Pneumothorax in otherwise healthy non-intubated patients suffering from COVID-19 pneumonia: a systematic review
Introduction
After the increasing report of cases of severe respiratory infections caused by a novel coronavirus, known as coronavirus 2 (SARS-CoV-2) in late 2019, the outbreak occurred during the following months was declared as a global pandemic by the World Health Organization (WHO) (1). The WHO named this spectrum of infections as coronavirus disease 2019 (COVID-19) (1). According to the WHO update on November 2020, there were almost 51 million confirmed cases and more than 1.26 million deaths attributed to COVID-19 in more than 219 countries (1). The respiratory symptoms can vary from a self-limited upper respiratory infection to a massive pulmonary involvement with respiratory failure, acute respiratory distress syndrome (ARDS) and a state of hypercoagulability (2,3). As experience was gained with this new disease combined with the more liberal use of imaging techniques, for diagnostic and screening purposes, different radiological patterns of COVID-19 pneumonia have been identified (3-5). Cases of spontaneous pneumothorax have been described in patients suffering from COVID-19 pneumonia (6-30). Pneumothoraces have been observed either as a first manifestation of the disease or in a later phase, especially in patients that necessitated orotracheal intubation and mechanical ventilation (31-49). The aim of this study is to systematically review all the cases of spontaneous pneumothorax that occurred in otherwise healthy patients with no underlying lung disease and who were not put under invasive mechanical ventilation and detect similarities and differences comparing to spontaneous pneumothorax encountered before the emergence of this new entity.
We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-208).
Methods
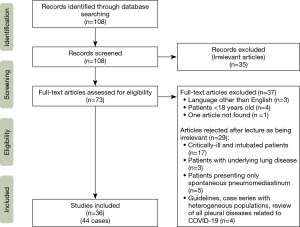
A PubMed research was conducted using the terms [Pneumothorax] AND [COVID-19] in November 2020. This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for a systematic review (PRISMA, www.prisma-statement.org) (50). Two reviewers (AA and PR) worked independently in order to conduct the research and the collection of data. Any discrepancies were resolved by consensus after discussion. Since this is a newly described entity, all the articles were published in 2020. The initial research resulted in 108 articles. After analysis of the titles and/or abstracts, 35 articles were excluded as being irrelevant to the topic. From the remaining 73 articles, 4 were excluded because the patients were less than 18 years old. Three additional articles were excluded because, even if they were relevant to the topic, they were published in another language than English. One article could not be retrieved. From the remaining 65 articles, after lecture and analysis, 29 more articles were excluded (articles reporting on critically ill patients that needed intubation and mechanical ventilation, a review of all pleural diseases related to COVID-19, a review relevant to the topic but describing a heterogeneous population of patients, articles reporting on patients with underlying lung disease, patients that presented only spontaneous pneumomediastinum and articles containing recommendations about the treatment of COVID-19-related pneumothorax). Finally, 36 articles (case reports and small case series) relevant to the topic were analyzed. The references of these articles were cross-checked in order to identify more cases. In total, 44 cases of spontaneous pneumothorax in otherwise healthy patients with no underlying lung disease that did not necessitate orotracheal intubation or invasive mechanical ventilation, before or after the diagnosis of pneumothorax were taken into account. The flowchart of study selection process is shown on Figure 1. Since the available data were extracted from small observational studies, no particular bias risk assessment was performed.
The following data were collected: age, sex, the side of the pneumothorax, the smoking habit, the time from onset of symptoms (or from hospital admission where the onset of symptoms was not clearly stated) to the diagnosis of pneumothorax, notable characteristics on computed tomography (CT) scan (especially the development of new bullous lesions) and the type of treatment. The presence of pneumomediastinum, subcutaneous emphysema or pneumopericardium in addition to the pneumothorax were added to the analysis. In cases where surgical treatment was performed, intraoperative and pathological findings were taken into account. Data concerning administration of corticosteroids and oxygen therapy (other than invasive mechanical ventilation) were retrieved. Whenever case series including intubated and non-intubated patients were encountered, only the latter were included in the analysis. Patients who presented an underlying lung pathology or other predisposing factors that could result in spontaneous pneumothorax were excluded.
In order to analyze the most homogeneous population possible, the intubated patients were deliberately excluded from the analysis. If the pneumothorax occurred while on mechanical ventilation (with or without concomitant ARDS) then ventilator-induced barotrauma could play a major role in its pathogenesis. On the other hand, if patients were intubated after the occurrence of pneumothorax, then its course and treatment are not the same as in non-intubated patients. It is a common practice not to operate on critically-ill patients but rather to leave in situ a chest tube for a prolonged period of time in order to treat a spontaneous pneumothorax (5).
Results
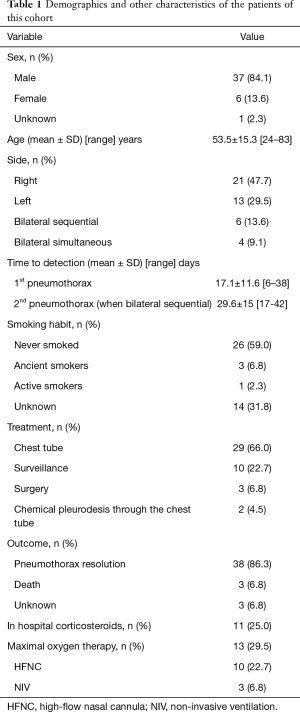
Case reports and cases series published in the medical literature were compiled in order to define similarities among patient characteristics, treatments received and outcomes. There were 37 male (84.1%) and 6 female (13.6%) patients. The gender of one patient (2.3%) was not reported. The mean age was 53.5 years. The majority of patients (59%) had never smoked. There were no data concerning smoking habit of 14 patients (31.8%). Twenty-one pneumothoraces occurred on the right side and 13 on the left. Six patients presented sequential bilateral and 4 spontaneous bilateral pneumothoraces. In 7 cases a tension pneumothorax occurred. The mean time from the onset of symptoms (or hospital admission) to the diagnosis of pneumothorax was 17.1 days. In case of bilateral sequential pneumothorax, the mean time for the diagnosis of the second pneumothorax was 29.6 days. In 16 cases (36.3%) the pneumothorax was associated with subcutaneous emphysema and/or pneumomediastinum. The majority of patients (66%) were treated only by chest tube thoracostomy, which most of the times resulted in a complete resolution of the pneumothorax. Simple surveillance was applied in 10 cases. Three patients underwent minimally invasive surgery. One additional patient was scheduled for surgery but there were no additional data reported. In two patients chemical pleurodesis through the chest tube was performed. In 14 cases (31.8%) air-filled lesions were detected on imaging. In 6 of these cases the lesions were bilateral. In the majority of cases (9 out of 14) they were localized in the lower lobes and in one case in the right middle lobe. In 5 cases the lesions were not present on the baseline CT scan upon admission. Eleven patients received corticosteroids during their hospital stay. Thirteen patients received maximal oxygen support [high-flow nasal cannula or non-invasive ventilation (NIV)]. Concerning the therapeutic outcome, in the majority of cases (86.3%) the pneumothorax was resolved. Three patients died. Among them, one patient (81 years old) died because of worsening of COVID pneumonia (10). Two patients (aged 40 and 67) died because of a multiorgan failure that occurred on admission day 60 and 18 days after the onset of symptoms respectively (35,44). There are no other data enlightening the causes of death and there are no data from autopsy studies. The outcome of three patients was unknown (two patients were still hospitalized by the time the case reports were submitted for publication). The demographics and other characteristics of the patients are demonstrated on Table 1.
Full table
Discussion
In the beginning of the SARS-CoV-2 pandemic there were scarce reports of pneumothorax attributed to this emerging virus. The more frequent imaging findings revealed bilateral and multilobar pulmonary involvement. Peripheral ground-glass opacities with vascular enlargement, consolidations, crazy paving and reticulations have been described (4). In some early cases series, pneumothorax was mentioned as a rather rare complication (6,7). While more experience with gained with this novel entity and with the routine use of CT scan, many other cases have been reported during the following months (9,10). In the different case series, the incidence is approximately 1%, however it is rather underestimated, since many cases are not reported (8).
The exact pathogenetic mechanism leading to spontaneous pneumothorax is not completely elucidated. However, the most probable mechanism is alveolar damage because of the inflammation and ischemic process (12-14,16). Common histopathological findings in COVID19 patients are capillary congestion and reactive pneumocyte changes indicating diffuse alveolar damage (12,13).
The de novo appearance of air-filled cavities (described as “cysts, bullae, pneumatoceles, etc.”) in healthy patients could further explain the pneumothorax by their spontaneous rupture (17-20). Coughing is frequently associated with COVID19 pneumonia and could be an aggravating factor, since forceful Valsalva maneuvers increase the intrathoracic pressure and lead to the rupture of these air-filled lesions (14,22). The atypical localization of these lesions (lower lobe predominance) reinforces the hypothesis that pneumothorax in patients suffering from COVID-19 pneumonia should be differentiated from ordinary cases. Another difference from ordinary cases is the high rate (22.7%) of bilateral pneumothoraces.
The fact that some of these lesions were seen in sequential CT scans in patients without respiratory comorbidities and in the absence of other concomitant infections should be recognized as a direct complication secondary to COVID-19 infection.
On the other hand, these cavities can disappear spontaneously with time, as demonstrated in the case reported by Fan et al. (15). A voluminous subpleural bulla, responsible for a spontaneous massive pneumothorax, completely resolved after approximately 3 months.
The aim of this systematic review is to contribute to the current knowledge regarding lung involvement in the form of parenchymal disruption and subsequent pneumothorax in healthy individuals. The series of cases that are reported present quite heterogeneous populations, with a mixture of previously healthy patients and patients with pre-existing comorbidities. The majority of these patients needed either oxygen therapy with or without non-invasive positive pressure or invasive mechanical ventilation. Patients that needed intubation and mechanical ventilation were excluded in order to eliminate the confounding factor of barotrauma, which is a well-known complication, especially in the setting of ARDS. In our analysis, the mean age for COVID-19 related pneumonia was 53.5 years. In the multicenter study of Martinelli et al., reporting on 60 patients with spontaneous pneumothorax, 72% had no respiratory comorbidities and 32% did not receive invasive or NIV. However, there were no separate data on different subgroups of non-ventilated patients that could permit us to include them in our analysis, even though it is the largest case series (9).
The authors conclude that spontaneous pneumothorax complicating COVID-19 pneumonia is not an independent factor for poor prognosis. For all the above reasons, we agree with Martinelli et al. and with Porcel who stress that the existence of a true secondary spontaneous pneumothorax due to SARS-CoV-2 should be recognized (9,11).
In our analysis 22.7% of the patients were treated conservatively and 66% with a chest tube placement with a favorable outcome. The size of the chest tube was not always mentioned and whenever it was stated it ranged from pigtail catheters to large-bore chest tubes. There were also patients that underwent surgery. In the case reported by Caviezel et al., a patient with no underlying lung disease and who never had non-invasive or invasive mechanical ventilation, underwent bilateral exploratory thoracoscopies with wedge resections (23). The surgical indications were failure of conservative treatment (chest tubes) on one side and early pneumothorax occurrence on the other side. Intraoperatively, bulla-like hematomas were discovered on the lower lobes whereas there were fibrin deposits on the surface of the lungs. Wedge resections of these hematomas were performed by using staplers with reinforced staple lines. Histopathological findings were compatible with fibrosis, fibrinous inflammatory changes, intra-alveolar hemorrhage and endothelitis. Even after surgical treatment there were postoperative prolonged air leaks on one side, which were finally spontaneously resolved after several weeks. According to the authors, the inflammatory process and the focal endothelitis with subsequent alveolar damage could trigger and maintain the postoperative prolonged air leaks.
Bellini et al. reported two other cases (24). Both patients had no history of respiratory pathology. Surgical indication was early pneumothorax recurrence after chest tube thoracostomy. Both patients underwent exploratory thoracoscopy with wedge resections associated with mechanical pleurodesis and partial pleurectomy. Intraoperative and histopathological findings were similar. The lung has lost its compliance and there were areas of atelectasis and vascular congestion. As a result, the lung was frail and bled easily at manipulation. The postoperative course was uneventful for both patients and the chest tubes were removed on the 6th postoperative day. At the microscopic level, interstitial pneumonia associated with vascular changes were observed. More specifically, multiple and extensive interstitial and endoalveolar blood extravasation was observed. It was associated with marked myo-intimal thickening and blood stasis, also diffuse microthrombi were seen.
Another patient from the series of Martinelli et al. underwent surgical treatment (non-intubated patient without further information) with bullectomy and pathological findings were quite atypical and globally similar to the abovementioned cases (9).
Two patients underwent chemical pleurodesis through the chest tube (29,33). One patient underwent talc slurry. For the other patient there was no precision about the agent used for chemical pleurodesis.
The treatment of spontaneous pneumothorax attributed to SARS-CoV-2 must not deviate from the standard of care before the pandemic, as surgical treatment in this particular setting feasible and safe. However, the initial treatment should be modified in order to prevent contamination through aerosolization. For that reason, there are recommendations regarding chest tube placement in patients with COVID-19 pneumonia. The British Thoracic Society (BTS) and the American Association for the Surgery of Trauma (AAST) proposed recommendations for tube thoracostomy good clinical practice (51,52).
Another issue is the timing until surgery in patients with COVID-19 infection. The American Society of Anesthesiology (ASA) and Anesthesia Patient Safety Foundation (APSF) made a joint statement on elective surgery and anesthesia for patients after COVID-19 infection (53). They state that “all elective operations should be delayed until the patient has met criteria for discontinuing isolation and COVID-19 transmission precautions and has entered the recovery phase” (53). Another study concluded that there are limited data about timing of surgery after COVID-19 infection (54). They propose that surgery should be delayed for at least four weeks after notification of a positive swab, but without robust data concerning patient subgroups and specific pathologies due to the small number of patients enrolled (54).
When revising these cases of pneumothorax in COVID-19-infected subjects, one could wonder if the use of corticosteroids is a risk factor for developing pneumothorax during this acute respiratory infection. Pneumothorax has been described in patients taking glucocorticosteroids and immunosuppressants for respiratory disease although in these articles other confounding factors, such as opportunistic infection, cavities, reticular abnormalities, and alveolopleural fistula, facilitating the development of pneumothorax are also mentioned (55-58). Glucocorticoids could increase the risk of infection and delay wound healing; however, a protective role has been suggested in patients with non-COVID-19 related inflammatory disease who are at risk of developing a pneumothorax (55,59-61). In these last patients, it has been reported that interrupting or reducing corticosteroids did not seem to improve their prognosis (55).
Initially most COVID-19 treatment guidelines stated that the use of glucocorticoids was either contraindicated or not recommended. Recently new data have been published about the use of dexamethasone. The RECOVERY-trial concluded that therapy with dexamethasone at a dose of 6 mg once daily for up to 10 days decreased 28-day mortality in patients with COVID-19 on respiratory support. Patients not requiring oxygen showed no benefit but had a possibility of harm with corticosteroid therapy (62). These findings were consistent with another study in ARDS which showed a decrease of 15% of mortality when a course of dexamethasone was given (63).
Although in the RECOVERY-study benefits were shown with dexamethasone, the meta-analysis of 73 comparative studies by Cano et al. showed that low-dose methylprednisolone was being the most common used corticosteroid in COVID-19. Corticosteroids were mainly used in severe disease as found in mechanically ventilated patients (35.3%), ICU patients (51.3%), and severe COVID-19 patients (40%) (62-64). An estimated 21.6% of COVID-19 patients received corticosteroids in their analysis. These authors also found that low-dose corticosteroids do not have a significant impact on the duration of SARS-CoV-2 viral shedding. This viral shedding in COVID-19 appears to be higher early in the illness and declines thereafter. The benefit on mortality in the RECOVERY-study, seems to be greater in patients with COVID-19 who were recruited after the first week of their illness. At that stage immuno-pathological processes are probably driving the course of the disease, with active viral replication playing a less important role (62).
In our analysis of pneumothoraces in COVID-19 patients, the administration of corticosteroids was reported in 11 (25%) patients. Methylprednisolone as well as dexamethasone were used. In the majority of case reports the exact dose and timing of administration of the corticosteroid, in relation to the onset of symptoms, was not mentioned.
When evaluating the risk for developing a pneumothorax during a COVID-19 infection, one could even hypothesize a potential increased risk when using corticosteroid therapy. Several studies report an increased risk of thromboembolic events when using corticosteroids in inflammatory diseases (64-69). Thrombosis in lung tissue could lead to micro-infarction, local necrosis and could increase the risk of air leaks. In our study, there was no causative role established between corticosteroids and development of air-filled lesions because only 3 patients that presented such anomalies on CT scan received corticosteroids during their hospital stay.
Other potential side effects in COVID-19 patients receiving corticosteroids include immune suppression and hyperglycemia which can both lead to bacterial, mycobacterial or fungal secondary infection (64). These could theoretically increase the risk of pneumothorax.
Another confounding factor in the development of pneumothorax in COVID-19, could have been the use of positive airway pressure. As previously mentioned, intubated patients were excluded from our analysis due to the possibility of a pneumothorax secondary to barotrauma. However, the risks and benefits in COVID-19 patients, of more gentle methods of oxygen administration with positive pressure, are largely unknown. These methods of NIV, can be bi-level or continuous positive airway pressure (BiPAP resp. CPAP), or high flow nasal canula (HFNC). Though the risk of barotrauma and subsequent complications are low with NIV, such cases have been reported in the literature (61,70,71).
Some authors suggest that extensive damage to lung parenchyma caused by COVID-19 makes low-risk patients susceptible to alveolar rupture with extension of air into the mediastinum, pleura, and subcutaneous tissues (61). BiPAP and CPAP use normally higher positive end-expiratory pressures compared to HFNC, and increase the risk of air leakage. Nevertheless, pneumothoraces related to HFNC have been reported in a pediatric population, although confounding factors such as pre-existing hyperinflation and prior surgery were present (72). In this pediatric population in the intensive care unit (ICU), no worsening of the air leak was seen under HFNC among the 6 preexisting pneumothoraces. A systematic review compared the safety and efficacy of HFNC with other forms of non-invasive respiratory support in preterm infants, and concluded that, following extubation, HFNC is associated with lower rates of pneumothorax and nasal trauma compared with nasal CPAP. Although these findings were seen in a pediatric population, it is interesting because the lungs in these preterm infants have reduced surfactant, a mechanism that has also been suggested in COVID-19 patients (73,74). SARS-CoV-2 enters and replicates in the alveolar type II cells impacting the production and turnover of pulmonary surfactants. This results in alveolar collapse and inflammation leading to increased capillary permeability, edema, and microvascular thrombosis; where the associated ARDS clinical picture closely resembles neonatal respiratory distress syndrome (NRDS), caused by surfactant deficiency (75).
In adults, only one case report of a localized right-side pneumothorax while on HFNC has been reported in a patient with hemophagocytic lymphohistiocytosis (HL). However, this patient had prior ARDS which required mechanical ventilation, and therefore a HL- or ARDS-associated lung abnormality, a trauma during endotracheal intubation or a barotrauma during mechanical ventilation are more likely risk factors for the subsequent development of pneumothorax. More reports on the use of NIV and HFNC in COVID-19 patients are needed to assess the risk of pneumothorax. Currently there are no data against using this kind of pressure and oxygen support.
Our study has some limitations. The cases reported in the literature present heterogeneous populations. Even though we tried to create a homogeneous population in our study by excluding a great deal of cases, it is still possible that some confounding factors could not be entirely eliminated, because some data were not reported by the authors. Consequently, the population of the present study consists of a small number of patients. As already mentioned, this entity is still underdetected (especially in the absence of clinical signs and symptoms as in the case of a small pneumothorax) and underreported. A limitation of most case reports is that the lungs were retrospectively assessed on chest roentgenogram and not CT (61). The reports did rarely mention pre-existing lung pathology such as emphysema in smokers, or interstitial lung disease. Although tobacco smoking is reported in several case reports, the consumption of cannabis or exposure to pollution (indoor or outdoor), known risk factors for developing pneumothorax (76,77), are mentioned in none of the reports. In addition, occupational exposure to pathogens is not mentioned. When discussing radiologic imaging in the reviewed articles, the presence of a COVID-19 associated alveolar consolidation with air bronchogram, is often not reported. The incidence of bacterial or mycobacterial secondary infection in the different case reports has not been reported. This is also the case for the presence of radiologically important infectious sequalae that could have been a risk factor for subsequent infections due to altered local immunologic defense. Therefore, it is with the current known information impossible to determine if pneumothorax is more likely in patients with pulmonary consolidations. The time from symptom onset to discovering a pneumothorax is 17.1 days, which is quite long. Respiratory symptoms of COVID-19 generally appear one week after initial upper airway symptoms, which could partially explain this finding, however, it is unknown how long the patients in these case reports were suffering from respiratory symptoms before the diagnosis of pneumothorax. In the case of respiratory symptoms this could be attributed to worsening COVID-19 respiratory insufficiency, to the pneumothorax, or both. Currently reports on side effects of corticosteroids in COVID-19 patients are lacking. The case reports used for this review, did not provide information about treatments taken by patients at the time of diagnosis of pneumothorax. Almost none of the authors discussed treatment changes, as compared to what the patients were usually taking, or initiating other treatments (e.g., antibiotics or inhaled bronchodilators). The reported mortality was rather low, however, due to lack of information it is unclear if the pneumothorax was the main reason, or if other factors contributed to this outcome.
Conclusions
Secondary spontaneous pneumothorax should be part of the differential diagnosis in patients suffering from COVID-19, especially in case of acute respiratory deterioration. The pathogenetic mechanism is different from the spontaneous pneumothorax frequently encountered in clinical practice before the pandemic. Imaging techniques especially CT scan should be repeated throughout the clinical course of the patients in order to detect newly developed pulmonary complications such as bullae or cysts. Surgical treatment is feasible in that particular setting and patients whose general condition permits, should be offered surgery according to the existing guidelines regarding spontaneous pneumothorax. National registries and databases are necessary in order to better understand the pathogenesis and complications of this novel entity. Future reports about COVID-19-associated pneumothoraces, should include the dose, duration and type of corticosteroid that was given to these patients. These reports should also include the timing of corticosteroid therapy in relation to the onset of the first symptoms. Finally, outcome parameters should be more clearly defined and discussed when reporting co-morbidities of COVID-19 such as pneumothorax.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-208
Peer Review File: Available at https://dx.doi.org/10.21037/jtd-21-208
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-208). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- The World Health Organization, Coronavirus disease 2019 (COVID-19) pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed November 11th 2020).
- Valencia DN. Brief Review on COVID-19: The 2020 Pandemic Caused by SARS-CoV-2. Cureus 2020;12:e7386 [Crossref] [PubMed]
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 2020;34:101623 [Crossref] [PubMed]
- Salehi S, Abedi A, Balakrishnan S, et al. Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients. AJR Am J Roentgenol 2020;215:87-93. [Crossref] [PubMed]
- Wang XH, Duan J, Han X, et al. High incidence and mortality of pneumothorax in critically Ill patients with COVID-19. Heart Lung 2021;50:37-43. [Crossref] [PubMed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. [Crossref] [PubMed]
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475-81. [Crossref] [PubMed]
- Polistina GE, Simioli F, Imitazione P, et al. Different presentation of pulmonary parenchymal disruption in COVID-19 pneumonia. Case series of Sub-Intensive Care Unit in Naples, Italy. Monaldi Arch Chest Dis 2020; [Crossref] [PubMed]
- Martinelli AW, Ingle T, Newman J, et al. COVID-19 and pneumothorax: a multicentre retrospective case series. Eur Respir J 2020;56:2002697 [Crossref] [PubMed]
- Zantah M, Dominguez Castillo E, Townsend R, et al. Pneumothorax in COVID-19 disease- incidence and clinical characteristics. Respir Res 2020;21:236. [Crossref] [PubMed]
- Porcel JM. Pleural diseases and COVID-19: ubi fumus, ibi ignis. Eur Respir J 2020;56:2003308 [Crossref] [PubMed]
- Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020;77:198-209. [Crossref] [PubMed]
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2. [Crossref] [PubMed]
- Shan S, Guangming L, Wei L, et al. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19: case report and literature review. Rev Inst Med Trop Sao Paulo 2020;62:e76 [Crossref] [PubMed]
- Fan Q, Pan F, Yang L. Spontaneous pneumothorax and subpleural bullae in a patient with COVID-19: a 92-day observation. Eur J Cardiothorac Surg 2020;58:858-60. [Crossref] [PubMed]
- Salah O, Faisal M, Alshahwani I, et al. Bilateral Hemopneumothorax in COVID-19. Cureus 2020;12:e10314 [PubMed]
- Berhane S, Tabor A, Sahu A, et al. Development of bullous lung disease in a patient with severe COVID-19 pneumonitis. BMJ Case Rep 2020;13:237455 [Crossref] [PubMed]
- Yamaya T, Baba T, Hagiwara E, et al. Pneumothorax in a COVID-19 Pneumonia Patient without Underlying Risk Factors. Intern Med 2020;59:2921-5. [Crossref] [PubMed]
- Yasukawa K, Vamadevan A, Rollins R. Bulla Formation and Tension Pneumothorax in a Patient with COVID-19. Am J Trop Med Hyg 2020;103:943-4. [Crossref] [PubMed]
- Liu K, Zeng Y, Xie P, et al. COVID-19 with cystic features on computed tomography: A case report. Medicine (Baltimore) 2020;99:e20175 [Crossref] [PubMed]
- Amoah K, Gunasekaran K, Rahi MS, et al. A case of secondary tension pneumothorax in COVID-19 pneumonia in a patient with no prior history of lung disease. SAGE Open Med Case Rep 2020;8:2050313X20967504.
- Hameed M, Jamal W, Yousaf M, et al. Pneumothorax In Covid-19 Pneumonia: A case series. Respir Med Case Rep 2020;31:101265 [Crossref] [PubMed]
- Caviezel C, Weiss L, Haessig G, et al. Case report of sequential bilateral spontaneous pneumothorax in a never-ventilated, lung-healthy COVID-19-patient. Int J Surg Case Rep 2020;75:441-5. [Crossref] [PubMed]
- Bellini R, Salandini MC, Cuttin S, et al. Spontaneous pneumothorax as unusual presenting symptom of COVID-19 pneumonia: surgical management and pathological findings. J Cardiothorac Surg 2020;15:310. [Crossref] [PubMed]
- Zayet S, Klopfenstein T, Mezher C, et al. Coronavirus disease 2019 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema, France. New Microbes New Infect 2020;38:100785 [Crossref] [PubMed]
- Rehman T, Josephson G, Sunbuli M, et al. Spontaneous Pneumothorax in an Elderly Patient With Coronavirus Disease (COVID-19) Pneumonia. Ochsner J 2020;20:343-5. [Crossref] [PubMed]
- Fahad AM, Mohammad AA, Al-Khalidi HA, et al. Spontaneous pneumothorax as a complication in COVID-19 male patient: A case report. Clin Case Rep 2020;8:3116-9. [Crossref] [PubMed]
- Sonia F, Kumar M. A Complication of Pneumothorax and Pneumomediastinum in a Non-Intubated Patient With COVID-19: A Case Report. Cureus 2020;12:e10044 [PubMed]
- Ahluwalia AS, Qarni T, Narula N, et al. Bilateral pneumothorax as possible atypical presentation of coronavirus disease 2019 (COVID-19). Respir Med Case Rep 2020;31:101217 [Crossref] [PubMed]
- Alhakeem A, Khan MM, Al Soub H, et al. Case Report: COVID-19-Associated Bilateral Spontaneous Pneumothorax-A Literature Review. Am J Trop Med Hyg 2020;103:1162-5. [Crossref] [PubMed]
- Chen X, Zhang G, Tang Y, et al. The coronavirus diseases 2019 (COVID-19) pneumonia with spontaneous pneumothorax: a case report. BMC Infect Dis 2020;20:662. [Crossref] [PubMed]
- Ferreira JG, Rapparini C, Gomes BM, et al. Pneumothorax as a late complication of COVID-19. Rev Inst Med Trop Sao Paulo 2020;62:e61 [Crossref] [PubMed]
- Janssen ML, van Manen MJG, Cretier SE, et al. Pneumothorax in patients with prior or current COVID-19 pneumonia. Respir Med Case Rep 2020;31:101187 [Crossref] [PubMed]
- do Lago VC, Cezare TJ, Fortaleza CMCB, et al. Does COVID-19 Increase the Risk for Spontaneous Pneumothorax? Am J Med Sci 2020;360:735-7. [Crossref] [PubMed]
- Mallick T, Dinesh A, Engdahl R, et al. COVID-19 Complicated by Spontaneous Pneumothorax. Cureus 2020;12:e9104 [PubMed]
- Khurram R, Johnson FTF, Naran R, et al. Spontaneous tension pneumothorax and acute pulmonary emboli in a patient with COVID-19 infection. BMJ Case Rep 2020;13:237475 [Crossref] [PubMed]
- González-Pacheco H, Gopar-Nieto R, Jiménez-Rodríguez GM, et al. Bilateral spontaneous pneumothorax in SARS-CoV-2 infection: A very rare, life-threatening complication. Am J Emerg Med 2021;39:258.e1-3. [Crossref] [PubMed]
- Borghesi A, Aggiusti C, Farina D, et al. COVID-19 Pneumonia: Three Thoracic Complications in the Same Patient. Diagnostics (Basel) 2020;10:498. [Crossref] [PubMed]
- Brogna B, Bignardi E, Salvatore P, et al. Unusual presentations of COVID-19 pneumonia on CT scans with spontaneous pneumomediastinum and loculated pneumothorax: A report of two cases and a review of the literature. Heart Lung 2020;49:864-8. [Crossref] [PubMed]
- Al-Shokri SD, Ahmed AOE, Saleh AO, et al. Case Report: COVID-19-Related Pneumothorax-Case Series Highlighting a Significant Complication. Am J Trop Med Hyg 2020;103:1166-9. [Crossref] [PubMed]
- Eperjesiova B, Hart E, Shokr M, et al. Spontaneous Pneumomediastinum/Pneumothorax in Patients With COVID-19. Cureus 2020;12:e8996 [PubMed]
- Hollingshead C, Hanrahan J. Spontaneous Pneumothorax Following COVID-19 Pneumonia. IDCases 2020;21:e00868 [Crossref] [PubMed]
- Spiro JE, Sisovic S, Ockert B, et al. Secondary tension pneumothorax in a COVID-19 pneumonia patient: a case report. Infection 2020;48:941-4. [Crossref] [PubMed]
- López Vega JM, Parra Gordo ML, Diez Tascón A, et al. Pneumomediastinum and spontaneous pneumothorax as an extrapulmonary complication of COVID-19 disease. Emerg Radiol 2020;27:727-30. [Crossref] [PubMed]
- Ucpinar BA, Sahin C, Yanc U. Spontaneous pneumothorax and subcutaneous emphysema in COVID-19 patient: Case report. J Infect Public Health 2020;13:887-9. [Crossref] [PubMed]
- Wang W, Gao R, Zheng Y, et al. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J Travel Med 2020;27:taaa062.
- Rohailla S, Ahmed N, Gough K. SARS-CoV-2 infection associated with spontaneous pneumothorax. CMAJ 2020;192:E510 [Crossref] [PubMed]
- Dennison J, Carlson S, Faehling S, et al. Case report: Spontaneous pneumothorax in resolved, uncomplicated COVID-19 Pneumonia-A literature review. Respir Med Case Rep 2020;31:101291 [Crossref] [PubMed]
- Sun R, Liu H, Wang X. Mediastinal Emphysema, Giant Bulla, and Pneumothorax Developed during the Course of COVID-19 Pneumonia. Korean J Radiol 2020;21:541-4. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [Crossref] [PubMed]
- British Thoracic Society. Pleural services during the COVID-19 pandemic-Revised. Available online: https://www.brit-thoracic.org.uk (accessed November 22nd 2020).
- Pieracci FM, Burlew CC, Spain D, et al. Tube thoracostomy during the COVID-19 pandemic: guidance and recommendations from the AAST Acute Care Surgery and Critical Care Committees. Trauma Surg Acute Care Open 2020;5:e000498 [Crossref] [PubMed]
- ASA and APSF joint statement on elective surgery and anesthesia for patients after COVID-19 infection. Available online: https://www.asahq.org/about-asa/newsroom/news-releases/2020/12/asa-and-apsf-joint-statement-on-elective-surgery-and-anesthesia-for-patients-after-covid-19-infection (accessed March 29th 2021).
- COVIDSurg Collaborative. Delaying surgery for patients with a previous SARS-CoV-2 infection. Br J Surg 2020;107:e601-2. [Crossref] [PubMed]
- Shi X, Zhang Y, Lu Y. Risk factors and treatment of pneumothorax secondary to granulomatosis with polyangiitis: a clinical analysis of 25 cases. J Cardiothorac Surg 2018;13:7. [Crossref] [PubMed]
- Manika K, Kioumis I, Zarogoulidis K, et al. Pneumothorax in sarcoidosis. J Thorac Dis 2014;6:S466-9. [PubMed]
- McKnight CL, Burns B. Pneumothorax. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan. 2020 Nov 16. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441885/
- Nishimoto K, Fujisawa T, Yoshimura K, et al. Pneumothorax in connective tissue disease-associated interstitial lung disease. PLoS One 2020;15:e0235624 [Crossref] [PubMed]
- Youssef J, Novosad SA, Winthrop KL. Infection Risk and Safety of Corticosteroid Use. Rheum Dis Clin North Am 2016;42:157-76. ix-x. [Crossref] [PubMed]
- Eastridge CE, Hamman JL. Pneumothorax complicated by chronic steroid treatment. Am J Surg 1973;126:784-7. [Crossref] [PubMed]
- Manna S, Maron SZ, Cedillo MA, et al. Spontaneous subcutaneous emphysema and pneumomediastinum in non-intubated patients with COVID-19. Clin Imaging 2020;67:207-13. [Crossref] [PubMed]
- RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021;384:693-704. [Crossref] [PubMed]
- Villar J, Ferrando C, Martínez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med 2020;8:267-76. [Crossref] [PubMed]
- Cano EJ, Fonseca Fuentes X, Corsini Campioli C, et al. Impact of Corticosteroids in Coronavirus Disease 2019 Outcomes: Systematic Review and Meta-analysis. Chest 2021;159:1019-40. [Crossref] [PubMed]
- Mishra GP, Mulani J. Corticosteroids for COVID-19: the search for an optimum duration of therapy. Lancet Respir Med 2021;9:e8 [Crossref] [PubMed]
- Majoor CJ, Sneeboer MM, de Kievit A, et al. The influence of corticosteroids on hemostasis in healthy subjects. J Thromb Haemost 2016;14:716-23. [Crossref] [PubMed]
- Stuijver DJF, Majoor CJ, van Zaane B, et al. Use of oral glucocorticoids and the risk of pulmonary embolism: a population-based case-control study. Chest 2013;143:1337-42. [Crossref] [PubMed]
- Johannesdottir SA, Horváth-Puhó E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med 2013;173:743-52. [Crossref] [PubMed]
- Brotman DJ, Girod JP, Posch A, et al. Effects of short-term glucocorticoids on hemostatic factors in healthy volunteers. Thromb Res 2006;118:247-52. [Crossref] [PubMed]
- Carron M, Freo U. Complications of non-invasive ventilation techniques: a comprehensive qualitative review of randomized trials. Br J Anaesth 2013;110:896-914. [Crossref] [PubMed]
- Piastra M, Morena TC, Antonelli M, et al. Uncommon barotrauma while on high-flow nasal cannula. Intensive Care Med 2018;44:2288-9. [Crossref] [PubMed]
- Baudin F, Gagnon S, Crulli B, et al. Modalities and Complications Associated With the Use of High-Flow Nasal Cannula: Experience in a Pediatric ICU. Respir Care 2016;61:1305-10. [Crossref] [PubMed]
- Han S, Mallampalli RK. The Role of Surfactant in Lung Disease and Host Defense against Pulmonary Infections. Ann Am Thorac Soc 2015;12:765-74. [Crossref] [PubMed]
- Leth-Larsen R, Zhong F, Chow VT, et al. The SARS coronavirus spike glycoprotein is selectively recognized by lung surfactant protein D and activates macrophages. Immunobiology 2007;212:201-11. [Crossref] [PubMed]
- Schousboe P, Wiese L, Heiring C, et al. Assessment of pulmonary surfactant in COVID-19 patients. Crit Care 2020;24:552. [Crossref] [PubMed]
- Martinasek MP, McGrogan JB, Maysonet A. A Systematic Review of the Respiratory Effects of Inhalational Marijuana. Respir Care 2016;61:1543-51. [Crossref] [PubMed]
- Park JH, Lee SH, Yun SJ, et al. Air pollutants and atmospheric pressure increased risk of ED visit for spontaneous pneumothorax. Am J Emerg Med 2018;36:2249-53. [Crossref] [PubMed]