
Feasibility of application of an absorbable topical collagen hemostat sheet (INTEGRAN®) for prevention of postoperative recurrence of pneumothorax in youths
Introduction
Primary spontaneous pneumothorax (PSP) is a common thoracic disease. It is not a life-threatening, but is a concern because of its high recurrence rate, which is approximately 50% after conservative therapy (1). Almost all patients with recurrent PSP need surgical intervention. However, wedge resection alone using video-assisted thoracic surgery (VATS) is associated with a high postoperative recurrence rate of 10–20% (2-4).
Due to these problems, several additional procedures are used in surgery to minimize postoperative recurrence. In Western countries, parietal pleural abrasion and pleurectomy are often used, with the intent to create adhesion of the chest wall with the lung surface and consequently prevent recurrence. However, pleural adhesion has disadvantages of reducing respiratory function and potentially interfering with future ipsilateral surgery. To address this disadvantage, staple-line coverage has been introduced, with the goal of thickening the visceral pleura and consequently preventing recurrence (5). Two materials, polyglycolic acid (PGA) and oxidized regenerated cellulose (ORC) sheets, are currently used, but both have disadvantages. A PGA sheet induces strong adhesion between the chest wall and lung surface, similarly to procedures for the parietal pleura (6,7). In contrast, an ORC sheet does not induce chest wall adhesion, but is associated with a much higher recurrence rate of 20% (8).
Recently, the efficacy of an absorbable topical collagen hemostat sheet (INTEGRAN®, Nippon Zoki Pharmaceutical Co., Ltd., Osaka, Japan) has been shown for prevention of air leak after lobectomy for primary lung cancer (9). In addition, no intrathoracic adhesion was observed in a patient who received a second surgery after lobectomy with use of INTEGRAN® (10). These features suggest that INTEGRAN® may be an ideal clinical material for staple-line coverage in PSP surgery, especially for young adults. However, there is no report of use of INTEGRAN® in PSP surgery to date. In addition, all INTEGRAN® is left in the thoracic cavity when used in the coverage technique, whereas most is removed intraoperatively when used as a topical hemostat. Therefore, we conducted this study to evaluate the safety of this material for staple-line coverage in young adults with PSP.
We present the following article in accordance with the TREND reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-274).
Methods
Study design
The single-arm prospective intervention study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Kanto Rosai Hospital on February 9, 2017 (No. 2016-19) and informed consent was taken from all the patients. Accumulation of case was started on April 1, 2017. The inclusion criteria were patients aged 16–25 years old with a surgical indication for PSP [high-risk patients for postoperative recurrence according to Sudduth et al. (11)], who underwent bullae resection with a surgical stapling device at Kanto Rosai Hospital, had no abnormal data in preoperative blood tests, and had an Eastern Cooperative Oncology Group performance status of 0 or 1. The exclusion criteria were secondary spontaneous pneumothorax (e.g., catamenial pneumothorax), synchronous bilateral pneumothorax, hemothorax >1,000 mL in the chest cavity or a shock vital sign (systolic blood pressure <80 mmHg and HR >100/min), history of ipsilateral thoracic surgery, hypersensitivity to bovine-derived products (e.g., insulin), and pregnancy. Written informed consent was obtained before surgery and the patient was temporarily registered. Actual registration was performed intraoperatively after confirmation of all criteria (Figure 1).

The primary endpoint of the rate of material-related adverse events (AEs) was defined as fever ≥38 °C continuing for ≥2 days; WBC >18,000/mm3 and/or CRP >15 mg/dL postoperatively; or acute empyema within 30 postoperative days. To distinguish an abnormal inflammatory reaction to INTEGRAN from a normal postoperative reaction, we used relatively high cut-off values for WBC and CRP. The study protocol is registered in the UMIN Clinical Trials Registry (UMIN000026530).
Surgical procedure (protocol treatment) and follow-up
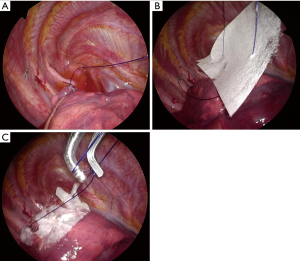
All procedures were performed by thoracic surgeons (H.A or H.K or A.K) at Kanto Rosai Hospital using a 2-port VATS approach (a 2.5-cm working window on the 3rd or 4th intercostal anterior axillary line and a 0.7-cm scope-insertion port on the 5th or 6th intercostal middle axillary line) under general anesthesia with double-lumen endotracheal tube intubation. After thorough observation of the entire visceral pleural surface, a leak test was conducted at an airway pressure of 20 cmH2O to check for air leak. Then, bullae were resected using a surgical stapling device (Endo GIA Universal, Medtronic, Minneapolis, MN, USA or Powered ECHELON FLEX, Johnson & Johnson, New Brunswick, NJ, USA). After resection, a sealing test was performed at an airway pressure of 20 cmH2O to confirm no air leak from the resected site. Then, both edges of the staple-line were ligated with a pre-tied loop ligature (ENDOLOOP, Johnson & Johnson). An INTEGRAN® sheet (0.2 g in weight and 10.0 cm × 5.0 cm in size) was then placed on the staple-line through the guide of the ENDOLOOP for both edges, and knotted with the ENDLOOP to fix the INTEGRAN® sheet (Figure 2). Surgery was finished with insertion of a chest tube. This tube was removed when air leak was absent after postoperative day (POD) 1 and patients was discharged on the day after removal of the chest tube. Preventive administration of antibiotics was only performed at the start of surgery and no additional antibiotics were administered.
Patients were followed as outpatients for at least for 1 year after surgery. Chest X-ray was performed routinely just after surgery, on POD 1, on the day after chest tube removal, on POD 14, after postoperative month (POM) 1 and 6, and when recurrence was suspected. Blood tests were performed on POD 1 and 14 and in POM 1, 6, and 12. Computed tomography (CT) with a lung field setting (window level −550 HU, window width 1,600 HU) and 1-mm slice thickness was performed in POM 12 to evaluate the formation of new bullae around the staple-line, which Choi et al. found to be a significant risk factor for recurrence (12). CT was also performed when postoperative recurrence was suspected.
Sample size and statistical analysis
Drug information for INTEGRAN® indicates that AEs (mainly fever and inflammatory reactions) occur in 2.5% of patients if INTEGRAN® is used as an absorbable topical hemostat. When used as a topical hemostat, most INTEGRAN® is removed intraoperatively after confirmation of hemostasis. However, all INTEGRAN® remains in the thoracic cavity when used for staple-line coverage. Thus, we estimated a threshold rate of material-related AEs of 20% and an expected rate of 4%. With 80% power and an alpha value of 0.05 (one-sided), the required sample size was calculated to be 23 patients using a binomial test. In anticipation of some censored cases, 25 patients were enrolled in the study.
The postoperative recurrence-free period was defined as the period between the date of surgery and the date of recurrence confirmed by chest X-ray or CT, or the date of the last outpatient follow-up. A recurrence-free survival curve was estimated using the Kaplan-Meier method. All statistical analyses were performed with IBM SPSS statistics 26 (IBM Corp., New York, NY, USA).
Results
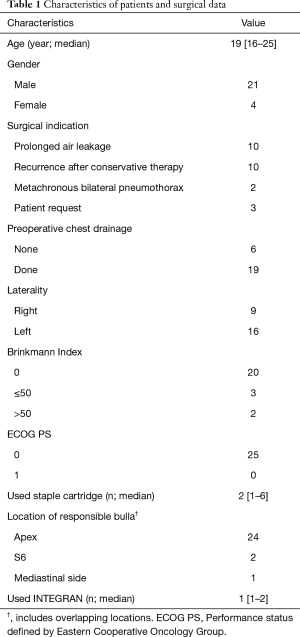
Participant flow was shown in Figure 1. The 25 subjects (21 men and 4 women) had a median age of 19 years old (range, 16 to 25 years old). The surgical indication for PSP was prolonged air leak (n=10), recurrence after conservative therapy (n=10), metachronous bilateral pneumothorax (n=2), and patient request (n=3). Cessation of the air leak was monitored for about 1 week after chest tube insertion in patients with PSP for the first time. Surgery was performed if the air leak continued beyond one week. The characteristics of the patients and operative data are shown in Table 1. In almost all patients, the resected bulla was in one region only, and only one INTEGRAN® sheet was used in staple-line covering.
Full table
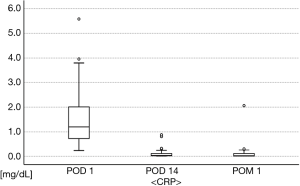
Regarding postoperative body temperatures (Figure 3), two patients had fever ≥38 °C: one had fever ≥38 °C on POD 1, but this decreased to <38 °C on the next day, while the other patient had fever ≥38 °C only on POD 2. In postoperative blood tests (Figures 4,5), the maximum WBC and CRP levels were 17,900/mm3 and 5.58 mg/dL, respectively, and no patients had abnormal WBC (>18,000/mm3) or CRP (>15 mg/dL) in POM 1. Acute empyema did not occur in any patients. Thus, the overall rate of material-related AEs was 0/25 (0.0%) in this study.


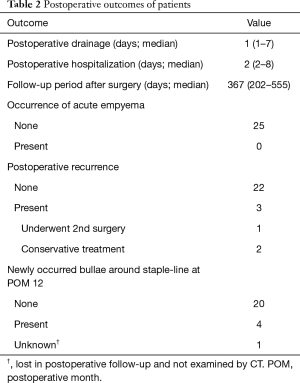
Postoperative outcomes are shown in Table 2. The median follow-up period after surgery was 367 days (range, 202 to 555 days). One patient was lost follow-up at POM 6 and was regarded as censored in the Kaplan-Meier method. There was recurrence of PSP in 3 patients, of whom one underwent a second surgery two months after the first surgery. The recurrence-free survival curve showed a 1-year recurrence-free rate of 88.0% postoperatively. In reoperation, only mild adhesion between the lung surface and chest wall was present, and this was dissected easily by a 2-port VATS approach. Also, the responsible bulla was not around the staple-line of the first surgery, but at the top of the S6 segment in this case. New bullae had developed around the staple-line in 4 of 24 patients (16.7%) on CT performed in POM 12. However, all 3 patients with postoperative recurrence had no bullae around the staple-line in this CT examination.
Full table
Discussion
PSP is not life threatening, but it is problematic because of its high recurrence rate (1), which is over 25% in young patients even after surgical treatment (13). Upon PSP recurrence, patients have to restrict their social activity due to the symptoms and the need for further treatment. This is a particular concern because PSP occurs more frequently in young adults, whose quality of life may be particularly affected by this loss of social activity. Therefore, prevention of recurrence is very important in treatment of PSP, especially for young patients.
Procedures such as parietal pleural abrasion and pleurectomy have been developed to minimize postoperative recurrence of PSP. In a meta-analysis, Sudduth et al. showed that wedge resection + chemical pleurodesis ± pleural abrasion was the most suitable procedure, with a recurrence rate of 1.7–2.8% (11). Thus, additional intraoperative procedures for the parietal pleura that promote adhesion between the chest wall and lung surface and consequently prevent recurrence are well accepted in Western countries and recommended by some guidelines (14,15). However, pleural adhesion reduces respiratory function and may interfere with future ipsilateral thoracic surgery, which is a particular concern in young patients who may eventually require a second surgery for PSP or for lung cancer.
To avoid these problems, staple-line coverage was first reported by Sakamoto et al. in 2004 as a procedure to thicken the visceral pleura and prevent new occurrence of bullae around the staple-line in wedge resection. The recurrence rate after staple-line coverage by a PGA sheet was 2.6%, compared to 9.5% after wedge resection only, and a pathologically proven grossly thickened visceral pleura with fibrosis was present in the PGA-covered area, which may prevent occurrence of new bullae around the staple-line (16). Subsequently, Lee et al. showed that staple-line coverage was non-inferior to mechanical pleurodesis and superior to intraoperative pleural abrasion in terms of postoperative recurrence (17,18). However, there is strong adhesion between the lung surface covered with PGA and the chest wall, a similar disadvantage to procedures for the parietal pleura (6,7).
An ORC sheet for staple-line coverage is used mainly in Japan, and this sheet thickens the visceral pleura with minimal or no chest wall adhesion (19). However, Ozawa et al. found that patients with an ORC covering had a much higher recurrence rate than patients with PGA covering (22.8% vs. 3.6%) (8). Thus, there is no consensus on the most suitable material for staple-line coverage for prevention of recurrence of PSP.
Recently, Miyamoto et al. and Fujiu et al. found that pleural covering by INTEGRAN® induces visceral pleural thickening without adhesion to the chest wall (9,10), which suggests that this material could be suitable for staple-line coverage in PSP surgery for young patients. INTEGRAN® is widely used as an absorbable topical hemostat. Collagen derived from the dermis of Australian calves is converted to pure atelocollagen by enzyme solubilization to remove telopeptides that cause antigenicity, and is then chemically cross-linked with a polyepoxy compound to form an insoluble and stable fiber. This raw material is formed into sheets as INTEGRAN®, which has low antigenicity because it contains no protein components other than collagen (20). INTEGRAN® also contains no blood products, which suggests that it might be safe for staple-line coverage without concerns of allergic reaction and blood-borne infections.
There has been no previous report of use of INTEGRAN® for staple-line coverage in PSP surgery. INTEGRAN® is widely used as a topical hemostat, but almost all is removed intraoperatively after confirmation of hemostasis. In contrast, all INTEGRAN® is left in the thoracic cavity when used for staple-line coverage. Thus, we conducted this study to establish the safety of use of INTEGRAN® as a material for staple-line coverage in PSP surgery, especially for young adults.
Drug information for INTEGRAN® indicates main AEs of fever, local inflammation, and hematoma when used as a topical hemostat. In our study, postoperative fever occurred in 2 patients, but none had fever for >24 hours. Moreover, no patients had an abnormal inflammatory reaction and acute empyema did not occur in any cases. These results suggest that 0.2 g of INTEGRAN® can be safely left in the intrathoracic cavity, and we consider that INTEGRAN® can be safely used for staple-line coverage.
The 1-year postoperative recurrence rate was 12.0% in our study, which appears to be inferior to that in previous reports. However, we consider the recurrence rate in our study is acceptable because our study was conducted in young patients, which is a high-risk cohort. Joharifard et al. found a postoperative recurrence rate of 40% in young children who underwent wedge resection with intraoperative pleural abrasion (21). In a meta-analysis, Sudduth et al. found a postoperative recurrence rate of about 10% in patients <25 years old even after wedge resection with intraoperative pleural abrasion (11). Noh et al. found a postoperative recurrence rate of 17.9% in patients aged <25 years old, despite treatment with wedge resection with staple-line coverage and/or pleurodesis (13). Thus, staple-line coverage with INTEGRAN® appears to be equivalent to other procedures for prevention of postoperative recurrence.
Formation of new bullae around the staple-line occurred in only 4 patients on CT in POM 12, and recurrence occurred only in patients without new bullae. These results may be explained by the hypothesis that INTEGRAN® thickens the visceral pleura and prevents rupture, even if new bullae formation occurs. However, we note that patients who had recurrence after staple-line coverage with INTEGRAN® showed mild adhesion of the INTEGRAN®-covered lung surface and the chest wall in second surgery, which differs from the findings in Miyamoto et al. and Fujiu et al. (9,10). Although the adhesion was mild and easily dissected by a VATS approach, this suggests that chest wall adhesion may still occur in use of INTEGRAN® for staple-line coverage. Regardless, this study was conducted to evaluate the safety of INTEGRAN® for staple-line coverage, and the number of cases and follow-up period were insufficient to evaluate the efficacy. Evaluation of the efficacy of INTEGRAN® for prevention of recurrence of PSP will require a prospective randomized study and we are now planning such a study.
Conclusions
In conclusion, this study showed that INTEGRAN®, a sheet-type absorbable topical collagen hemostat, can be safely used as material to cover a staple-line at the resection margin in surgery for young patients with PSP. To evaluate the efficacy of INTEGRAN® for prevention of recurrence of PSP, a further prospective randomized study is required.
Acknowledgments
Funding: This work was supported by research funds to promote hospital functions from the Japan Organization of Occupational Health and Safety.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-274
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-274
Peer Review File: Available at https://dx.doi.org/10.21037/jtd-21-274
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-274). HA reports funding from Japan Organization of Occupational Health and Safety. HA serves as an unpaid editorial board member of Journal of Thoracic Disease. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The single-arm prospective intervention study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Kanto Rosai Hospital on February 9, 2017 (No. 2016-19) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sadikot RT, Greene T, Meadows K, et al. Recurrence of primary spontaneous pneumothorax. Thorax 1997;52:805-9. [Crossref] [PubMed]
- Horio H, Nomori H, Kobayashi R, et al. Impact of additional pleurodesis in video-assisted thoracoscopic bullectomy for primary spontaneous pneumothorax. Surg Endosc 2002;16:630-4. [Crossref] [PubMed]
- Muramatsu T, Ohmori K, Shimamura M, et al. Staple line reinforcement with fleece-coated fibrin glue (TachoComb) after thoracoscopic bullectomy for the treatment of spontaneous pneumothorax. Surg Today 2007;37:745-9. [Crossref] [PubMed]
- Nakanishi K. Long-term effect of a thoracoscopic stapled bullectomy alone for preventing the recurrence of primary spontaneous pneumothorax. Surg Today 2009;39:553-7. [Crossref] [PubMed]
- Ebana H, Hayashi T, Mitani K, et al. Oxidized regenerated cellulose induces pleural thickening in patients with pneumothorax: possible involvement of the mesothelial-mesenchymal transition. Surg Today 2018;48:462-72. [Crossref] [PubMed]
- Takeshita S, Muramatsu T, Shimamura M, et al. The Usefulness of Various Reinforcement Materials on Re-operation for Spontaneous pneumothorax. J Nihon Univ Med Ass 2014;73:183-5.
- Isaka T, Kanzaki M, Kikkawa T, et al. In vivo and in vitro degradation behavior of artificial materials used for sealing pulmonary air leak. Jpn J Chest Surg 2011;25:466-71. [Crossref]
- Ozawa Y, Sakai M, Ichimura H. Covering the staple line with polyglycolic acid sheet versus oxidized regenerated cellulose mesh after thoracoscopic bullectomy for primary spontaneous pneumothorax. Gen Thorac Cardiovasc Surg 2018;66:419-24. [Crossref] [PubMed]
- Miyamoto H, Sakao Y, Sakuraba M, et al. The effects of sheet-type absorbable topical collagen hemostat used to prevent pulmonary fistula after lung surgery. Ann Thorac Cardiovasc Surg 2010;16:16-20. [PubMed]
- Fujiu K, Miyamoto H. Microscopic findings of sheet-type collagen applied to air leaks after pulmonary resection. Ann Thorac Cardiovasc Surg 2012;18:212-5. [Crossref] [PubMed]
- Sudduth CL, Shinnick JK, Geng Z, et al. Optimal surgical technique in spontaneous pneumothorax: a systematic review and meta-analysis. J Surg Res 2017;210:32-46. [Crossref] [PubMed]
- Choi SY, Kim DY, Suh JH, et al. New bullae formation in the staple line increases the risk of recurrent pneumothorax following video-assisted thoracoscopic surgery bullectomy for primary spontaneous pneumothorax. J Thorac Dis 2018;10:4287-92. [Crossref] [PubMed]
- Noh D, Lee S, Haam SJ, et al. Recurrence of primary spontaneous pneumothorax in young adults and children. Interact Cardiovasc Thorac Surg 2015;21:195-9. [Crossref] [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- Sakamoto K, Takei H, Nishii T, et al. Staple line coverage with absorbable mesh after thoracoscopic bullectomy for spontaneous pneumothorax. Surg Endosc 2004;18:478-81. [Crossref] [PubMed]
- Lee S, Park SY, Bae MK, et al. Efficacy of polyglycolic acid sheet after thoracoscopic bullectomy for spontaneous pneumothorax. Ann Thorac Surg 2013;95:1919-23. [Crossref] [PubMed]
- Lee S, Kim HR, Cho S, et al. Staple line coverage after bullectomy for primary spontaneous pneumothorax: a randomized trial. Ann Thorac Surg 2014;98:2005-11. [Crossref] [PubMed]
- Kurihara M, Kataoka H, Ishikawa A, et al. Latest treatments for spontaneous pneumothorax. Gen Thorac Cardiovasc Surg 2010;58:113-9. [Crossref] [PubMed]
- Charriere G, Bejot M, Schnitzler L, et al. Reactions to a bovine collagen implant. Clinical and immunologic study in 705 patients. J Am Acad Dermatol 1989;21:1203-8. [Crossref] [PubMed]
- Joharifard S, Coakley BA, Butterworth SA. Pleurectomy versus pleural abrasion for primary spontaneous pneumothorax in children. J Pediatr Surg 2017;52:680-3. [Crossref] [PubMed]


