
Outcomes of surgically managed primary lung sarcomas: a National Cancer Database analysis
Introduction
Primary lung sarcoma (PLS) represents an uncommon variant of primary lung cancer, accounting for less than 0.5% of all reported primary lung malignancies (1,2). PLS is a heterogeneous group of mesenchymal tumors, classified based on tissue origin as well as distinct histological and biological features (3,4). The diagnosis of PLS is established only after more common pathology, such as pulmonary sarcomatoid carcinoma and metastatic extrapulmonary sarcoma, have been excluded. Owing to its rarity, the current characterization of these tumors has been limited to data from small case reports and retrospective tumor registries (1,2,5-15).
Several clinical series suggest that PLS is associated with more aggressive behavior and inferior survival compared to other primary lung malignancies, with an expected 3-year survival of 17% to 50% following surgery (1,8,16,17). PLS is also associated with inferior outcomes compared to other soft-tissue sarcomas, including worse 5-year overall survival (OS) compared to extremity soft tissue sarcomas (35% vs. 71%, P<0.001) (16). Despite the lack of definitive evidence supporting a standardized management for PLS, patients undergoing complete surgical resection have shown significantly better survival compared to non-resected and positive margins cases (2,11,18). Although these patients are highly selected, consideration for aggressive surgical resection as the first line treatment for management is impacted by the lack of effective options in terms of either neoadjuvant or adjuvant therapy (radiation and/or chemotherapy) for PLS (11,16).
The opportunity to examine these low frequency tumors in a comprehensive dataset such as the National Cancer Database (NCDB) is ideal. Therefore, we sought to analyze and examine the influence of prognostic clinical and pathological characteristics in PLS and their impact on OS after surgical resection.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jtd-21-1).
Methods
Data source
The NCDB is a hospital-based tumor registry managed by the Commission on Cancer of the American College of Surgeons and the American Cancer Society, which captures approximately 70% of all newly diagnosed lung cancer cases in the United States. This dataset contains detailed information on patient demographics, tumor staging, treatment, and survival (19). The data used in this analysis is derived from the 2015 version of the de-identified clinically populated NCDB participant user file. The Institutional Review Board of Yale University School of Medicine approved this study with consent waived. The NCDB is not responsible for the conclusions drawn from the analytic or statistical methodology employed by the investigator (20). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) pertaining to the ethical principles for medical research involving human subjects.
Study population
We performed a retrospective cohort analysis of the NCDB. The database was queried for adult patients (>18 years old) diagnosed with pathologically confirmed PLS from 2004–2014. The number of cases report during this time period determined the study size. To minimize the impact of outliers, we limited this study to patients with invasive tumors, no prior cancer history, and complete follow-up. Only microscopically confirmed histological subtypes were included (21,22). Thirty-seven distinct histological subtypes were extracted from the NCDB using the appropriate codes from the International Classification of Diseases for Oncology 3rd edition (ICD-O-3; Table S1).
Primary tumor location was limited to the lung and bronchus (C340-C349) (23). Concurrently managed patients with non-small cell lung cancer (NSCLC) treated with surgery during the same treatment period were included as a comparator to the PLS cohort for sociodemographic features and unadjusted outcomes (same cohort selection criteria for both groups, Figure S1). NSCLC adenocarcinoma was chosen as the comparator as it is the most common lung cancer histology and to limit the heterogeneity of outcomes in the comparator. In a similar fashion, patients diagnosed with pulmonary sarcomatoid carcinoma (giant-cell carcinoma, spindle-cell carcinoma, pleomorphic carcinoma, carcinosarcoma, and pulmonary blastoma) were identified and compared to the PLS cohort as these tumors are often difficult to distinguish from PLS.
Data elements
The primary outcome of this analysis was OS, defined as months from surgical resection to death or censor (December 2014). A complete list of demographics, tumor characteristics, and therapeutic metrics are available online (24). Independent covariates of interest including age, sex, race, Charlson-Deyo comorbidity index score (modified by NCDB to three groups = 0, 1, 2, ≥3), income (median income of patient’s zip code area), education (expressed as percentage of adults with no high school diploma), insurance status, facility type and location, year of diagnosis, resection margin, cancer sequence, histological subtype, tumor grade, tumor size, tumor site, and therapeutic approach were evaluated. Patients were categorized into four surgically managed groups for analysis (surgery alone, surgery and chemoradiation, surgery and chemotherapy, and surgery and radiotherapy). The NCDB does not report traditional American Joint Committee on Cancer (AJCC) stage or collaborative stage for PLS. Tumor grade was included and dichotomized into two groups: “low” (grade 1 and 2) or “high” (grade 3 and 4) (25,26). In the context of prior reports, the distribution of tumor size was dichotomized into groups less than or greater than 5 cm (larger tumors) and margin of resection into groups of R0 versus non-R0 or unknown margin. Nodal status was categorized as positive, negative, or not examined/unknown subcategories. Patients with missing surgical information were excluded.
Statistical analysis
Kaplan-Meier survival curves were created for several stratified subsets including therapeutic strategy, tumor grade, tumor size, nodal status, surgical margins status, and extent of resection. Survival curves were compared using the log-rank test, and the chi-square test was used to compare categorical variables. OS was defined as the time from surgery date to date of death from any cause, or date of last follow-up. To determine the independent predictors of survival, Cox proportional hazards regression models were built and adjusted for patient, tumor, and treatment characteristics of interest. A sensitivity analysis was performed to define the presence of a survival benefit with neoadjuvant or adjuvant therapy. Two-tailed P-values of <0.05 was considered statistically significant. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Results
We identified 695 patients with PLS, 23.1% of which were histologically classified as synovial sarcoma (including ‘not otherwise specified’ (NOS), biphasic, and spindle cell subtypes), 13.1% as malignant solitary fibrous tumor, 11.2% as sarcoma (NOS), and 11.5% as leiomyosarcoma (including NOS and epithelioid subtypes; Table S1). Sociodemographic, therapeutic, and clinical/pathological characteristics are outlined in Table 1. For PLS, mean age at diagnosis was 57.7 years (range, 18–90 years), with 40.3% of surgically managed patients diagnosed between 45–64 years of age. Most PLS patients were privately insured (50.7%). PLS patients were more likely to be from areas of higher income and education. The majority of PLS tumors were located in the lower lobe (39%) and for patients whose tumor grade was known, the majority were high-grade tumors. Only 8.6% of patients had identifiable nodal involvement following resection while 61.7% had tumors greater than 5 cm. The majority of patients were managed with surgical resection alone (64.3%), whereas 7.6% underwent surgery and adjuvant chemoradiation, 19.4% were treated with surgery and adjuvant chemotherapy alone, and 8.6% surgery and adjuvant radiotherapy alone. An R0 resection was accomplished in 73.2% of cases undergoing surgery. Most patients underwent anatomic resection (76.6%), including 19.4% that required pneumonectomy.
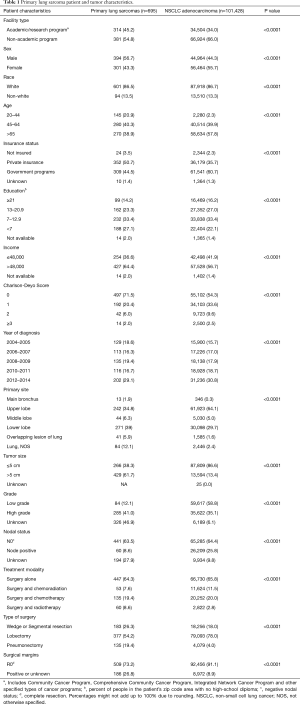
Full table
Unadjusted survival analysis
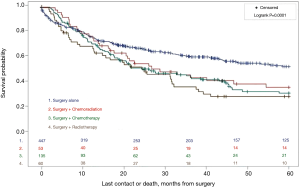
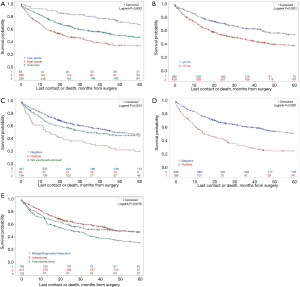
With a median follow up of 28.2 months (interquartile range, 1.8–107.5 months), the 5-year cumulative OS probability for the entire PLS study group was 44%. Surgically managed patients treated with surgical resection alone had the best 5-year OS (51%); compared to those treated with surgery and adjuvant radiation, chemotherapy or chemoradiotherapy (Figure 1). Survival analyses suggest differential outcome based on tumor grade: high grade tumors were associated with inferior OS (19%) compared to low or unknown grade (33.7% and 66.8% respectively, P<0.0001; Figure 2A). Tumors size greater than 5 cm was also associated with inferior OS compared to tumors less than 5 cm (38% vs. 54.2%; P<0.001; Figure 2B). In the unadjusted model, N0 regional lymph node status was associated with superior OS compared to N1+ nodal status (44% vs. 20%, P<0.001; Figure 2C), and an R0 resection improved OS compared to non-R0 resection (51.2% vs. 24.8% P<0.001; Figure 2D). Patients undergoing pneumonectomy had inferior OS compared to those managed with lobectomy or wedge/segmental resection (30% vs. 47% vs. 46%, P=0.001; Figure 2E). Of the most common PLS histologies, malignant solitary fibrous tumors had the best 5-year survival compared to leiomyosarcomas and synovial sarcomas (65.5% vs. 49.6% vs. 40.3% respectively, Figure S2).
Adjusted survival comparisons
Within the multivariable Cox proportional analysis of surgically-managed patients, risk for mortality was higher with increasing age (HR 1.64; 95% CI: 1.13–2.38; P=0.009), increasing number of comorbidities (HR 1.86; 95% CI: 1.24–2.81; P=0.002), high-grade tumors (HR 2.51; 95% CI: 1.59–3.97; P<0.0001), tumor size greater than 5 cm (HR 1.36; 95% CI: 1.06–1.75; P=0.01), positive nodal status (HR 1.93; 95% CI: 1.34–2.77, P=0.0004) and positive surgical margins (HR 1.93; 95% CI: 1.49–2.50; P<0.001). Female gender was also associated with favorable OS. In this model, patients treated with a combination of surgery and adjuvant chemotherapy exhibited worse OS (HR 1.41; 95% CI: 1.05–1.88, P=0.01) compared to surgery alone (Table 2).
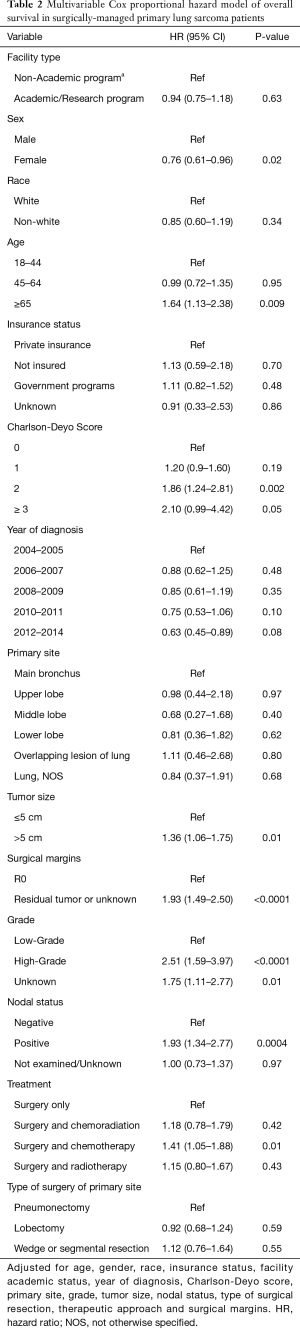
Full table
The PLS study group was then compared to 101,428 surgically managed NSCLC adenocarcinoma patients treated during the same study period (same cohort selection criteria). Patients with PLS were younger at presentation and consequently had fewer comorbidities. A larger proportion of patients were males (56.7% vs. 44.3%) with ‘larger’ tumor sizes (61.7% vs. 13.4%), and tumors that were more likely to be high-grade (41% vs. 35.1%). PLS patients were less likely to have node positive disease (Table 1). Kaplan-Meier survival analysis demonstrated five-year cumulative OS was worse for PLS (44%) compared to adenocarcinoma (53.6%, P<0.0001, Figure S3) among surgically managed patients. We then compared the survival outcomes of the PLS study group to patients with surgically managed sarcomatoid carcinomas. A total of 2,091 patients diagnosed with sarcomatoid carcinomas were identified in the same study period. Patients with sarcomatoid carcinomas had a worse 5-year survival (37.7%) than PLS (44%; Figure S4).
Discussion
Current data on PLS outcomes are limited to small case series and heterogeneous registry data that is poorly informative to a clinician or surgeon managing these patients. This retrospective analysis examined the impact of clinicopathological characteristics and therapeutic strategies on the survival of 695 patients with PLS. To our knowledge, this analysis represents the largest population-based survival study of PLS to the date. Our results are consistent with previous reports that demonstrate PLS is associated with a slight male predominance, a higher incidence in younger patients (<65 years), and large tumor sizes (1,2,8). In this study, the most common histologic subtypes identified were synovial sarcoma (including NOS, biphasic and spindle cell subtypes), malignant solitary fibrous tumor and leiomyosarcoma (including NOS and epithelioid subtypes), and sarcoma NOS. These findings are consistent with previous retrospective studies including a Surveillance, Epidemiology, and End Results (SEER) study that defined pertinent clinical data in this cancer subset (16). Also consistent with prior literature, a recognizable pattern of poor prognosis was identified for PLS patients with a high-grade malignancy, large tumor size (>5 cm), Charlson-Deyo score ≥2, and increasing age. Female gender was associated with improved survival. Five-year OS of PLS patients was significantly worse compared to surgically treated patients with NSCLC adenocarcinoma.
Another interesting point of comparison are pulmonary sarcomatoid carcinomas, which are a group of five poorly differentiated NSCLC histologies (giant-cell carcinoma, spindle-cell carcinoma, pleomorphic carcinoma, carcinosarcoma, and pleomorphic carcinoma). In some cases, these rare, highly malignant carcinomas are difficult to distinguish from PLS and have been found to have poor prognosis (27). Our data demonstrated that patients with sarcomatoid carcinomas had a worse 5-year survival than both NSCLC and PLS. Thus, accurate identification of sarcomatoid carcinomas and PLS must be performed for appropriate treatment.
Although previous studies have reported unsatisfactory response rates for nonsurgical modalities (5-year OS <20%), PLS represent a heterogeneous group of tumors without efficacious chemotherapy or radiation options and little in the way of consensus recommendations for management (1). Our findings confirm superior OS for surgical patients in whom an R0 resection was achieved in both unadjusted and adjusted models. This data is consistent with previous reports on the association between OS and complete surgical resection (1,2,5-15). In our study population, R0 resection was achievable in 73.2% of cases. Therefore, we recommend consideration for aggressive surgical resection for PLS patients in whom an R0 resection is achievable.
Surgical resection alone was the predominant therapeutic modality utilized in this study group despite the aggressive and recurrent natural history of PLS. Given the heterogeneity and small overall sample size of our cohort, it is difficult to recommend a specific treatment modality for PLS in general. In the adjusted model, surgery with adjuvant chemotherapy was found to have a worse survival compared to surgery alone (HR 1.05–1.88, P=0.01). This result is different than results from a prior meta-analysis that included patients with soft tissue sarcoma, which suggested a similar survival benefit to adjuvant chemotherapy following complete resection (28). It is possible that the small sample size, coupled with variation in outcomes of the different PLS histologies may have contributed to this discrepancy. Further investigation is warranted to define the optimal chemotherapeutic regimens that might maximize this benefit following PLS resection.
The current study contains several limitations that impact the derived results. First, the NCDB does not capture specific details on radiation and/or chemotherapy treatment modalities which may impact our analysis, such as the agents used, dosimetry, tolerability, or toxicity. There are likely patient and disease specific factors that impact treatment decisions which cannot be identified in this analysis, including the risk of selection bias and the potential for divergent outcomes associated with heterogeneous pathology within the PLS umbrella. In addition, the NCDB does not report AJCC or collaborative stage for PLS, and pathologic nodal and metastatic status were not available for all patients. Therefore, a staging analysis was not possible. Lung-cancer specific survival and recurrence are also not available in the NCDB. Given the rare nature of PLS, this cohort contains a heterogenous population with 37 different histologic subtypes, and as such, our results may not be equally generalizable to all patients with this diagnosis. Finally, there are inherent limitations common to all retrospective database analyses. Notably, there is no record of the physician decision-making process from which each therapy was selected, which in turn may affect the survival outcomes of each treatment modality. We also cannot exclude the possibility of bias in the data, for example, we noted that PLS patients are more likely to be treated at an academic or research program than NSCLC patients which may allude to a referral bias.
Conclusions
In conclusion, PLS represents a rare and aggressive group of tumors with significantly inferior long-term survival compared to patients with NSCLC. Similar to patients with NSCLC, tumor size and grade, nodal status, increasing age, male gender, and increasing comorbidities were associated with poorer prognosis. Consistent with NSCLC recommendations, we believe surgical resection alone and consideration for adjuvant therapy is advisable, even for patients with evidence of local nodal involvement, recognizing the prognosis of these more advanced cases is inferior to node negative disease (29-31). Additional studies are necessary to define optimal chemotherapeutic regimens for this cancer subset. Future therapeutic strategies, including the role of immunotherapy, may be particularly relevant in this surgical population given the high risk for recurrence associated with PLS.
Acknowledgments
The NCDB states, “the data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible or the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.”
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jtd-21-1
Peer Review File: Available at http://dx.doi.org/10.21037/jtd-21-1
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-21-1). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Yale University School of Medicine approved this study with consent waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Etienne-Mastroianni B, Falchero L, Chalabreysse L, et al. Primary sarcomas of the lung: a clinicopathologic study of 12 cases. Lung Cancer 2002;38:283-9. [Crossref] [PubMed]
- Régnard JF, Icard P, Guibert L, et al. Prognostic factors and results after surgical treatment of primary sarcomas of the lung. Ann Thorac Surg 1999;68:227-31. [Crossref] [PubMed]
- Zambo I, Vesely K. WHO classification of tumours of soft tissue and bone 2013: the main changes compared to the 3rd edition. Cesk Patol 2014;50:64-70.
- Litzky LA. Pulmonary sarcomatous tumors. Arch Pathol Lab Med 2008;132:1104-17. [Crossref] [PubMed]
- Martini N, Hajdu SI, Beattie EJ Jr. Primary sarcoma of the lung. J Thorac Cardiovasc Surg 1971;61:33-8. [Crossref] [PubMed]
- Cameron EW. Primary sarcoma of the lung. Thorax 1975;30:516-20. [Crossref] [PubMed]
- Nascimento AG, Unni KK, Bernatz PE. Sarcomas of the lung. Mayo Clin Proc 1982;57:355-9. [PubMed]
- Janssen JP, Mulder JJ, Wagenaar SS, et al. Primary sarcoma of the lung: a clinical study with long-term follow-up. Ann Thorac Surg 1994;58:1151-5. [Crossref] [PubMed]
- Corpa-Rodríguez ME, Mayoralas-Alises S, García-Sánchez J, et al. Postoperative course in 7 cases of primary sarcoma of the lung. Arch Bronconeumol 2005;41:634-7. [Crossref] [PubMed]
- Keel SB, Bacha E, Mark EJ, et al. Primary pulmonary sarcoma: a clinicopathologic study of 26 cases. Mod Pathol 1999;12:1124-31. [PubMed]
- Porte HL, Metois DG, Leroy X, et al. Surgical treatment of primary sarcoma of the lung. Eur J Cardiothorac Surg 2000;18:136-42. [Crossref] [PubMed]
- Kim YD, Lee CH, Lee MK, et al. Primary alveolar soft part sarcoma of the lung. J Korean Med Sci 2007;22:369-72. [Crossref] [PubMed]
- Cabuk D, Ustuner B, Akgul AG, et al. Primary synovial sarcoma of lung. Korean J Thorac Cardiovasc Surg 2014;47:306-9. [Crossref] [PubMed]
- Deokar KK, Kunjir NG, Ghorpade S. Primary ewings sarcoma of the lung. J Clin Diagn Res 2015;9:XD01-XD03. [PubMed]
- Petrov DB, Vlassov VI, Kalaydjiev GT, et al. Primary pulmonary sarcomas and carcinosarcomas--postoperative results and comparative survival analysis. Eur J Cardiothorac Surg 2003;23:461-6. [Crossref] [PubMed]
- Spraker MB, Bair E, Bair R, et al. An analysis of patient characteristics and clinical outcomes in primary pulmonary sarcoma. J Thorac Oncol 2013;8:147-51. [Crossref] [PubMed]
- Attanoos RL, Appleton MA, Gibbs AR. Primary sarcomas of the lung: a clinicopathological and immunohistochemical study of 14 cases. Histopathology 1996;29:29-36. [Crossref] [PubMed]
- Bacha EA, Wright CD, Grillo HC, et al. Surgical treatment of primary pulmonary sarcomas. Eur J Cardiothorac Surg 1999;15:456-60. [Crossref] [PubMed]
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017;3:1722-8. [Crossref] [PubMed]
- American College of Surgeons. National Cancer Database [WWW document]. Accessed 8-10-2018. Available online: https://www.facs.org/quality-programs/cancer/ncdb
- Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: An analysis of 26,758 cases. Int J Cancer 2006;119:2922-30. [Crossref] [PubMed]
- Corey RM, Swett K, Ward WG. Epidemiology and survivorship of soft tissue sarcomas in adults: a national cancer database report. Cancer Med 2014;3:1404-15. [Crossref] [PubMed]
- International Classification of Diseases for Oncology: Third edition. First revision 2013. Accessed 8-10-2018. Available online: http://codes.iarc.fr/home
- American College of Surgeons. National cancer Database. Data dictionary PUF 2015. PUF data dictionary items. Accessed 8-10-2018. Available online: https://www.facs.org
- Resio BJ, Chiu AS, Hoag J, et al. Primary Salivary Type Lung Cancers in the National Cancer Database. Ann Thorac Surg 2018;105:1633-9. [Crossref] [PubMed]
- Thiels CA, Bergquist JR, Krajewski AC, et al. Outcomes of Primary Colorectal Sarcoma: A National Cancer Data Base (NCDB) J Gastrointest Surg 2017;21:560-8. Review. [Crossref] [PubMed]
- Steuer CE, Behera M, Liu Y, et al. Pulmonary Sarcomatoid Carcinoma: An Analysis of the National Cancer Data Base. Clin Lung Cancer 2017;18:286-92. [Crossref] [PubMed]
- Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113:573-81. [Crossref] [PubMed]
- Eberhardt WE, De Ruysscher D, Weder W, et al. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol 2015;26:1573-88. [Crossref] [PubMed]
- Lackey A, Donington JS. Surgical management of lung cancer. Semin Intervent Radiol 2013;30:133-40. [Crossref] [PubMed]
- Vansteenkiste J, Crino L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [Crossref] [PubMed]


