. Cancer 2018;124:775-84. [Crossref] [PubMed]
- Vyfhuis MAL, Bentzen SM, Molitoris JK, et al. Patterns of Care and Survival in Stage III NSCLC Among Black and Latino Patients Compared With White Patients. Clin Lung Cancer 2019;20:248-257.e4. [Crossref] [PubMed]
- Enewold L, Thomas A. Real-World Patterns of EGFR Testing and Treatment with Erlotinib for Non-Small Cell Lung Cancer in the United States. PLoS One 2016;11:e0156728 [Crossref] [PubMed]
- Gutierrez ME, Choi K, Lanman RB, et al. Genomic Profiling of Advanced Non-Small Cell Lung Cancer in Community Settings: Gaps and Opportunities. Clin Lung Cancer 2017;18:651-9. [Crossref] [PubMed]
- Illei PB, Wong W, Wu N, et al. ALK testing trends and patterns among community practices in the united states. JCO Precis Oncol 2018;1-11. [Crossref]
- Kehl KL, Lathan CS, Johnson BE, et al. Race, Poverty, and Initial Implementation of Precision Medicine for Lung Cancer. J Natl Cancer Inst 2019;111:431-4. [Crossref] [PubMed]
- Lynch JA, Berse B, Rabb M, et al. Underutilization and disparities in access to EGFR testing among Medicare patients with lung cancer from 2010 - 2013. BMC Cancer 2018;18:306. [Crossref] [PubMed]
- Palazzo LL, Sheehan DF, Tramontano AC, et al. Disparities and Trends in Genetic Testing and Erlotinib Treatment among Metastatic Non-Small Cell Lung Cancer Patients. Cancer Epidemiol Biomarkers Prev 2019;28:926-34. [Crossref] [PubMed]
- Riaz F, Presley CJ, Chiang AC, et al. Disparities in broad-based genomic sequencing for patients with advanced non-small cell lung cancer. J Geriatr Oncol 2019;10:669-72. [Crossref] [PubMed]
- Chou YT, Farley JF, Stinchcombe TE, et al. The Association Between Medicare Low-Income Subsidy and Anticancer Treatment Uptake in Advanced Lung Cancer. J Natl Cancer Inst 2020;112:637-46. [Crossref] [PubMed]
- Duma N, Idossa DW, Durani U, et al. Influence of Sociodemographic Factors on Treatment Decisions in Non-Small-Cell Lung Cancer. Clin Lung Cancer 2020;21:e115-29. [Crossref] [PubMed]
- Kehl KL, Hassett MJ, Schrag D. Patterns of care for older patients with stage IV non-small cell lung cancer in the immunotherapy era. Cancer Med 2020;9:2019-29. [Crossref] [PubMed]
- Maguire FB, Morris CR, Parikh-Patel A, et al. Disparities in Systemic Treatment Use in Advanced-stage Non-Small Cell Lung Cancer by Source of Health Insurance. Cancer Epidemiol Biomarkers Prev 2019;28:1059-66. [Crossref] [PubMed]
- O'Connor JM, Seidl-Rathkopf K, Torres AZ, et al. Disparities in the Use of Programmed Death 1 Immune Checkpoint Inhibitors. Oncologist 2018;23:1388-90. [Crossref] [PubMed]
- Verma V, Haque W, Cushman TR, et al. Racial and Insurance-related Disparities in Delivery of Immunotherapy-type Compounds in the United States. J Immunother 2019;42:55-64. [Crossref] [PubMed]
- Ascha MS, Funk K, Sloan AE, et al. Disparities in the use of stereotactic radiosurgery for the treatment of lung cancer brain metastases: a SEER-Medicare study. Clin Exp Metastasis 2020;37:85-93. [Crossref] [PubMed]
- Chen Y, Criss SD, Watson TR, et al. Cost and Utilization of Lung Cancer End-of-Life Care Among Racial-Ethnic Minority Groups in the United States. Oncologist 2020;25:e120-9. [Crossref] [PubMed]
- Cole AP, Nguyen DD, Meirkhanov A, et al. Association of Care at Minority-Serving vs Non-Minority-Serving Hospitals With Use of Palliative Care Among Racial/Ethnic Minorities With Metastatic Cancer in the United States. JAMA Netw Open 2019;2:e187633 [Crossref] [PubMed]
- Kann BH, Park HS, Johnson SB, et al. Radiosurgery for Brain Metastases: Changing Practice Patterns and Disparities in the United States. J Natl Compr Canc Netw 2017;15:1494-502. [Crossref] [PubMed]
- Modh A, Doshi A, Burmeister C, et al. Disparities in the Use of Single-fraction Stereotactic Radiosurgery for the Treatment of Brain Metastases From Non-small Cell Lung Cancer. Cureus 2019;11:e4031 [Crossref] [PubMed]
- Saphire ML, Prsic EH, Canavan ME, et al. Patterns of Symptom Management Medication Receipt at End-of-Life Among Medicare Beneficiaries With Lung Cancer. J Pain Symptom Manage 2020;59:767-777.e1. [Crossref] [PubMed]
- Dillman RO, Herndon J, Seagren SL, et al. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst 1996;88:1210-5. [Crossref] [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [Crossref] [PubMed]
- Aupérin A, Le Péchoux C, Pignon JP, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol 2006;17:473-83. [Crossref] [PubMed]
- Keedy VL, Temin S, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) Mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol 2011;29:2121-7. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn 2013;15:415-53. [Crossref] [PubMed]
- Ettinger DS, Bepler G, Bueno R, et al. Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw 2006;4:548-82. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn 2018;20:129-59. [Crossref] [PubMed]
- El-Telbany A, Ma PC. Cancer genes in lung cancer: racial disparities: are there any? Genes Cancer 2012;3:467-80. [Crossref] [PubMed]
- Decision Memo for Next Generation Sequencing (NGS) for Medicare Beneficiaries with Advanced Cancer (CAG-00450N). Available online: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=290. Accessed November 29, 2020.
Local Coverage Determination for Molecular Pathology Procedures (L35000) - Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2010;8:740-801. [Crossref] [PubMed]
- Howlader N, Forjaz G, Mooradian MJ, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med 2020;383:640-9. [Crossref] [PubMed]
- Sul J, Blumenthal GM, Jiang X, et al. FDA Approval Summary: Pembrolizumab for the Treatment of Patients With Metastatic Non-Small Cell Lung Cancer Whose Tumors Express Programmed Death-Ligand 1. Oncologist 2016;21:643-50. [Crossref] [PubMed]
- Hanna N, Johnson D, Temin S, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484-515. [Crossref] [PubMed]
- O'Connor JM, Fessele KL, Steiner J, et al. Speed of Adoption of Immune Checkpoint Inhibitors of Programmed Cell Death 1 Protein and Comparison of Patient Ages in Clinical Practice vs Pivotal Clinical Trials. JAMA Oncol 2018;4:e180798 [Crossref] [PubMed]
- Ferrell B, Koczywas M, Grannis F, et al. Palliative care in lung cancer. Surg Clin North Am 2011;91:403-17. ix. [Crossref] [PubMed]
- Orlovic M, Smith K, Mossialos E. Racial and ethnic differences in end-of-life care in the United States: Evidence from the Health and Retirement Study (HRS). SSM Popul Health 2019;7:100331 [Crossref] [PubMed]
- Hoffman KM, Trawalter S, Axt JR, et al. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A 2016;113:4296-301. [Crossref] [PubMed]
- Meghani SH, Byun E, Gallagher RM. Time to take stock: a meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med 2012;13:150-74. [Crossref] [PubMed]
- Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 1994;330:592-6. [Crossref] [PubMed]
- Jesus OTV, Qiu A, Carretero E, et al. Barriers to quality end of life care for latinos: hospice healthcare professionals’ perspective (S732). J Pain Symptom Manage 2013;45:436-7. [Crossref]
- Duma N, Durani U, Molina J, et al. Abstract 1641: Utilization of palliative therapies among Hispanics with stage IV non-small cell lung cancer. In: Clinical Research (Excluding Clinical Trials). American Association for Cancer Research; 2018:1641.