Options for temporary mechanical circulatory support
Background
Temporary mechanical circulatory support (MCS) refers to a group of devices generally used for less than 30 days to maintain adequate organ perfusion by compensating for a failure of the pumping mechanism of the heart. Beginning with the first clinical use of intra-aortic balloon pumps (IABPs) in 1968, MCS devices have traditionally been used for treating patients with acute circulatory collapse, post-cardiotomy syndrome, or as a bridge to more definitive therapy (1). However, over the last several decades the scope of applications has widened, and the availability of easily deployable devices has increased significantly. Furthermore, the field has seen a paradigm shift in the use of temporary MCS from reactive to prophylactic support prior to high-risk percutaneous interventions (HR-PCI), ablations, or transcatheter valve replacements. Consequently, analysis of national trends in a 4-year period from 2007-2011 showed the use of percutaneous devices for short-term MCS had increased by 1,511%, and the use of non-percutaneous devices increased by 101% (2).
The increased availability and rapid adoption of new temporary MCS devices necessitate physicians to become familiar with these commonly used technologies. This review will examine the different options for temporary MCS devices placed both percutaneously and via median sternotomy including the IABP, veno-arterial-extracorporeal membrane oxygenation (VA-ECMO), TandemHeart® (CardiacAssist, Pittsburg, PA, USA) Impella® and BVS 5000® (both Abiomed Inc., Danvers, MA, USA), CentriMag® and Thoratec percutaneous ventricular assist device (pVAD)® (both Thoratec Corporation, Pleasanton, CA, USA).
Indications
Temporary MCS are differentiated from long-term devices in that they are indicated when recovery is expected (bridge-to-recovery), or if the outcome is uncertain and more time is needed prior to making a definitive decision (bridge-to-decision) (3). For the purpose of this review, temporary MCS refer to devices that are generally used for less than 30 days. The indications for temporary MCS can be classified using both hemodynamic parameters and specific clinical conditions as shown in Table 1.
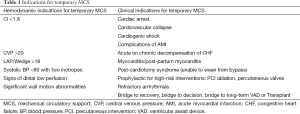
Full table
General contraindications to temporary MCS
Prior to initiating temporary MCS, it is important to consider situations that may preclude a patient from achieving benefit from MCS, such as documented irreversible neurologic damage or disseminated malignancy. Patients with bleeding disorders or platelet counts less than 40 k may not be able to tolerate the anticoagulation required with temporary MCS; therefore, a contraindication to anticoagulation is generally a contraindication to temporary MCS placement. Furthermore, severe peripheral vascular disease (PVD) may prevent arterial cannulation or result in peripheral ischemia. Device specific contraindications are shown below in Table 2 and will be described in detail in Section 2 and Section 3.
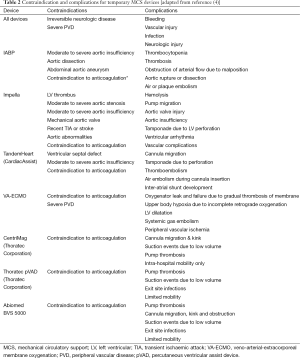
Full table
Classification (as per approach)
Temporary MCS can be classified per the approach used for placement. Devices placed percutaneously described in this review include the IABP, Impella, TandemHeart, and VA-ECMO. Devices placed via median sternotomy described in this review include CentriMag, Thoratec pVAD, and Abiomed BVS 5000. The following two sections will provide an overview of the mechanism of action, hemodynamics, device-specific contraindications, complications, daily management considerations, and briefly discuss clinical data for select devices.
Percutaneous temporary MCS
Intra-aortic balloon pump (IABP)
Overview of mechanism of action, and hemodynamics
The IABP is the most widely used and most affordable form of MCS (5). It is frequently placed for cardiogenic shock complicating acute myocardial infarction (AMI), coronary perfusion prior to revascularization, prior to HR-PCI, and as a bridge-to-decision or therapy with a higher level of MCS. The IABP is a counter-pulsation device and is comprised of an 8-9 Fr inflatable balloon catheter and a pump. The balloon catheter is typically placed via the femoral artery to sit in the descending aorta distal to the left subclavian artery and proximal to the renal arteries. For patients who need long-term support alternative insertion approaches including axillary/subclavian have been described (6).
During diastole the intra-aortic balloon fills with helium obstructing the lumen of the descending aorta. This increases diastolic pressure resulting in increased coronary perfusion. During systole, the balloon rapidly deflates creating a vacuum effect thereby decreasing afterload and subsequently reducing myocardial oxygen consumption. The IABP is unique in its ability to improve coronary perfusion. However, unlike other forms of MCS, the IABP does not provide significant increases in peripheral tissue perfusion or cardiac output in liters/minute.
Of note, the triggering of inflation and deflation is synchronized with the cardiac cycle, which can be based either on pressure triggers or the electrocardiogram (ECG). When triggered on ECG, diastolic inflation occurs roughly in the middle of the t-wave or during repolarization of the heart (7). By utilizing the cardiac cycle as a triggering mechanism, inadequate or improper balloon inflation or deflation can occur in the setting of arrhythmias, poor ECG quality, or tachycardia.
Device specific contraindications, complications, and daily management considerations
The primary contraindications to IABP use include aortic valve regurgitation, which may worsen secondary to diastolic balloon inflation, and aortic dissection or aneurysm. The most common severe complication is limb ischemia which occurs in roughly 1% of patients (8). Daily management considerations include ensuring optimal placement of the device via daily chest X-rays and optimizing IABP triggering to the cardiac cycle (5).
Clinical data
Clinical data regarding the use of IABP for cardiogenic shock complicating AMI has failed to show conclusive benefit in prospective randomized studies. A 2009 meta-analysis of seven randomized trials (n=1,009) by Sjauw et al., examining IABP use in ST-elevation myocardial infarction showed neither an improved left ventricular ejection fraction (LVEF) nor 30-day survival benefit, and was associated with higher stroke and bleeding rates (9). The randomized, multicenter IABP-Shock II Trial of 600 patients with cardiogenic shock complicating AMI also did not show any significant differences between the IABP and non-IABP group with respect to the primary end point of 30-day mortality (10). Furthermore, no significant differences were seen in any of the secondary end-points including time to hemodynamic stabilization, length of stay in ICU, serum lactate levels, renal function, or length of stay in intensive care unit. As a result, the 2013 AAC/AHA guidelines for IABP use in the setting of cardiogenic shock complicating AMI were downgraded from IA/B to IIA/B recommendation.
In spite of this, it remains the most commonly deployed MCS device with over 60,000 implants performed yearly. The likely benefit is from increased coronary perfusion and hence, it still has a role to play in acute coronary syndromes and when the severity of the cardiovascular stability does not warrant escalation to “true MCS”. It is a versatile device that is easy to insert even at bedside without any radiographic or echocardiographic control, lending it useful in relatively inexperienced hands.
Impella® 2.5, CP and 5.0 (Abiomed Inc.)
Overview of Mechanism of Action and Hemodynamic Effects
The Impella system is a miniaturized, continuous flow, axial pump contained within a single pigtail catheter. Utilizing the Archimedes-screw principle, blood is pumped from the left ventricle to the ascending aorta by rotating a screw-shaped surface inside a small, hollow pipe that traverses the aortic valve. The single pigtail catheter (sized 12-21 Fr depending on the model) is placed via the femoral artery, in a retrograde fashion, so that the inlet to pump sits in the left ventricle and the outlet in the ascending aorta. Three commonly used versions currently available provide a maximum flow of 2.5 L/min (Impella 2.5 via 12 Fr), 3.3 L/min (Impella CP via 14 Fr) and 5.0 L/min (Impella 5.0 via 21 Fr). The Impella 2.5 and Impella CP can be placed percutaneously while the Impella 5.0 is placed via surgical cut down.
By pumping blood from the left ventricle into the ascending aorta, the Impella system increases the forward flow of blood. This improves mean arterial pressure, peripheral tissue perfusion and slightly reduces PCWP. This pump-assisted forward blood flow also reduces stroke volume, directly unloading the left ventricular (LV) and thus reducing myocardial oxygen consumption. Compared to IABP, the Impella provides a greater increase in cardiac output.
The Impella RP was recently approved in 2015 for right-sided support utilizing an inlet area in the inferior vena cava and an outlet in the pulmonary artery; however experience with this device is limited.
Device specific contraindications, complications, and daily management considerations
Primary contraindications to Impella use include the presence of LV thrombus or mechanical aortic valve. Relative contraindications include aortic valve stenosis or regurgitation. Special considerations should be made in patients with biventricular failure, as sufficient right ventricular function is required to maintain LV preload and hemodynamic support. Furthermore, considerations should be made for patients with a pre-existing VSD, as the Impella may cause increased right-to-left shunting and subsequent hypoxemia.
Within the first 24 hours of use, hemolysis secondary to mechanical erythrocyte shearing has been reported in approximately 5-10% of patients (11). Device repositioning may alleviate this complication; however, persistent hemolysis leading to acute kidney injury is an indication for removal. Other complications include aortic valve injury, papillary muscle snaring and shearing, and tamponade secondary to pump migration causing LV perforation. Also, post-MI patients may be prone to arrhythmia as the presence of the pigtail catheter may irritate an arrhythmogenic LV wall. Daily management includes adjusting pump speed to ensure adequate flow and optimizing pump positioning which can be confirmed via echocardiography. The device is FDA approved for up to 6 hours of use, however clinically it has been used up to several days.
Clinical data
The prospective PROTECT II trial, randomized 452 patients undergoing HR-PCI with complex 3-vessel disease or unprotected left main coronary artery disease and LVEF <35% to either Impella 2.5 or IABP placement (12). While there was not a statistically significant difference in the primary endpoint of a 30-day composite of adverse events, there was a trend toward less adverse events in the Impella group (34.3 Impella vs. 42.2% IABP, P=0.092). In the correct patient population, the Impella appears as safe as IABP but offers advantages of improved hemodynamics based on the mechanism of action when compared to the IABP.
TandemHeart (CardiacAssist)
Overview of Mechanisms of Action and Hemodynamics Effects
The TandemHeart system is an extracorporeal, centrifugal, continuous flow pump, which can be used for left, right, and biventricular failure. For LV support, the pump aspirates oxygenated blood from the left atrium and pumps it into the femoral artery thereby bypassing the left ventricle. Placement of the inflow cannula into the left atrium occurs via a 21 Fr catheter into the femoral vein. This cannula is then advanced to the right atrium with subsequent transeptal puncture into the left atrium. The trans-septal cannula contains 14 side holes and a large end hole that facilitate aspiration of left atrial blood to the pump. The outflow cannula to the femoral artery consists of a 15-19 Fr cannula.
Of note, flow via the pump to the femoral artery is additive to the patient native LV output from the heart. The size of the femoral arterial cannula determines the maximal flow; a 15 Fr cannula provides approximately 3.5 L/min of additional flow, while the 19 Fr cannula can provide up to 5 L/min of additional flow. With this LA to FA ventricular bypass system, both the heart and the pump work together in parallel, or in tandem, to provide flow to the aorta (in contrast to the Impella that works in series with the heart). While pump function is additive to the function of the heart, it is important to note that native heart LV ejection is reduced when the pump is in use secondary to reduced LV preload.
The hemodynamics effects of the TandemHeart are a function of the ability to redirect blood flow from the left atrium to the femoral artery. As more blood is redirected from the LA to the femoral artery, LV preload and stroke volume decrease, which in turn reduces LV workload and myocardial oxygen demand (13). By working in tandem with the heart, peripheral tissue perfusion is increased. For RV support, the inflow cannula is placed in the right atrium and the outflow cannula is placed in the pulmonary artery.
Device specific contraindications, complications, and daily management considerations
Adequate functioning of the TandemHeart is dependent on left atrial volume; thus, in the setting of poor right ventricular (RV) function a concomitant right ventricular assist device (RVAD) may be needed to maintain left atrial volume. Generally, a VSD and severe aortic regurgitation are contraindications to use, however there is limited experience in this setting (7). As a consequence of utilizing a transeptal puncture from right atrium to left atrium, the presence of a right or left atrial thrombus is a contraindication to use as it may result in thromboembolism into the systemic circulation.
Important complications are related to the transeptal puncture and placement of an unanchored cannula into the left atrium. During cannula insertion, air embolisms may be introduced into systemic circulation. Furthermore, cannula migration into the LA wall can lead to tamponade secondary to perforation. Conversely, cannula migration back into RA can lead to inter-atrial shunt development. Finally, thromboembolism is a significant concern and activated clotting times of approximately 300 are generally required.
Daily management considerations include ensuring optimal placement of the device, avoiding patient maneuvering that may dislodge the cannula, and optimizing afterload and pump speed to maintain adequate flow. The TandemHeart is approved for up to 6 hours; however it has been used on the order of days clinically.
Clinical Data
Burkhoff et al., randomized 33 patients within 24 hours of developing cardiogenic shock to IABP (n=14) or TandemHeart (n=19) (14). Patients with TandemHeart showed improved cardiac indices and decreased pulmonary capillary wedge pressures, however, differences in overall 30-day survival were not statistically significant between groups. A retrospective study in 2012 by Kar et al., evaluated 117 patients with severe cardiogenic shock refractory to IABP and/or vasopressor use (15). Placement of the TandemHeart resulted in statistically significant improvements of hemodynamic measures including increases in cardiac index, systolic blood pressure (BP), urine output and decreases in pulmonary capillary wedge pressure, lactic acid levels, and creatinine levels.
Veno-arterial-extracorporeal membrane oxygenation (VA-ECMO)
Overview of mechanism of action and hemodynamics effects
VA-ECMO is an extracorporeal device that utilizes both a centrifugal, continuous flow pump to provide MCS and a membrane oxygenator to facilitate carbon dioxide and oxygen exchange. VA-ECMO allows for full biventricular cardiopulmonary support and has recently seen increased utilization nationally (16). Unlike the TandemHeart, VA-ECMO can be placed quickly at the bedside if needed. VA-ECMO is placed percutaneously using an 18-21 Fr cannula into the femoral vein that aspirates deoxygenated blood to the pump and membrane oxygenator. After gas exchange, the oxygenated blood is actively pumped, with flows up to 6 L/min, into the systemic circulation via a 15-17 Fr cannula placed in the femoral artery.
Important hemodynamic effects of VA-ECMO are due to the active pumping of venous blood into the arterial circulation. The increased arterial blood volume results in increased afterload, which consequently increases myocardial oxygen demand. This is in contrast to the Impella, which directly reduces LV volume and pressure (i.e., unloads the LV) and TandemHeart, which also reduces LV stroke work, albeit indirectly via LA unloading and reduced LV preload. The negative consequence of VA-ECMO on myocardial protection are theoretic, but may potentially be avoided by LV venting or unloading with an IABP or Impella (17).
Device specific contraindications, complications, and daily management considerations
Contraindications to EMCO are similar to other types of percutaneous MCS devices. In the setting of severe peripheral arterial disease, central cannulation should be considered over peripheral cannulation. Also, in patients with severe aortic insufficiency, a venting strategy can be considered to avoid increased ventricular wall stress. Complications include bleeding, thrombosis of circuit, incomplete retrograde oxygenation leading to cerebral, coronary, and upper extremity hypoxia, infection, and systemic gas embolism. As mentioned previously, increased afterload in the setting of severe LV dysfunction can lead to LV distension and possibly pulmonary edema (17). In select cases, a second, antegrade 5-6 Fr arterial sheath can be spliced into arterial outflow cannula to ensure flow to distal extremities in order to avoid limb ischemia. Daily management includes adjustment of flow rates to optimize tissue perfusion, LV monitoring with arterial line waveform and frequent echocardiography, and aggressive diuresis as many patients are fluid overloaded when ECMO is initiated. While FDA approved for up to 32 days, it is traditionally only used for several days.
Clinical data
No randomized trials using ECMO currently exist. A meta-analysis by Cheng et al., of 1,866 patients receiving ECMO for treatment of cardiogenic shock and cardiac arrest showed a survival to hospital discharge between 20.8-65.4% (18). Schmidt et al., developed a survival calculator based on analysis of 3,846 patients with refractory cardiogenic shock treated with ECMO between 2003 and 2013 (19). Improved survival rates are associated with treatment of patients with cardiogenic shock from myocarditis, refractory VT/VF or post heart or lung transplantation. Other factors such as decreased age significantly improve predicted survival particularly for patients less than 63 years of age. The surgical calculator can be found at http://www.save-score.com/.
Temporary MCS placed via median sternotomy
CentriMag® (Thoratec Corporation)
The CentriMag is a centrifugal, continuous flow extracorporeal pump, and is one of the most common surgically implanted devices for temporary MCS (20). The pump contains a magnetically levitated Impella that is contact-free without mechanical bearings or seals. It is designed to minimize friction and heat generation, and to reduce shear force on RBCs to prevent hemolysis. The CentriMag provides LV, RV or Bi-V support depending on cannula placement. For LV support, a 28 Fr cannula is placed in the LA using simple purse string sutures. Blood is aspirated into the pump and is returned to the aorta via a 20-22 Fr cannula at a rate up to 5-7 L/min. For RV support, the inflow cannula is placed in the RA and returned to the body via PA. The CentriMag can also be used as part of an ECMO circuit and has FDA approval for 6 hours of use for LV support, and up to 30 days for right ventricular support. However, CentriMag use over 30 days as a bridge-to-solution has been reported clinically with acceptable survival without significant increases in device-related complications (21).
The primary contraindication is patients who are unable to be treated with anticoagulation. A retrospective review by Takayama et al., of 143 patients who received CentriMag as bridge-to-decision therapy showed 69% survival at 30 days and 49% survival at one year, with major bleeding events occurring in 33%, and cerebrovascular accidents occurring 14% of patients (22). A larger meta-analysis and systematic review of 999 patients by Borisenko et al., showed survival rates while on support between 62-83%, and 30-day survival between 41-66% depending on indication for placement (20). Rates of complications in the meta-analysis were highest for bleeding requiring exploration occurring in 28% (95% CI: 23-32), followed by renal complication at 28% (95% CI: 22-36), and infections in 24% (95% CI: 19-30). Rates of thrombosis were 7% (95% CI: 5-11) and neurological complications 7% (95% CI: 4-11), with hemolysis occurring in 3% (95% CI: 1-6), and device failure 0.08% (95% CI: 0.0-0.5). There are no randomized trails examining the use of CentriMag.
Thoratec pVAD® (Thoratec Corporation)
Thoratec pVAD is a paracorporeal, pulsatile, pneumatically driven pump that is surgically placed. Individual pumps can be used for LV and RV support separately, or two pumps can be used together for Bi-V support. The pumps are positioned outside of the body on the anterior abdominal wall. Each pump consists of a 65 mL stroke volume pumping chamber with two mechanical disk valves that maintain unidirectional blood flow. The pumping chamber is compressed by alternating positive and negative air pressure to achieve ejection at a rate of 40-110 beats/min and a flow between 1.3-7.2 L/min. The pump can be operated in three modes: synchronous to EKG, fixed rate, or fill-to-empty, with the most common being the fill-to-empty mode (3). In this mode, the pump rate is determined by the VAD filling.
The pump sits outside the body, thus the only internal component is the cannula. This facilitates use in patients of nearly all sizes. Placement typically requires cardiopulmonary bypass. For LV support, the inflow cannula can be placed in the apex of the left ventricle and the outflow is typically placed in the ascending aorta. For RV support, the inflow cannula is placed in the right atrium, and the outflow cannula is placed in the pulmonary artery.
The primary contraindication to use is patients who are unable or unwilling to be treated with anticoagulation. It is approved for both short-term and long-term use, and as a bridge-to-transplant and a bridge-to-recovery. The use of this device is waning and is restricted to few sites in the United States.
Abiomed BVS 5000 (Abiomed Inc.)
The Abiomed BVS 5000 is a paracorporeal, pulsatile, pneumatically driven pump that is surgically placed. Individual pumps can be used for LV and RV support separately, or two pumps can be used together for Bi-V support. Each pump is composed of two chambers; the atrial chamber fills passively by gravitational force and is connected to the ventricle chamber by a trileaflet valve. The pumping mechanism is automated and self-regulating. During systole, compressed air enters the ventricular chamber and compresses the blood filled bladder delivering a constant stroke volume of 80 mL. While Abiomed BVS 5000 can provide flows up to 6 L/min, the flow is primarily determined by the amount of drainage passively received by the atrial chamber, which is a function of a patient’s volume status. Therefore, in the setting of decreased pump flow, volume resuscitation of the patient would increase passive filling of the device and improve pump flow back to the patient. The ease of use is the primary advantage of this device. The Abiomed BVS 5000 is approved for all types of recoverable heart failure. For left heart support the inflow cannula is placed in the LA with outflow to the thoracic aorta. For right heart support the inflow cannula is placed in RA and the outflow cannula is placed in the pulmonary artery. The use of this device is also waning and is limited to a few sites in the United States.
Management decisions post-op
Immediate post-op
If signs of cardiac recovery are present, such as improved cardiac indices, LVEF, lactate levels, or PCWP, then the patient should be weaned from temporary MCS. However, if hemodynamic parameters or clinical signs fail to show improvement, then considerations should be made for long-term MCS or withdrawal of support (for patients with permanent neurological damage).
The most important perioperative management soon after insertion or implementation of temporary MCS is the control of bleeding. This may require frequent trips to the OR until all bleeding is under control. We do not start anticoagulation until all bleeding has ceased. In absence of bleeding, the anticoagulation consists of aspirin with heparin infusion (PTT goal of 40-80 seconds). In select cases we would use a dual antiplatelet agent such as Plavix. We do not use Coumadin in temporary MCS cases for the simple reason of being able to reverse anticoagulation promptly in case of bleeding.
Patients frequently need some initial pressor support, however, continued pressor support beyond 24 hours should prompt search for an undiagnosed sepsis, with aggressive culture and coverage with broad-spectrum antibiotics due to high rates of infection. We frequently keep a low dose beta-agonist onboard (either low dose dopamine or epinephrine with some milrinone) for pulsatility of native ventricles to wash the biological valves and prevent stasis within ventricles. The old idea of giving “rest” to the ventricles merely leads to intraventricular clot formation and limits further therapy and leads to complications such as CVA. Aggressive cardioversion from any atrial flutter or atrial fibrillation is a good strategy to avoid any stasis within the cardiac chambers.
Optimization
The most important aspect in the management of these patients is to start optimizing them as soon as they are stable from the perioperative or peri-insertion stage. This includes judicious use of diuretics (including infusion of furosemide and boluses of chlorothiazide) to reduce volume overload. Merely putting a biventricular assist device (BIVAD) or RVAD/ECMO is unlikely to bring the filling pressures down; one needs to aggressively diuresis the patient while maintaining forward flow of the pump. In situations such as acute renal injury, a prompt institution of CVVH either via dedicated line or via the circuit of the pump is essential. We do not wait more than two hours in the case of anuria to institute CVVH in these patients, the recovery in these patients from acute renal injury is surprisingly quick if CVVH is instituted early on and volume status is optimized. As one adds additional pumps such as the roller pumps in CVVH circuit, one must be watchful of platelet consumption. At all times platelet count greater than 50 k should be maintained.
Weaning protocol
Our protocol includes initial assessment by bedside turning down flows and rpms while watching pulse pressure and degree of pulsatility in absence of any optimization maneuvers. If this step shows adequate pulse pressure (>30 mm of Hg) and central venous pressure (CVP) that doesn’t rise more than 2 cm under maximally optimized conditions, then the patient should ideally have a swan placement and a transthoracic echo assessment where the rpms are turned down serially under adequate anticoagulation. We usually do this in the presence of the surgeon, heart failure cardiologist, and an expert cardiologist experienced in echocardiographic interpretations. Chamber sizes and mixed venous saturations and hemodynamic data are collected while visual estimation of ejection fraction and tricuspid annular plane systolic excursion (TAPSE) is done. Also important is the concordance of the both right and left ventricle and how weaning of one chamber support affects another and vice versa. One does not need to turn down the pumps completely; we seldom if ever need to reduce rpms below 1,000-1,500 (CentriMag or Tandem Heart).
If the patient is able to maintain hemodynamics and adequate cardiac output as per hemodynamics, with good echocardiographic signs of recovery, then we proceed to the next step, if not, we resume the support and continue to support the patient with a view to assessing function again in a week’s duration.
The next step of weaning is carried out in the operating room under TEE guidance and with adequate anticoagulation on board to have ACT >300 seconds. Usually the support is completely withdrawn and pumps stopped for duration of 5-10 minutes, and patient’s hemodynamics is observed continuously. Any deterioration or continual need for escalation of inotropes or pressors is a contraindication to proceeding for explantation, but if the patient stays stable then explantation should proceed.
Conclusions
The utilization of temporary MCS has evolved significantly over the last several decades. Physicians and surgeons are now equipped with an array of temporary support devices that are being used both to treat and prevent cardiovascular collapse and improve hemodynamic parameters in a wide variety of clinical situations. While data is lacking that directly compares temporary MCS devices, selection is typically guided by availability of devices and patient-specific factors and contraindications. Further studies that directly compare devices are needed to provide better guidance on device selection and placement.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kantrowitz A, Tjonneland S, Freed PS, et al. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA 1968;203:113-8. [PubMed]
- Stretch R, Sauer CM, Yuh DD, et al. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol 2014;64:1407-15. [PubMed]
- Bellumkonda L, Bonde P. Ventricular assist device therapy for heart failure--past, present, and future. Int Anesthesiol Clin 2012;50:123-45. [PubMed]
- Gilotra NA, Stevens GR. Temporary mechanical circulatory support: a review of the options, indications, and outcomes. Clin Med Insights Cardiol 2015;8:75-85. [PubMed]
- Abnousi F, Yong CM, Fearon W, et al. The evolution of temporary percutaneous mechanical circulatory support devices: a review of the options and evidence in cardiogenic shock. Curr Cardiol Rep 2015;17:40. [PubMed]
- Estep JD, Cordero-Reyes AM, Bhimaraj A, et al. Percutaneous placement of an intra-aortic balloon pump in the left axillary/subclavian position provides safe, ambulatory long-term support as bridge to heart transplantation. JACC Heart Fail 2013;1:382-8. [PubMed]
- Rihal CS, Naidu SS, Givertz MM, et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol 2015;65:e7-e26. [PubMed]
- Severi L, Vaccaro P, Covotta M, et al. Severe intra-aortic balloon pump complications: a single-center 12-year experience. J Cardiothorac Vasc Anesth 2012;26:604-7. [PubMed]
- Sjauw KD, Engström AE, Vis MM, et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J 2009;30:459-68. [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287-96. [PubMed]
- Lauten A, Engström AE, Jung C, et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circ Heart Fail 2013;6:23-30. [PubMed]
- O'Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation 2012;126:1717-27. [PubMed]
- Kapur NK, Paruchuri V, Urbano-Morales JA, et al. Mechanically unloading the left ventricle before coronary reperfusion reduces left ventricular wall stress and myocardial infarct size. Circulation 2013;128:328-36. [PubMed]
- Burkhoff D, Cohen H, Brunckhorst C, et al. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J 2006;152:469.e1-8.
- Kar B, Gregoric ID, Basra SS, et al. The percutaneous ventricular assist device in severe refractory cardiogenic shock. J Am Coll Cardiol 2011;57:688-96. [PubMed]
- Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J 2015;61:31-6. [PubMed]
- Koeckert MS, Jorde UP, Naka Y, et al. Impella LP 2.5 for left ventricular unloading during venoarterial extracorporeal membrane oxygenation support. J Card Surg 2011;26:666-8. [PubMed]
- Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014;97:610-6. [PubMed]
- Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015;36:2246-56. [PubMed]
- Borisenko O, Wylie G, Payne J, et al. Thoratec CentriMag for temporary treatment of refractory cardiogenic shock or severe cardiopulmonary insufficiency: a systematic literature review and meta-analysis of observational studies. ASAIO J 2014;60:487-97. [PubMed]
- Mohite PN, Zych B, Popov AF, et al. CentriMag short-term ventricular assist as a bridge to solution in patients with advanced heart failure: use beyond 30 days. Eur J Cardiothorac Surg 2013;44:e310-5. [PubMed]
- Takayama H, Soni L, Kalesan B, et al. Bridge-to-decision therapy with a continuous-flow external ventricular assist device in refractory cardiogenic shock of various causes. Circ Heart Fail 2014;7:799-806. [PubMed]


