
Transcatheter aortic valve replacement using the new Evolut-Pro system: a prospective comparison with the Evolut-R device
Introduction
Transcatheter aortic valve replacement (TAVR) has become the default therapy for patients with severe aortic stenosis who are at increased risk for surgical aortic valve replacement (SAVR) (1,2). The CoreValve system, introduced in 2003, was the first-generation self-expandable valve. The second generation Evolut R (EVR) self-expanding bioprothesis was introduced in 2014 providing a reduced rate of paravalvular leak (PVL) and higher device success. EVR included a 14-F deliver catheter, a modified nitinol design at the annulus that optimizes radial expansive force, a longer porcine pericardial sealing skirt, and a nitinol delivery catheter capsule that allows re-sheathing and recapturing the prosthesis during deployment (3). The latest iteration of this self-expandable valve is the Evolut Pro (EVP) system. This novel valve consists of an external pericardial wrap designed to reduce PVL while retaining the benefits of the previous generation, including a low delivery profile, self-expansion, and the ability to recapture and reposition the valve (4). Compared with the previous valve generation, a larger introducer sheath (16F) is needed, and a minimum vessel diameter of 5.5 mm (5 mm for EVR) is required.
The efficacy of the EVP design has been very recently tested by the investigators of the EvolutPRO clinical study (5). This was a non-randomized, single-arm prospective registry at eight centers in the USA enrolling only 60 well-selected patients. Patients were prospectively followed for 30 days and the primary efficacy endpoint was absence or trace aortic regurgitation. This registry demonstrated that the EVP system provided excellent survival and hemodynamics with minimal residual aortic regurgitation. However, the mid-term clinical results of this novel valve remain unknown.
This retrospective study aimed to compare short and mid-term functional and clinical outcomes of the new EVP with the established EVR system in a series of consecutive patients.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-20-2409).
Methods
Patient selection
Eighty-three consecutive patients with severe symptomatic aortic stenosis were recruited after successful transfemoral TAVR using either the EVR or EVP bioprosthesis between June 2015 and October 2018. Therapeutic options in all these patients were previously discussed in a dedicated Heart Team meeting. Clinical and anatomic selection criteria were: patients presenting symptomatic (NYHA class >2) severe aortic stenosis (defined as aortic valve area [AVA] <1 cm2 or indexed AVA <0.6 cm2/m2) who were deemed intermediate or high risk (STS >3) for SAVR. Anatomic exclusion criteria included severe mitral regurgitation and presence of an aortic annulus size outside the limits recommended for the implant (perimeter derived diameter <18 mm or >26 mm).
The prosthesis sizing was based on systematic measurements obtained by computed tomography (CT). Data acquisition was prospective and included baseline demographics and comorbidities, preprocedural imaging data from transthoracic echocardiography (TTE) and CT, procedural transesophageal echocardiography (TEE), angiographic parameters, and post-interventional data. Prior to valve implantation, coronary angiography was performed in all patients to rule out or eventually treat relevant coronary artery disease.
Clinical results were systematically evaluated in-hospital, at 30-day and at mid-term follow-up. The adverse major clinical endpoints (MACE) were defined according to the Valve Academic Research Consortium (VARC)-2 criteria: all causes mortality, stroke, cardiologic complications [myocardial infarction, heart failure, TAVR endocarditis, and pacemaker implantation], major bleeding, and major vascular complications (6). A composite endpoint, including cardiovascular death, stroke, reintervention and pacemaker implantation, was also analyzed.
CT scan and TTE measurements
All patients underwent a preprocedure ECG-triggered multislice CT scan with contrast administration. Images were analyzed in end-systole to measure the perimeter and annular diameters, the sinus of Valsalva diameters and height, and the height of the coronary arteries. The images were systematically analyzed using a cardiac application on dedicated workstations by two independent observers. The largest (Dmax) and the smallest (Dmin) aortic annulus diameters were measured. The mean (Dmean) diameter was derived by averaging the largest and smallest diameter. The circularity of aortic annulus was defined using the “eccentricity index” using the formula (1 − Dmin/Dmax) (7). The degree of the aortic valve calcification was semi-quantitatively classified into no calcification, mild calcification (small calcium spots), moderate calcification (larger calcium spots), and severe calcification (extensive calcification), as previously described (8). To assess the congruence between the aortic annulus and the device, a “cover index”, expressed as a ratio of: 100 × [(prosthesis diameter − multislice CT annulus diameter)/prosthesis diameter], was calculated (9).
Finally, in order to explore the value of the difference between CoreValve prosthesis size and annular size, the difference between the nominal CoreValve bioprosthesis size and mean aortic annulus diameter, expressed as prosthesis diameter − CT mean annular diameter, was also calculated (10). Following a predefined protocol, pre-discharge TTE was obtained in all patients. PVL was graded by a single and independent experienced operator, blinded to angiographic data and procedural results. Multiple parameters were assessed, including regurgitation color jet density and width, the circumferential extent of turbulent regurgitation jet around the aortic annulus, descending and abdominal aorta diastolic flow reversal on pulsed wave Doppler, and pressure half-time on the continuous wave Doppler signal, as previously defined (11). The PVL was classified as none, mild, moderate and severe. Significant PVL was defined as a VARC-2 score more than mild (6,11).
Procedure
During the procedure, all patients were under either general anesthesia or sedation, as previously decided in agreement with the anesthesiologist. TEE was used (in those under general anesthesia or when required in sedated patients) for procedural guidance and immediate assessment of PVL. In addition, hemodynamic assessment by simultaneous measurements of left ventricular (LV) and aortic root pressure was performed before and after valve implantation.
A transfemoral approach was selected whenever feasible. Immediate hemodynamic measurements, aortic root angiography and echocardiographic assessment were systematically obtained in all patients to elucidate the presence and severity of PVL.
Statistical analysis
For the statistical analysis, the 83 patients were divided in two groups according to the type of valve implanted. Baseline characteristics, procedural and post-interventional results and follow up clinical outcomes were compared. Potential predictors of PVL were also analyzed. The data are expressed as mean ± SD. Differences between means were compared using Student’s t-test after assessing normality. Differences in categorical variables were analyzed using Chi-square tests. For the evaluation of the mid-term clinical follow up, survival curves were calculated according to the Kaplan-Meier method. To provide more detailed information about mid-term follow up, Kaplan-Meier analysis was done comparing events since procedure versus events after discharge. To account for the different follow-up time (longer patients treated with EVR) only the 1-year clinical follow-up was compared between the 2 groups. The level of statistical significance was set at P<0.05. All statistical analyses were performed using SPSS Statistics version 18.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional committee board of Hospital La Princesa (No. 3961) and individual consent for this retrospective analysis was waived.
Results
Baseline characteristics
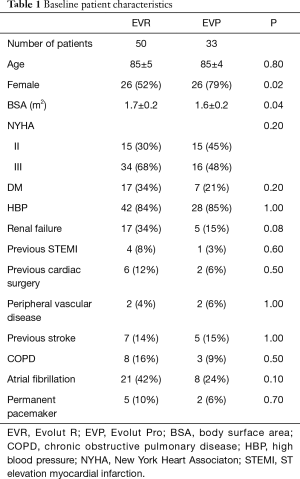
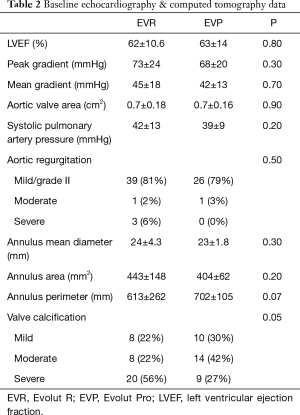
A total of 83 patients received either the EVR (n=50) or the EVP (n=33). The mean age was 84 years in both groups. The percentage of women was lower in the EVR group (52% vs. 79%; P=0.02) (Table 1). All the patients had severe aortic stenosis (mean AVA, EVR 0.73±0.18 cm2vs. EVP 0.73±0.16 cm2). TTE before the procedure demonstrated that almost 50% of patients had mild aortic regurgitation, while severe aortic regurgitation was observed only in 3 patients of the EVP group. Most patients had some degree of mitral regurgitation, more frequently graded as mild (Table 2). The aortic annulus showed similar geometry in the 2 cohorts with no differences in the remaining anatomic parameters. However, the prevalence of severe valve calcification was higher in EVR group (56% vs. 27%; P=0.05) (Table 2).
Full table
Full table
Implantation data
General anesthesia was selected in 88% of EVR cases and 45% of EVP cases. An iliofemoral access route was used in almost all patients (EVR 94% vs. EVP 100%). The percentage of predilation (EVR 14% vs. EVP 6%; P=0.3) as well as postdilation (EVR 35% vs. EVP 27%; P=0.6) was similar in both groups. Vascular complications were numerically more frequent in the EVR group (19% versus 6%; P=0.1). A minority of patients (EVR 6% vs. EVP 9%; P=0.7) suffered episodes of transient severe hypotension during the procedure and 1 patient in the EVR group suffered a cardiac tamponade, due to wire-induced ventricular perforation, and eventually died. The appearance of new onset conduction abnormalities was higher in the EVP group (EVR 17% vs. EVP 39%; P=0.01), including 8 patients developing left bundle branch block and 5 high-degree atrioventricular block.
Immediately after the procedure, mild PVL was detected in most patients (on angiography: 63% EVR vs. 79% EVP; P=0.3; on echocardiography: 54% EVR vs. 68% EVP; P=0.5). However, severe PVL was observed in 1 patient treated with EVR.
Early outcomes
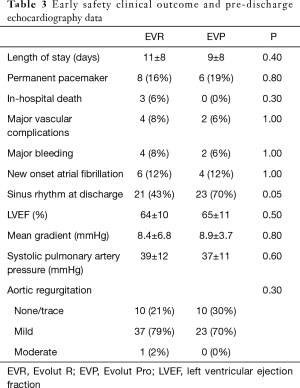
The duration of hospital stay was similar in the two groups (EVR 11±8 vs. EVP 9±8 days; P=0.4). Despite a more frequent rate of conduction abnormalities detected during procedure in the EVP group, there were not differences in the final requirement for permanent pacemaker implantation (PPI) (EVR 16% vs. EVP 19%; P=0.8). The percentage of new onset atrial fibrillation was 12% in both groups. Vascular and bleeding complications were uncommon (Table 3). There were not major adverse cardiac or cerebrovascular events during hospitalization. There were no post-procedure deaths in the EVP group but 3 patients in the EVR group eventually died during hospitalization due to non-cardiac causes [deterioration of preexisting very severe respiratory insufficiency (n=2) and preexisting severe hepatic failure (n=1)] (Table 3).
Full table
Pre-discharge echocardiographic findings
Pre-discharge TTE was performed in all patients (mean time from valve implantation 5±6.8 days). Marked improvements in aortic valve hemodynamics were found in both groups (Table 3). Among the 6 patients with bicuspid aortic valve, 4 received the EVP Bioprotheses, without differences in the presence of PVL post-TAVR. The incidence of mild PVL was similar with the two Bioprotheses (EVR 79% vs. EVP 70%; P=0.4). Moderate PVL was observed only in one patient treated with EVR (the patients with severe PVL immediately after the intervention) (Table 3).
Early PVL predictors
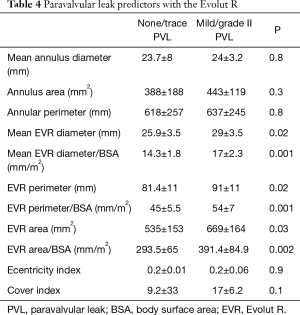
In the EVR group, the presence of PVL was directly related to prosthesis size. Notably, non PVL was more frequently observed when relatively smaller EVR were implanted (according to diameter, area and perimeter, in absolute values and also when corrected by body surface area) (Table 4). However, these correlations were not observed in the EVP group. In addition, other factors such as mean aortic annulus, eccentricity index, cover index, degree of annular calcification, mean annular and LVOT diameter, and baseline aortic regurgitation were not related to the occurrence of PVL in any group (Table 4).
Full table
30-day clinical follow up
After discharge, no additional mortality occurred in any group during the first month. Three patients required readmission within 30 days due to heart failure (1 EVR and 2 EVP). No patients suffered a cerebrovascular accident or required a PPI during the first month (Figure 1).
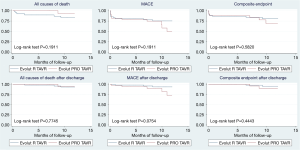
Mid- term clinical follow-up
The median survival time was 11±0.5 months for the patients with EVR, and 12±0.2 months in the EVP group. A total of 24% and 33% of patients in the EVR and EVP group, respectively, experienced MACE at 1-year clinical follow-up (P=0.3). Among EVR patients, there were 7 all-causes deaths (those previously described during hospitalization, one due to cerebral hematoma, one of unknown cause, and one due to severe respiratory insufficiency). However, only one non-cardiac death occurred in the EVP group (Figure 1). When analyzing events after discharge, MACE was numerally more frequent (in the EVP group) (15% vs. 6%; P=0.07). Events include 2 rehospitalization due to heart failure, prosthesis-related endocarditis (successfully treated with medical management), stroke and femoral artery pseudoaneurysm requiring surgical intervention. Only one patient (EVR group) required a PPI after discharge (Figure 1). At late follow up, 60% of patients in both groups were in NYHA class I.
Discussion
The most important findings of our study are as follows. First, there is little information in the literature describing the mid-term clinical outcomes in patients treated with the novel EVP (12). Our current findings provide important additional data in this regard. Second, although hemodynamically relevant PVL was not observed in any patient, a high rate of residual mild PVL was common (70% of patients). This phenomenon was comparable in the EVP and EVR groups. Third, although classical anatomic factors predicted the occurrence of mild PVL in the EVR group, these factors failed to be associated with this problem in the EVP group.
Other findings were also of interest. Overall, the improvement in most relevant hemodynamic parameters was similar in both groups and these findings are in agreement with some preliminary reports (13). In addition, although some post-procedural complications (especially conduction abnormalities) were more prevalent among patients treated with EVP prosthesis, these did not translate into a higher need of PPI or worse clinical outcomes. Finally, vascular and bleeding complications were infrequent in both groups and no patient died during hospitalization in the EVP group. Last but not least, at mid-term clinical follow-up (median survival time: EVR 11±0.5 months, EVP 12±0.2 months) the rate of MACE was similar in the 2 groups (EVR: 24%, EVP: 33%; P=0.3).
Previous studies
The CoreValve system was the first-generation self-expandable valve introduced to the market. Although multiple studies demonstrated positive results (14,15), first-generation TAVR devices were far from perfect because of vascular complications, need for PPI, PVL, stroke, and other procedure-related complications (16). The next-generation device, EVR System was set out to mitigate these challenges. Several studies demonstrated higher overall survival with significantly lower rates of vascular complications, bleeding events, need for PPI, and moderate to severe PVL (17-19). However, there have been growing concerns regarding the higher incidence of PVL with TAVR as compared with SAVR and its association with poor outcomes. To better address this event, very recently the EVP system was introduced in the clinical area. Currently, however, information on the performance of this novel valve remains limited and with non-uniform results.
Firstly, the initial EvolutPRO single-arm registry, including 60 well-selected patients, demonstrated high in-hospital survival rates (98.3%). None to trace PVL was reported in 72.4% of patients while the remaining 27.6% experienced mild PVL. In that series no patient had moderate or severe PVL post procedure (5). Later, a German group reported a matched comparison of the EVP and EVR devices (20). They concluded that both prostheses provide excellent hemodynamic performance with no detectable differences between them. Notably, the incidence of mild PVL was also very low and similar (16% vs. 15% in EVR and EVP group, respectively). In this sense, several recent studies have not observed differences in the rate of PVL between EVR and EVP (12,21). These findings are comparable to those obtained in our study and corroborate data obtained in the real world. In contrast, another study recently published showed lower rates of PVL with EVP at one month of implantation (22).
The overall incidence of residual PVL after TAVR has been described in many studies reaching up to 80% of patients, mostly classified as mild (23). This is largely concordant with our current results. In the FORWARD study, mild PLV after EVR was detected in 30.9% (24) whereas in the EvolutPRO clinical study (5), mild PVL was observed in 27.6% of patients. However, the low incidence of mild PVL observed in the German study (16%) was surprising and noticeably lower than in the rest of the studies cited. A difference concerning to our study is that these investigators did not performed TEE during the procedure which is particularly useful for the assessment of acute aortic regurgitation immediately post-TAVR (25). In our study, as previously described, TEE was only used in the cases in which the patients were treated under general anesthesia. However, we should acknowledge that TEE was less frequently used in the EVP group. Accordingly, it remains possible that a lower rate of PVL could have been obtained in the EVP group if the use of TEE would have been more systematic. Moreover, despite of the propensity score matching performed in the German study (according to logEuroSCORE and CT-derived valve characteristics), the 2 groups remained unbalanced for other relevant characteristics (the presence of none/mild PVL, the AVA, and prevalence of atrial fibrillation). In this regard, baseline characteristics were also unbalanced in our series and this could help to explain, at least in part, the different results.
Many studies support the importance of a mid and long-term clinical follow-up after TAVR (26-30). The EVP has been recently introduced in the market and, therefore, information on its mid-term results is critical to better understand the clinical value of this novel device. Only one previous paper has reported the 1-year clinical follow up, with similar survival between both protheses (12). However, our study not only ratifies this finding, but also examines information on events other than mortality, including in MACE and composite endpoint, during mid-term follow-up. We believe that these novel data complement the current evidence and further refine the medium-term clinical outcome of patients treated with these devices.
Another important point is related to the need of new PPI. Some studies show lower PPI rates with EVP compared with EVR (5,12). In contrast, our study and others find no difference in this respect (21,22). With regard to other complications included in the VARC-2 criteria, no significant differences were found in any of the articles comparing the two prostheses.
As previously described, several studies (17-19) demonstrated the advantages of EVR with respect to its predecessor. However, our study, do not support the superiority of the novel EVP system regarding reduction of mild degrees of PVL. Larger series will be required to ascertain whether this novel device is indeed able to reduce the incidence of clinically relevant residual PVL as compared with its predecessor. This information appears critical considering the expected widespread use of this device in low-risk patients (31,32).
There were several limitations. First of all, this is a single center observational retrospective analysis of 83 consecutive patients undergoing transfemoral self-expandable TAVR. Although all data were prospectively obtained following a detailed and comprehensive protocol, we cannot exclude the possibility of selection bias. Therefore, it cannot be considered as a true head-to-head comparison of the two TAVR systems. Second, baseline characteristics of the two groups showed some differences that should be taken into consideration when the study results are interpreted. In addition, the potential influence of unmeasured confounders cannot be completely excluded considering the observational study design. Finally, the limited number of patients treated, and the lack of long-term clinical follow-up prevent drawing definitive conclusions regarding rare complications and long-term clinical outcomes. However, in the ever-evolving field of interventional heart valve therapy our findings accurately reflect the initial experience with this new system in “real world” patients undergoing TAVR.
Conclusions
The EVP new design appears to be safe and effective for the treatment of patients with severe aortic stenosis with excellent results at mid-term clinical follow up. Although hemodynamic results appear to be similar to those obtained with the EVR, the novel EVP device is still associated with a significant rate of residual mild PVL that appears to be comparable to that observed with EVR. While classical anatomic factors predict the occurrence of mild PVL with the EVR valve, these do not appear to be related with the presence of PVL with the EVP valve.
Acknowledgments
The authors would like to thank the invaluable help of the Radiology Department of Hospital La Princesa.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-20-2409
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-20-2409
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-20-2409). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional committee board of Hospital La Princesa (No. 3961) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:2440-92. [Crossref] [PubMed]
- Vahanian A, Lung B. The new ESC/EACTS guidelines on the management of valvular heart disease. Arch Cardiovasc Dis 2012;105:465-7. [Crossref] [PubMed]
- Popma JJ, Reardon MJ, Khabbaz K, et al. Early Clinical Outcomes After Transcatheter Aortic Valve Replacement Using a Novel Self-Expanding Bioprosthesis in Patients With Severe Aortic Stenosis Who Are Suboptimal for Surgery: Results of the Evolut R U.S. Study. JACC Cardiovasc Interv 2017;10:268-75. [Crossref] [PubMed]
- Mahtta D, Elgendy I, Bavry A. From CoreValve to Evolut PRO: Reviewing the Journey of Self-Expanding Transcatheter Aortic Valves. Cardiol Ther 2017;6:183-92. [Crossref] [PubMed]
- Forrest JK, Mangi AA, Popma JJ, et al. Early Outcomes With the Evolut PRO Repositionable Self-Expanding Transcatheter Aortic Valve With Pericardial Wrap. JACC Cardiovasc Interv 2018;11:160-8. [Crossref] [PubMed]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403-18. [Crossref] [PubMed]
- Leipsic J, Gurvitch R, Labounty T, et al. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging 2011;4:416-29. [Crossref] [PubMed]
- Delgado V, Ng A, Van de Veire N, et al. Transcatheter aortic valve implantation: role of multi-detector row computed tomography to evaluate prosthesis positioning and deployment in relation to valve function. Eur Heart J 2010;31:1114-23. [Crossref] [PubMed]
- Détaint D, Lepage L, Himbert D, et al. Determinants of significant paravalvular regurgitation after transcatheter aortic valve: implantation impact of device and annulus discongruence. JACC Cardiovasc Interv 2009;2:821-7. [Crossref] [PubMed]
- Willson AB, Webb JG, Labounty TM, et al. 3-dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement: a multicenter retrospective analysis. J Am Coll Cardiol 2012;59:1287-94. [Crossref] [PubMed]
- Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for Transcatheter Aortic Valve Implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol 2011;57:253-69. [Crossref] [PubMed]
- Kalogeras K, Ruparelia N, Kabir T, et al. Comparison of the self-expanding Evolut-PRO transcatheter aortic valve to its predecessor Evolut-R in the real world multicenter ATLAS registry. Int J Cardiol 2020;310:120-125. [Crossref] [PubMed]
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95. [Crossref] [PubMed]
- Reardon MJ, Adams DH, Kleiman NS, et al. 2-Year Outcomes in Patients Undergoing Surgical or Self-Expanding Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2015;66:113-21. [Crossref] [PubMed]
- Yakubov SJ, Adams DH, Watson DR, et al. 2-Year Outcomes After Iliofemoral Self-Expanding Transcatheter Aortic Valve Replacement in Patients With Severe Aortic Stenosis Deemed Extreme Risk for Surgery. J Am Coll Cardiol 2015;66:1327-34. [Crossref] [PubMed]
- Sherif MA, Abdel-Wahab M, Stöcker B, et al. Anatomic and procedural predictors of paravalvular aortic regurgitation after implantation of the Medtronic CoreValve bioprosthesis. J Am Coll Cardiol 2010;56:1623-9. [Crossref] [PubMed]
- Giannini C, De Carlo M, Tamburino C, et al. Transcathether aortic valve implantation with the new repositionable self-expandable Evolut R versus CoreValve system: A case-matched comparison. Int J Cardiol 2017;243:126-31. [Crossref] [PubMed]
- Landes U, Bental T, Barsheshet A, et al. Comparative Matched Outcome of Evolut-R vs. CoreValve Transcatheter Aortic Valve Implantation. J Invasive Cardiol 2017;29:69-74. [PubMed]
- Schulz E, Jabs A, Gori T, et al. Transcatheter aortic valve implantation with the new-generation Evolut R: Comparison with CoreValve(R) in a single center cohort. Int J Cardiol Heart Vasc 2016;12:52-6. [Crossref] [PubMed]
- Hellhammer K, Piayda K, Afzal S, et al. The Latest Evolution of the Medtronic CoreValve System in the Era of Transcatheter Aortic Valve Replacement: Matched Comparison of the Evolut PRO and Evolut R. JACC Cardiovasc Interv 2018;11:2314-22. [Crossref] [PubMed]
- Rao G, Sheth S, Donnelly J, et al. Early Real-World Experience with CoreValve Evolut PRO and R Systems for Transcatheter Aortic Valve Replacement. J Interv Cardiol 2019;2019:1906814 [Crossref] [PubMed]
- Forrest JK, Kaple RK, Tang GHL, et al. Three Generations of Self-Expanding Transcatheter Aortic Valves: A Report From the STS/ACC TVT Registry. JACC Cardiovasc Interv 2020;13:170-9. [Crossref] [PubMed]
- Généreux P, Head SJ, Hahn R, et al. Paravalvular leak after transcatheter aortic valve replacement: the new Achilles' heel? A comprehensive review of the literature. J Am Coll Cardiol 2013;61:1125-36. [Crossref] [PubMed]
- Grube E, Van Mieghem N, Bleiziffer S, et al. Clinical Outcomes With a Repositionable Self-Expanding Transcatheter Aortic Valve Prosthesis: The International FORWARD Study. J Am Coll Cardiol 2017;70:845-53. [Crossref] [PubMed]
- Lerakis S, Hayek S, Douglas P. Paravalvular aortic leak after transcatheter aortic valve replacement: current knowledge. Circulation 2013;127:397-407. [Crossref] [PubMed]
- Chakos A, Wilson-Smith A, Arora S, et al. Long term outcomes of transcatheter aortic valve implantation (TAVI): a systematic review of 5-year survival and beyond. Ann Cardiothorac Surg 2017;6:432-43. [Crossref] [PubMed]
- Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation 2011;123:299-308. [Crossref] [PubMed]
- Moat NE, Ludman P, de Belder MA, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol 2011;58:2130-8. [Crossref] [PubMed]
- Avanzas P, Pascual I, Munoz-Garcia A, et al. Long-term Follow-up of Patients With Severe Aortic Stenosis Treated With a Self-expanding Prosthesis. Rev Esp Cardiol (Engl Ed) 2017;70:247-53. [Crossref] [PubMed]
- Codner P, Orvin K, Assali A, et al. Long-Term Outcomes for Patients With Severe Symptomatic Aortic Stenosis Treated With Transcatheter Aortic Valve Implantation. Am J Cardiol 2015;116:1391-8. [Crossref] [PubMed]
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med 2019;380:1695-705. [Crossref] [PubMed]
- Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med 2019;380:1706-15. [Crossref] [PubMed]


