The BCL11A-XL expression predicts relapse in squamous cell carcinoma and large cell carcinoma
Introduction
Some cases of human lymphoblastic leukemia related to chromosomal translocation t(2;14) (p13;q32) and the comparative genomic hybridization analysis approved that 2p13 abnormalities associated with human malignant lymphoma (1,2). According to these findings, the B cell leukemia 11A (BCL11A) gene was considered as a proto-oncogene of malignant hematopoietic diseases, also further studies showed that it is essential for pre-B-cell development, lymphocyte maturation, and goblin switching (3-6). The BCL11A gene also has three mRNA transcripts: BCL11A-XL, BCL11A-L and BCL11A-S (7). The BCL11A-L and S isoforms show 98.7% identity and 99.2% similarity to the mouse Evi9-a and Evi9-c, respectively (8). BCL11A-XL protein isoform was specifically generated in human and was restricted expression in bone marrow, lymphoid tissue and brain (3,7). Also BCL11A-XL expressed in a range of tumor-derived cell lines, such as primary mediastinal B-cell lymphoma (PMBLs), germinal center B-cell diffuse large B-cell lymphoma (GCB-DLBCLs) and activated B-cell diffuse large B-cell lymphoma (ABC-DLBCLs) (9). Function studies showed BCL11A-XL was a DNA-sequence-specific transcriptional repressor that associated with itself and with other BCL11A isoforms, as well as with the BCL6 proto-oncogene. So BCL11A-XL might play an essential role in tumor development (7,9).
BCL11A gene involvement in solid tumors has been rarely investigated. Khaled et al. reported BCL11A becomes an oncogene of triple-negative breast cancer and its overexpression promotes tumor formation (10). Our previous results also demonstrated that BCL11A protein expression levels were specifically upregulated in non-small cell lung cancer (NSCLC) tissues, especially in squamous cell carcinoma (SCC) and large cell carcinoma (LCC). Multivariate analysis showed that BCL11A was an independent prognostic factor for both disease-free survival (DFS) and overall survival (OS) (11). We investigated the protein isoforms of BCL11A in NSCLC and analysed the relationship between isoforms and clinicopathological parameters.
Materials and methods
Tissue samples and clinicopathological characteristics
Specimens were all BCL11A overexpression selected from Jiang et al.’s database and obtained informed consent from 40 NSCLC cases (27 SCCs, 8 LCCs and 5 adenocarcinomas (ACs)] who underwent potentially curative surgery at Guangdong Lung Cancer Institute between 2003 and 2008 (11). Form each samples, seven sections were prepared, one section for pathological assessment, the other three for negative control. Hemotoxylin and eosin (H&E) staining was performed on sections of each tissue to determine the percentage of tumor cells by two independent pathologists. Only those samples with tumor content ≥80% were allowed to enter this study. This study was approved by the Institutional Review Board (IRB) of Guangdong General Hospital. The staging and histological classifications were based on the World Health Organization (WHO) system. A follow-up evaluation was performed according to standard follow-up protocol. The median follow-up period was 73.9 months (range, 3.27-130.1 months).
Immunohistochemistry (IHC) antibodies
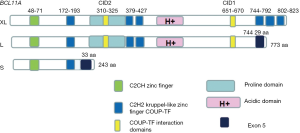
The primary mouse monoclonal antibody BCL11A/123 (Active Motif, USA) was raised against a recombinant protein corresponding to amino acids 637-835 of BCL11A-XL isoform protein (9). The other two primary mouse monoclonal antibodies BCL11A (ab19487 and ab18688; Abcam, USA) were applied against human BCL11A-L and S isoforms. The ab19487 antibody which epitope is in core of amino acids 172-434 can identify the BCL11A-XL and L isoforms (12). The Figure 1 shows the specificity of the three antibodies. We can use the exclusive method to distinguish the two isoforms. The ab18688 antibody which epitope is in the core of amino acids 1-171 can identify all the three isoforms (13). The previous study showed that the S isoform locates in cytoplasm while the XL and L are both in the nucleus (3,14). So in theory we can distinguish the three isoforms from each other. The second antibody was Mouse IgG (GeneTex, USA) labeled by enzyme horseradish peroxidase.
IHC on tissue samples
Immunohistochemical staining process was performed according to the protocol provided by DAKO (DakoCytomation, Glostrup, Denmark) (15). Primary antibodies were applied to the sections at a dilution of 1:100 at 4 °C temperature overnight. Those in the control group underwent the same way by add PBS. The sections were counterstained with Harris’s hematoxylin. Each tumor was assigned a score according to the intensity of the nucleic or cytoplasmic staining (0= no staining, 1= weak staining, 2= moderate staining, and 3= strong staining) and to the proportion of stained tumor cells (0=0%, 1=1-10%, 2=11-50%, 3=51-80%, and 4=81-100%), and judged by two pathologists, independently (15). The final immunoreactive score was determined by multiplying the intensity scores by the extent of positivity scores of stained cells, with a minimum score of 0 and a maximum score of 12 (11). Tumors with scores ≥2 were classified into the positive BCL11A isoforms expression group, while the others were classified into the negative group.
Statistical analysis
All analyses were performed using SPSS 13.0 software. The chi-square test was used to compare qualitative variables, and those with an expected frequency of <5 were analyzed by Fisher’s exact test. A non-parametric test was used to analyze quantitative data. Chi-square tests were used to assess the association of BCL11A-XL level with clinical variables. Survival curves between subgroups divided according to BCL11A-XL expression level were analyzed using the Kaplan-Meier method, and significant differences among subgroups were compared by log-rank test. A multivariate analysis was performed using the stepwise method. Hazards ratios (HR) and 95% confidence intervals (CI) were calculated using Cox proportional hazards models. P values <0.05 were considered statistically significant.
Results
The isoforms of the BCL11A in NSCLC tissues
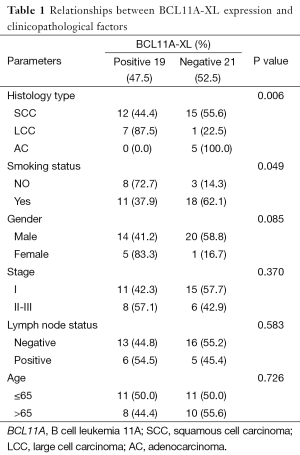
In our study we found that the BCL11A-XL protein isoform differentially expressed in SCC, LCC and AC. For the total 40 cases, 19 (47.5%) cases had positive BCL11A-XL expression and 21 (52.5%) cases had negative BCL11A-XL expression. For the 19 BCL11A-XL positive patients, SCC accounts for 63.2% (12/19), LCC accounts for 36.8% (7/19) and no positive case for AC (Table 1). The subcellular location was in the nucleus, which is accordance with other study results (7,9) (Figure 2). The BCL11A-L and S isoforms were positive for all 40 cases with no histology difference in my research (data not shown).
Full table
Correlation analysis between the expression of BCL11A-XL and patient clinicopathological characteristics
According to the results, the BCL11A-XL protein isoform was differentially expressed in SCC, LCC and AC (AC carcinoma). So the correlation analysis was performed to explore whether BCL11A-XL expression was related to clinicopathological variables in patients with NSCLC. The result showed that BCL11A-XL expression had no relationship with gender, clinical stage, lymph node status and age, but it did correlate with histology (P=0.006) and smoking status (P=0.049) (Table 1).
BCL11A-XL protein expression correlates with DFS in early stage patients with NSCLC
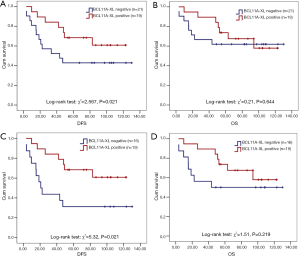
For all 40 patients subjected to immunohistochemical staining for BCL11A-XL, 19 (47.5%) had positive BCL11A-XL expression and 21 (52.5%) had negative BCL11A-XL expression. In all patients, the DFS and OS were both no statistically significant different between BCL11A-XL positive and negative groups (P>0.1) (Figure 3A,B). However, in the subgroup of patients with SCC and LCC, BCL11A-XL expression was predictive of better DFS (χ2=5.32, P=0.021) (Figure 3C). The median DFS of subgroup patients without BCL11A-XL expression was 20.8 months, but in the BCL11A-XL positive expression group, only 36.8% of patients relapsed at the endpoint of follow-up. Although no relationship between BCL11A-XL expression and OS was found in SCC and LCC patients, there was a tendency towards decreased survival in patients whose tumors lack of BCL11A-XL (P>0.1) (Figure 3D). The Cox regression survival analysis indicated that BCL11A-XL expression was an independent marker of DFS (HR 0.246; 95% CI, 0.065-0.939, P=0.040) in patients with NSCLC (Table 2), but not for OS.

Full table
Discussion
The current study analyzed the protein isoforms of BCL11A in NSCLC. The result demonstrated that BCL11A-XL protein differentially expressed in SCC and LCC. Statistical analyses demonstrated that BCL11A-XL expression was strongly associated with histology with NSCLC. Moreover, the survival analysis found that patients with BCL11A-XL expression had better DFS outcomes.
The BCL11A gene was first detected in B-cell chronic lymphocytic leukemia and considered as a proto-oncogene of malignant hematological diseases (14,15). Jiang et al. reported for the first time the role of BCL11A overexpression in predicting survival and relapse in early stage NSCLC (11). And according to the previous research (Jiang et al.), there were no advanced patients expressed BCL11A protein. So our study is also the first description of BCL11A-XL isoform in early stage lung cancer and its function as a prognostic factor for DFS. Pulford et al. detected the BCL11A-XL isoform in both normal and malignant tissues. The results showed BCL11A-XL expression was only observed in CD20-positive B cells and in a variety of B-cell tumors but was undetectable in the myeloma cases (9). We demonstrated that the BCL11A-XL protein was an independent prognostic factor of DFS for SCC and LCC patients. The survival analysis also showed that there was a tendency towards decreased survival in patients whose tumors lacked BCL11A-XL. Because most of the patients were diagnosed ten years ago, there were not enough tissues to detect the EGFR, ALK and KRAS mutation genes to exclude the survival difference were not caused by these mutation status. Maybe these mutation genes should be detected in further studies.
It is also need to explore the potential mechanisms of abnormal BCL11A activation in NSCLC. Luo et al. revealed that the BCL11A high expression in Burkitt lymphoma cell line (NAB-2) was associated with Epstein-Barr virus integration in human genome chromosome 2p13 (16). Many studies also reported that a subset of pulmonary squamous-cell carcinomas and ACs show EBV associated, especially lymphoepithelioma-like carcinoma (17-19). Our current findings show that BCL11A-XL was abundant in SCC and LCC, further studies might need to examine whether EBV associate with high expression of BCL11A in lung cancer. The BCL11A protein has three isoforms and all of them associated to malignancies (3,9,14.15). Compare to the other two isoforms, the BCL11A-XL protein was differently expressed in NSCLC tissues. In the future, we might do further researches to explore the BCL11A-XL function in NSCLC patients.
In summary, we report the BCL11A isoforms in NSCLC at the protein level for the first time. Thus, further studies need to investigate the corresponding mRNA transcripts in order to if the isoforms could promote or suppress NSCLC. Also, this study was performed in a limited number of NSCLC histology type and other samples should be analyzed to validate this results.
Conclusions
Activation of the BCL11A-XL may be a potential prognostic biomarker of NSCLC, and the BCL11A-XL isoform may play an important role in the tumorigenesis of SCC and LCC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Inaba T, Oku N, Gotoh H, et al. Philadelphia chromosome positive precursor B-cell acute lymphoblastic leukemia with a translocation t(2;14)(p13;q32). Leukemia 1991;5:719-22. [PubMed]
- Houldsworth J, Mathew S, Rao PH, et al. REL proto-oncogene is frequently amplified in extranodal diffuse large cell lymphoma. Blood 1996;87:25-9. [PubMed]
- Satterwhite E, Sonoki T, Willis TG, et al. The BCL11 gene family: involvement of BCL11A in lymphoid malignancies. Blood 2001;98:3413-20. [PubMed]
- Liu P, Keller JR, Ortiz M, et al. Bcl11a is essential for normal lymphoid development. Nat Immunol 2003;4:525-32. [PubMed]
- Yu Y, Wang J, Khaled W, et al. Bcl11a is essential for lymphoid development and negatively regulates p53. J Exp Med 2012;209:2467-83. [PubMed]
- Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 2008;322:1839-42. [PubMed]
- Liu H, Ippolito GC, Wall JK, et al. Functional studies of BCL11A: characterization of the conserved BCL11A-XL splice variant and its interaction with BCL6 in nuclear paraspeckles of germinal center B cells. Mol Cancer 2006;5:18. [PubMed]
- Satterwhite E, Sonoki T, Willis TG, et al. The BCL11 gene family: involvement of BCL11A in lymphoid malignancies. Blood 2001;98:3413-20. [PubMed]
- Pulford K, Banham AH, Lyne L, et al. The BCL11AXL transcription factor: its distribution in normal and malignant tissues and use as a marker for plasmacytoid dendritic cells. Leukemia 2006;20:1439-41. [PubMed]
- Khaled WT, Choon Lee S, Stingl J, et al. BCL11A is a triple-negative breast cancer gene with critical functions in stem and progenitor cells. Nat Commun 2015;6:5987. [PubMed]
- Jiang BY, Zhang XC, Su J, et al. BCL11A overexpression predicts survival and relapse in non-small cell lung cancer and is modulated by microRNA-30a and gene amplification. Mol Cancer 2013;12:61. [PubMed]
- Anti-Ctip1 antibody [14B5] (ab19487). Available online: http://www.abcam.com/ctip1-antibody-14b5-ab19487.html
- Anti-Ctip1 antibody [15E3AC11] (ab18688). Available online: http://www.abcam.com/Ctip1-antibody-15E3AC11-ab18688.html
- Nakamura T, Yamazaki Y, Saiki Y, et al. Evi9 encodes a novel zinc finger protein that physically interacts with BCL6, a known human B-cell proto-oncogene product. Mol Cell Biol 2000;20:3178-86. [PubMed]
- Weniger MA, Pulford K, Gesk S, et al. Gains of the proto-oncogene BCL11A and nuclear accumulation of BCL11A(XL) protein are frequent in primary mediastinal B-cell lymphoma. Leukemia 2006;20:1880-2. [PubMed]
- Luo WJ, Takakuwa T, Ham MF, et al. Epstein-Barr virus is integrated between REL and BCL-11A in American Burkitt lymphoma cell line (NAB-2). Lab Invest 2004;84:1193-9. [PubMed]
- Grinstein S, Preciado MV, Gattuso P, et al. Demonstration of Epstein-Barr virus in carcinomas of various sites. Cancer Res 2002;62:4876-8. [PubMed]
- Wöckel W, Höfler G, Popper HH, et al. Lymphoepithelioma-like carcinoma of the lung. Pathol Res Pract 1995;191:1170-4. [PubMed]
- Jafarian AH, Omidi-Ashrafi A, Mohamadian-Roshan N, et al. Association of Epstein Barr virus deoxyribonucleic acid with lung carcinoma. Indian J Pathol Microbiol 2013;56:359-64. [PubMed]