
Usefulness of lavage and drainage using video-assisted thoracoscopic surgery for Boerhaave’s syndrome: a retrospective analysis
Introduction
Spontaneous rupture of the esophagus, first described by Boerhaave in 1724, is a life-threatening condition characterized by disruption of the distal esophagus due to barotrauma that results in contamination of the mediastinum and pleural cavity with gastric contents. Boerhaave’s syndrome has a high mortality rate (14–40%) due to respiratory failure, septic shock, and multiple organ failure (1-3). The barogenic nature of the rupture probably explains the wide mediastinal contamination, empyema, and pneumothorax that can cause respiratory failure, sepsis, and shock. Surgical treatment is the basis of therapy, but there is no established surgical procedure (1-3). In our emergency department, we performed primary repair with thoracotomy as a treatment for Boerhaave’s syndrome until 2004. In 2004, a patient developed respiratory failure and septic shock during thoracotomy and died. Therefore, since 2004, we changed from primary repair with thoracotomy to laparotomy and postoperative computed tomography (CT)-guided drainage, and all patients survived. However, mechanical ventilator days and the length of intensive care unit (ICU) stay remained prolonged. We added lavage and drainage using video-assisted thoracoscopic surgery (VATS) to primary repair with laparotomy in 2011. Since lavage and drainage using VATS can be performed while checking the contaminated site, more sufficient lavage and drainage is possible compared to CT-guided drainage. The purpose of this study was to elucidate the usefulness of lavage and drainage using VATS for Boerhaave’s syndrome.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-2445).
Methods
Study design and population
From April 2004 to September 2018, 18 patients with Boerhaave’s syndrome presented to our department. Figure 1 shows the study design. Six of the 18 patients were treated conservatively and were excluded from the analysis. These 6 patients were stable in respiratory and hemodynamic state, and were not in sepsis. 3 of 6 patients had limited contamination of mediastinum. Therefore, we had decided that conservative treatment was possible. We performed fasting, total parenteral nutrition, administration of antibiotics, nasogastric tube decompression, and chest tube drainage for these patients. All 6 patients improved and were discharged. Primary repair was performed with laparotomy in 12 patients. These patients were divided into two groups according to the drainage method for the mediastinal and thoracic cavities after laparotomy. From 2004 to 2011, CT-guided drainage was performed in 6 patients (D group). On the other hand, from 2011 to 2018, lavage and drainage were performed using VATS in 6 patients (VATS group). The medical records of the included patients were reviewed retrospectively for survival rate, anastomotic leakage, operation time, PaO2/FiO2 (P/F) ratio after surgery, ventilator-free days (VFDs), length of ICU stay, and length of hospital stay.
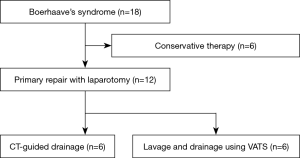
Primary repair with laparotomy (transabdominal approach)
The transabdominal approach was used for rupture of the lower thoracic esophagus. In all cases, the perforation site was the left wall of the lower thoracic esophagus. Laparotomy was done with an upper midline incision. The left lateral segment of the liver was mobilized, and the lesser omentum was opened. The left crus of the diaphragm were dissected, and the lower thoracic esophagus could be encircled and the perforation site identified. The primary repair was done by an entire layer or 2-layer suturing with absorbable thread. A fundic patch or omental patch was considered for reinforcement of the sutured site. Gastrostomy to reduce gastrointestinal pressure and a feeding jejunostomy were performed. The drain was inserted into the mediastinum near the sutured site of the esophagus through the foramen of Winslow from the right flank.
Lavage and drainage using VATS
After primary repair with laparotomy, the patient’s position was converted to the right decubitus position, and differential lung ventilation with a double-lumen endobronchial tube was performed. For VATS, a total of three trocars were inserted. First, the 10-mm camera port was introduced in the fifth intercostal space at the midaxillary line. Then, the two 5-mm ports were introduced in the third intercostal space at the anterior axillary line and the seventh intercostal space at the posterior axillary line. The purpose of the VATS was only lavage and drainage for the mediastinum and the thoracic cavity. Advanced surgical techniques such as primary suture were not required. If the contamination of the mediastinum and thoracic cavity were severe by food residue, a small thoracotomy and lavage with a large amount of saline were performed. Because it was difficult to remove food residue only by VATS. Two chest drainage tubes were inserted to the dorsal lung area and above the left diaphragm.
Statistical analysis
Data are reported as the medians [interquartile range (IQR)] or as proportions. All data were analyzed with SPSS version 21.0 software (SPSS, Inc., Chicago, IL, USA). Comparisons of the two groups were performed with the Mann-Whitney and Fisher’s exact tests for the variables of interest.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Kitasato University Ethics Committee (approval number B18-252). The committee waived the need for informed consent owing to the retrospective observational design of study.
Results
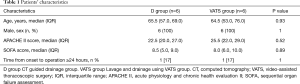
Table 1 shows the characteristics of the patients in both groups. There were no significant differences in age, sex, the acute physiology and chronic health evaluation II (APACHE II) score, the sequential organ failure assessment (SOFA) score, and time from onset to operation. Table 2 shows the surgical procedures of both groups. There were no significant differences in the rate of surgical procedures between the two groups: layer to layer suture, fundic patch, omental patch, gastrostomy, and jejunostomy.
Full table

Full table
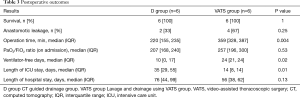
Table 3 shows the postoperative outcomes. All patients survived in both groups. The rate of anastomotic leakage and the P/F ratio after surgery were high in the VATS group compared with the D group, but there were no significant differences. Length of hospital stay was shorter in the VATS group compared with the D group, but there was no significant difference.
Full table
Operation time was significantly prolonged in the VATS group compared with the D group {359 [328, 387] vs. 220 [155, 235] min, P=0.004}. VFDs were extended significantly {24 [21, 24] vs. 10 [0, 17] days, P=0.02}, and length of ICU stay was shortened significantly {14 [8, 14] vs. 35 [29, 55] days, P=0.01} in the VATS group compared with the D group.
Discussion
Spontaneous esophageal rupture often causes respiratory failure and septic shock in the perioperative period. Primary repair with thoracotomy is most commonly performed as the surgical treatment, but it is an excessively invasive procedure and affects respiratory and circulatory status (1-3). In our emergency department, we changed the surgical approach from thoracotomy to laparotomy, because a patient developed respiratory failure and severe shock during thoracotomy and then died. Khan et al. (4) first reported a transabdominal approach for spontaneous rupture of the esophagus. They successfully managed four patients using this procedure. The advantages of primary repair with laparotomy are as follows. The reinforcement, such as a fundic patch or an omental patch, can be easily performed. Gastrostomy to reduce gastrointestinal pressure and feeding jejunostomy can also be performed. The effect on respiratory function is less than thoracotomy (5). The disadvantages of primary repair with laparotomy are as follows. Laparotomy is indicated only for rupture of the lower thoracic esophagus. Open or percutaneous drainage may be required for postoperative mediastinal abscess and empyema. The usefulness of CT-guided drainage for mediastinal abscess has often been reported. Gobien et al. (6) reported a series of 12 patients with mediastinal abscess, and six of them were successfully drained by CT-guided drainage. Recently, Arellano et al. (7) also reported that they performed CT-guided drainage for 23 patients with mediastinal abscess. Since the success rate was 95.6%, they concluded that it was a very useful method.
Recently, there have been several reports of minimally invasive surgical treatment for Boerhaave’s syndrome by endoscopic closure, laparoscopy, or thoracoscopy (8-14). In particular, thoracoscopic surgery has become widespread for esophageal cancer. It is less invasive than thoracotomy, especially with respect to postoperative pulmonary function. Thoracoscopic surgery has been performed for benign diseases such as Boerhaave’s syndrome. Thoracoscopic drainage for Boerhaave’s syndrome was first reported by Hutter et al. (15). The initial aim of thoracoscopic surgery for Boerhaave’s syndrome was to irrigate and drain the mediastinum and thoracic cavity. Lawrence et al. (16) reported the usefulness of thoracoscopic drainage for 30 patients with empyema. Later, primary repair with VATS was reported with the progress of endoscopic surgery (17-24). Primary repair with VATS can be performed by esophageal surgeons who usually perform esophagectomy for esophageal cancer (22-24), but it is a very difficult surgical procedure for general surgeons.
The advantages of lavage and drainage using VATS are as follows. It is a less invasive procedure than thoracotomy. We can sufficiently lavage and drain the thoracic cavity and mediastinum while checking the contaminated site. On the other hand, sufficient lavage and drainage cannot be performed with CT-guided drainage. Primary repair with thoracoscopic surgery requires a high level of surgical technique, whereas lavage and drainage using VATS can be performed relatively easily. The disadvantages of VATS are as follows. It requires conversion to the right decubitus position and differential lung ventilation after laparotomy, which affects respiratory and circulatory status. Since we perform VATS in addition to laparotomy, operation time becomes longer.
Recently, some successful endoscopic treatments for Boerhaave’s syndrome have been reported (8,10). Endoscopic clipping, stent may be useful. For particularly elderly individuals with hemodynamic and/or respiratory instability, less invasive endoscopic treatment may be preferred to more invasive surgical treatment. However, randomized trials haven’t been conducted to compare endoscopic treatment with surgical treatment. Even now, the gold standard treatment for Boerhaave’s syndrome is surgical repair. Therefore, we choose it for operable patients.
This study has several limitations. Boerhaave’s syndrome is rare, so only a small number of patients could be included in the present analysis. In this study, the percentages of anastomotic leakage were high. At this point, we haven’t found the cause of it. It is necessary to increase number of cases and investigate the cause of it in the future. In addition, it was a retrospective, historical study and not a randomized, controlled study. The surgical treatment for Boerhaave’s syndrome has not been established, then various surgical treatments have been performed at various centers. Especially, it depends on the surgeon’s surgical skill. Multicenter retrospective observational study is desired; however, it is difficult for these reasons.
Conclusions
Lavage and drainage using VATS compared with CT-guided drainage after esophageal repair with laparotomy extended VFDs and shortened the length of ICU stay. With VATS, sufficient lavage and drainage of the thoracic cavity and mediastinum can be achieved. VATS is an effective treatment method for Boerhaave’s syndrome.
Acknowledgments
We would like to thank Forte Science Communications (forte-science.co.jp) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-2445
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-20-2445
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-2445
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-2445). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Kitasato University Ethics Committee (approval number B18-252). The committee waived the need for informed consent owing to the retrospective observational design of study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pezzetta E, Kokudo T, Uldry E, et al. The surgical management of spontaneous esophageal perforation (Boerhaave's syndrome) ‒ 20 years of experience. Biosci Trends 2016;10:120-4. [Crossref] [PubMed]
- Sulpice L, Dileon S, Rayar M, et al. Conservative surgical management of Boerhaave's syndrome: experience of two tertiary referral centers. Int J Surg 2013;11:64-7. [Crossref] [PubMed]
- Shen G, Chai Y, Zhang GF. Successful surgical strategy in a late case of Boerhaave's syndrome. World J Gastroenterol 2014;20:12696-700. [Crossref] [PubMed]
- Khan AZ, Forshaw MJ, Davies AR, et al. Transabdominal approach for management of Boerhaave's syndrome. Am Surg 2007;73:511-3. [Crossref] [PubMed]
- Haveman JW, Nieuwenhuijs VB, Kobold JP, et al. Adequate debridement and drainage of the mediastinum using open thoracotomy or video-assisted thoracoscopic surgery for Boerhaave's syndrome. Surg Endosc 2011;25:2492-7. [Crossref] [PubMed]
- Gobien RP, Stanley JH, Gobien BS, et al. Percutaneous catheter aspiration and drainage of suspected mediastinal abscesses. Radiology 1984;151:69-71. [Crossref] [PubMed]
- Arellano RS, Gervais DA, Mueller PR. Computed tomography-guided drainage of mediastinal abscesses: clinical experience with 23 patients. J Vasc Interv Radiol 2011;22:673-7. [Crossref] [PubMed]
- Gomez-Esquivel R, Raju GS. Endoscopic closure of acute esophageal perforations. Curr Gastroenterol Rep 2013;15:321. [Crossref] [PubMed]
- Tellechea JI, Gonzalez JM, Miranda-García P, et al. Role of Endoscopy in the Management of Boerhaave Syndrome. Clin Endosc 2018;51:186-91. [Crossref] [PubMed]
- Ishikawa Y, Tagami T, Hirashima H, et al. Endoscopic Treatment of Boerhaave Syndrome Using Polyglycolic Acid Sheets and Fibrin Glue: A Report of Two Cases. J Nippon Med Sch 2017;84:241-5. [Crossref] [PubMed]
- Ishikawa Y, Tagami T, Hirashima H, et al. Endoscopic Treatment of Boerhaave Syndrome Using Polyglycolic Acid Sheets and Fibrin Glue: A Report of Two Cases. J Nippon Med Sch 2017;84:241-5. [Crossref] [PubMed]
- Do YW, Lee CY, Lee S, et al. Successful Management of Delayed Esophageal Rupture with T-Tube Drainage Using Video-Assisted Thoracoscopic Surgery. Korean J Thorac Cardiovasc Surg 2016;49:478-80. [Crossref] [PubMed]
- Kimberley KL, Ganesh R, Anton CK. Laparoscopic repair of esophageal perforation due to Boerhaave syndrome. Surg Laparosc Endosc Percutan Tech 2011;21:e203-5. [Crossref] [PubMed]
- Landen S, El Nakadi I. Minimally invasive approach to Boerhaave's syndrome: a pilot study of three cases. Surg Endosc 2002;16:1354-7. [Crossref] [PubMed]
- Hutter JA, Fenn A, Braimbridge MV. The management of spontaneous oesophageal perforation by thoracoscopy and irrigation. Br J Surg 1985;72:208-9. [Crossref] [PubMed]
- Lawrence DR, Ohri SK, Moxon RE, et al. Thoracoscopic debridement of empyema thoracis. Ann Thorac Surg 1997;64:1448-50. [Crossref] [PubMed]
- Lawrence DR, Ohri SK, Moxon RE, et al. Primary esophageal repair for Boerhaave's syndrome. Ann Thorac Surg 1999;67:818-20. [Crossref] [PubMed]
- Ashrafi AS, Awais O, Alvelo-Rivera M. Minimally invasive management of Boerhaave's syndrome. Ann Thorac Surg 2007;83:317-9. [Crossref] [PubMed]
- Ikeda Y, Niimi M, Sasaki Y, et al. Thoracoscopic repair of a spontaneous perforation of the esophagus with the endoscopic suturing device. J Thorac Cardiovasc Surg 2001;121:178-9. [Crossref] [PubMed]
- Scott HJ, Rosin RD. Thoracoscopic repair of a transmural rupture of the oesophagus (Boerhaave's syndrome). J R Soc Med 1995;88:414p-5p. [PubMed]
- Cho JS, Kim YD, Kim JW, et al. Thoracoscopic primary esophageal repair in patients with Boerhaave's syndrome. Ann Thorac Surg 2011;91:1552-5. [Crossref] [PubMed]
- Nakano T, Onodera K, Ichikawa H, et al. Thoracoscopic primary repair with mediastinal drainage is a viable option for patients with Boerhaave's syndrome. J Thorac Dis 2018;10:784-9. [Crossref] [PubMed]
- Okamoto H, Onodera K, Kamba R, et al. Treatment of spontaneous esophageal rupture (Boerhaave syndrome) using thoracoscopic surgery and sivelestat sodium hydrate. J Thorac Dis 2018;10:2206-12. [Crossref] [PubMed]
- Nakano T, Sato C, Sakurai T, et al. Thoracoscopic esophageal repair with barbed suture material in a case of Boerhaave's syndrome. J Thorac Dis 2016;8:E1576-80. [Crossref] [PubMed]


