
Thirty years of esophageal cancer surgery in Oulu University Hospital
Introduction
Esophageal cancer is the 7th most common cause of cancer-related death worldwide (1). The prognosis is poor, as 5-year overall survival remains below 20% (2). Esophagectomy provides the best opportunity for a cure in regional esophageal carcinoma although it involves a high operative risk (3). Surgical techniques have improved over the decades, but mortality rates after surgery are still 7% at 90 days and 25% at one year after surgery (4).
Esophagectomy is usually performed by either transthoracic resection, divided into Ivor-Lewis (laparotomy, right thoracotomy, and intrathoracic anastomosis) and McKeown (laparotomy, thoracotomy, cervicotomy and cervical anastomosis) esophagectomy, or transhiatal esophagectomy, including laparotomy and anastomosis through cervical incision (5). The benefit of transthoracic approach is increased lymph node yield and possibly improved prognosis, compared to transhiatal approach (6). Ivor Lewis technique might decrease the risk of pulmonary complications, recurrent laryngeal nerve injury, strictures and anastomotic leaks, compared to McKeown approach (7-9) and anastomotic leak rates compared to transhiatal approach (5). Minimally invasive esophagectomy (MIE) and hybrid MIE (hMIE) techniques have gained popularity with the aim of reducing postoperative morbidity (10,11). Although MIE is associated with longer learning curve, MIE could be associated with a better long-term survival compared to open esophagectomy (4,12-14). Four randomized controlled trials have compared MIE and open esophagectomy, suggesting reduced pulmonary complications and vocal cord paralysis, and improved quality of life with MIE without compromising long term oncological outcomes (15-18). Multimodality treatment has significantly improved survival and has become a standard of care for resectable locally advanced esophageal or esophagogastric junctional cancer (19).
Recently, the international benchmark for esophagectomy was published to monitor performance in surgery. The aim of this study was to review the evolution of esophageal cancer treatment in Northern Finland, with special interest in any differences in results, complications and survival between different techniques used through the years. Currently used guideline-based treatment is compared with international benchmark values.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-520).
Methods
Study design
This study was a retrospective cohort study from a single tertiary care hospital, between years 1987 and 2020, including all elective esophagectomies for cancer and dysplasia. The patients who underwent esophagectomy due to a benign or emergency indication were excluded. Follow-up ended December 31, 2019 in patients operated between years 1987 and 2016. In the last period 2017–2020 due to the short median follow-up time, end of follow-up was set at 1 year after surgery, or February 28, 2021 in patients operated after March 1, 2020.
Patients
Clinical data was collected from patient records. The study was conducted according to the guidelines of the Declaration of Helsinki (as revised in 2013) and was approved by the hospital district. The data collection was approved by the Oulu University Hospital Ethics Committee (EETTMK: 81/2008). The need for written informed consents was waived by National Authority for Medicolegal Affairs (VALVIRA) due to its retrospective nature.
Co-morbidity data was collected according to Royal College of Surgeons Charlson Score excluding esophageal cancer (20). Clinical preoperative staging and postoperative staging was performed according to the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC), 8th edition (21). Neoadjuvant- and adjuvant therapy data for each patient and type of treatment were obtained.
Positive resection margin, the number of examined lymph nodes and readmission rate were reported as in a previous study (22). R0 resection was defined as complete resection with at least 1 mm resection margin.
The complications were collected according to International consensus on standardization of data collection for complications associated with esophagectomy (23,24). Benchmark values were obtained from the study by Schmidt et al. in which complication rates were examined from a selected low-comorbidity group of 332 patients in 13 different high volume centers around the globe (24). Complications were categorized according to the Clavien-Dindo classification (25) divided into minor (Clavien-Dindo grade 1–2) and major complications (Clavien-Dindo grade ≥ IIIa).
Mortality data was confirmed from the nationwide and obligatory Cause of Death registry held by Statistics Finland, which has 100% coverage for dates of death in Finland, and combined to the patient records using the immutable personal identification number assigned to all residents in Finland.
Operative approach
Open esophagectomy included pyloromyotomy and jejunostomy feeding tube insertion. Cervical anastomosis was handsewn, whereas intrathoracic anastomosis technique varied according to surgeon preference, most often side-to-end anastomosis with a circular stapler. The MIE technique included wrapping of omental flap around the anastomosis, endoscopic pyloric dilatation and selective nasojejunal feeding tube placement. Intrathoracic anastomosis was preferred over cervical anastomosis. Side-to-end anastomosis with a circular stapler was usually performed.
Statistical analysis
The changes and results of esophageal cancer treatment over time with introduction of MIE in our center. Treatment strategies are compared to current guidelines including staging and use of neoadjuvant therapy, and benchmark values including postoperative morbidity, hospital stay, readmissions and 90-day mortality (24), and long-term survival was compared to previous national studies (4,26). Survival was studied using the life table method and plotted using Kaplan-Maier curves. Chi square test was used when comparing categorical variables. Continuous variables were compared by t-test and Mann-Whitney U test as appropriate. Numeric values are presented and mean (standard deviation) and median (interquartile range). Statistical analyses were performed using IBM SPSS version 27 (Armonk, NY, USA).
Results
Patients
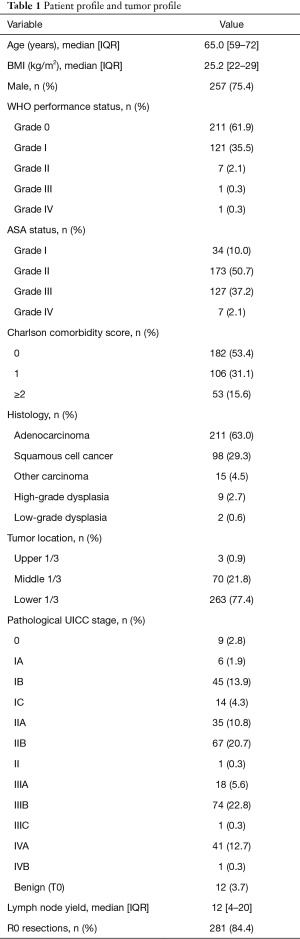
A total of 341 patients underwent esophagectomy for cancer in Oulu University Hospital during 1987–2020 (Table 1, Figure 1). The median age of patients was 65.0 years (IQR, 59–72 years) of which 257 (75.4%) were male (Table 1, Figure 1A). The majority had WHO performance status of Grade 0 (n=211, 61.9%), American Society of Anesthesiologists (ASA) class II (n=173, 50.7%), and 182 (53.4%) patients had Charlson comorbidity score 0 (Table 1, Figure 1B).
Full table
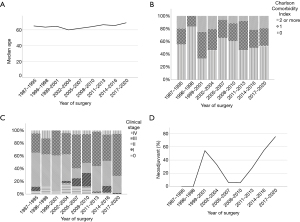
A total of 211 (63.0%) patients had adenocarcinoma and 98 (29.3%) squamous cell carcinoma (Table 1, Figure 2). Temporal changes in histology are presented in Figure 2A. Most of the tumors were located in the lower third of esophagus (77.4%) (Table 1). The dominant pathological UICC stage was IIIb (n=74, 22.8%) (Table 1, Figure 2B). Changes over time in clinical and pathological stage distribution are shown in Figures 1C and 2B.
Preoperative staging
The most common clinical T-stage was T3 (46.5%) and suspected positive lymph nodes were seen in 16.2% of patients in preoperative imaging. The percentages of clinical stage are shown in Figure 1C. A total of 238 (70.2%) patients had a clinically locally advanced disease and 4 (1.2%) advanced disease. Use of PET-CT was introduced in 2006, since then PET-CT was performed to 50 (14.7%) patients. Endoscopic ultrasound was first performed in 2003, and since then EUS was used selectively in 9 (2.6%) patients EUS.
Upstaging in the yp/pTNM compared to cTNM occurred in 22.3% (n=72) of the patients. Over time, upstaging occurred as follows: 11.9%, 0.0%, 9.1%, 21.7%, 12.9%, 28%, 36.7%, 26.1%, 21.8% in years 1987–1995, 1996–1998, 1999–2000, 2002–2004, 2005–2007, 2008–2010, 2011–2013, 2014–2016, 2017–2020.
Preoperative therapy
One hundred and eight patients received neoadjuvant therapy (31.8%) of which 61 (56.5%) was chemotherapy and 48 (44.4%) chemoradiotherapy (Table 2). However, the use of neoadjuvant treatment was originally introduced in 1999 mainly in squamous cell carcinoma, and guideline-based regular use followed in 2017–2020 where 41 (83.7%) patients with locally advanced or advanced esophageal carcinoma received recommended neoadjuvant therapy (Figure 1D).
Full table
Surgical approach
Transhiatal resection was performed in 167 (49.3%) patients, while transthoracic Ivor Lewis (IL) in 129 (38.1%) patients and transthoracic McKeown in 42 (12.4%) patients (Table 2, Figure 2C). MIE was performed on 49 (14.5%) patients of which three were converted to hybrid procedures (laparoscopy and thoracotomy). The median duration of surgery was 327 min (IQR, 249–405 min). Blood was transferred to 15.7% patients during surgery and to 19.7% postoperatively. Colon was used as a substitute in 8 patients. The median number of lymph nodes examined was 12 (IQR, 4–20) (Table 1). Lymph node yield in transhiatal operations were 6 (IQR, 1–14) and in transthoracic operations 17 (IQR, 10–25). The number of examined lymph nodes increased from 1987 to 2016, but decreased after introduction of MIE (Figure 2D). Positive resection margin was observed in 7.5% (Table 1), which was the highest after transhiatal resection (9.3%) the lowest after McKeown (2.6%). Over time R0 resections were observed as follows: 81.8%, 71.4%, 95.5%, 91.7%, 71.9%, 74.5%, 88.0%, 84.8% and 93.0% (1987–1995, 1996–1998, 1999–2001, 2002–2004, 2005–2007, 2008–2010, 2011–2013, 2014–2016, 2017–2020).
Perioperative complications and reoperations
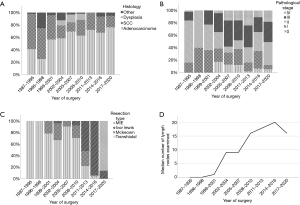
Complication data is presented in Figure 3. The overall complication rate was 72.7% while major complication rate (Clavien-Dindo grade ≥ IIIa) was 39.1% (Figure 3A). Pneumonia was the most common complication (n=82, 24.1%) and overall pulmonary complication rate was 40.6%. Incidence of pulmonary complications decreased over time (Figure 3B). The second most common complication was pleural effusion requiring additional drainage procedure 18.8% (n=64) and occurred most frequently after McKeown technique 32.5% (n=13). Anastomotic leak was observed in 12.3% and are shown periodically in Figure 3C and stratified by surgical technique in Figure 4. Anastomotic leak rate after handsewn and stapler assisted anastomosis was 14% (n=22) and 12.2% (n=20). Atrial dysrhythmia occurred in 5.3% (n=18) of patients.

The median hospital stay was 11 (IQR, 9–15) days and median ICU stay was 1 (IQR 1–2) days. Postoperative ICU treatment was indicated in 74.3% (n=252) of patients, of which 52.8% (n=133) were transferred routinely to ICU for one night. The changes in median hospital stay over time is shown in Figure 3D and changes sorted by surgical techniques in Figure 4. Reoperations were performed on 65 (19.2%) patients during 90 days postoperatively.
Readmissions and short-term mortality
Thirty-day readmission rate was 6.5%. Overall 90-day hospital readmission rate was 17.4% and occurred most frequently in earlier time periods, 23.3%, 8.3%, 12.5%, 8.3%, 34.4%, 17.6%, 20%, 17.4%, 8.8% (1987–1995, 1996–1998, 1999–2001, 2002–2004, 2005–2007, 2008–2010, 2011–2013, 2014–2016, 2017–2020). Adjuvant treatment was given to 117 (36.1%) patients with no changes over time. The 30- and 90-day survival rates are shown in Figure 5.
Long term outcomes
The prevalence of strictures requiring endoscopic dilatation was 21.9% and repeat dilations defined as three dilatations or more was 6.8%. In neck anastomosis dilatation and repeated dilatations were needed in 28.8% and 9.6%, and in intrathoracic anastomosis 10.9%, 2.3%, respectively. Recurrent nerve palsy developed to 14.7% (n=50), with incidence of 23.0% after neck anastomosis, and 1.6% with intrathoracic anastomosis. The incidence of recurrent nerve palsy decreased after the transition from transhiatal to transthoracic approach, as shown in Figure 4. Median follow-up time was 21 months (IQR, 10–70 months) in the surviving patients. The 1-, 3- and 5-year survival rates are shown in Figure 5.
Introduction of MIE
The first MIE was performed in September 2017. Before starting the MIE programs a team of three surgeons trained in MIE techniques through visitations and fellowship programs in other centers performing MIE. After the transition to MIE only three resections have been performed with open intention. MIE with intrathoracic anastomosis is now the most common technique in our center (Figure 2C). Conversion to thoracotomy occurred in 6.3% (n=3) and to laparotomy in 0.0% (n=0).
The median duration of surgery was longer in MIE group compared to open esophagectomy (477 vs. 304 min). Clavien-Dindo grade 5 complications (death) has occurred in one patient in MIE group (2.0%), while in the open esophagectomy group 5.9% (n=17) died during postoperative period (Figure 3A). Overall pulmonary complications (40.8% vs. 40.1%) and anastomotic leak rates (12.2% vs. 12.1%) were similar.
Discussion
The present study covers a 34-year period, providing comprehensive information on the development of esophagectomies and learning curve for each surgical technique at a medium volume center. Currently used guideline based treatment protocol has resulted with comparable outcomes to the international benchmark values, even though learning curve is included.
A strength of our study is the access to all patient records providing superior coverage compared to registry data. Long-term follow-up and survival information was acquired from Statistics Finland with no missing information. Our study had also some limitations. The retrospective study design prevented the inclusion of some minor complications, due to insufficient records, especially in older cases. However, at least all major complications have been recorded and included. The Oulu University Hospital is the only tertiary care center treating esophageal cancer in Northern Finland (approximately 50% of the geographical area of Finland), and captures >90% of the esophagectomies in the area during the study period.
We compared our data set to a benchmark data set to identify areas in need of improvement and tendencies in different time periods. There may be some reasons for differences between our center, and the benchmark study. The median age in our cohort was 65 years, while in the benchmark study by Schmidt et al. (24) it was 58 years. The benchmark values were defined for low comorbidity patients in the benchmark study, while our study included all consecutive patients. Higher age is associated with increased risk for postoperative complications (27). Poorer performance status and ASA status is thought to be related to poorer prognosis (28,29). WHO performance status in the patients in our cohort was worse compared to the benchmark study and ASA status ≥3 included 37.2% of patients while in the benchmark study there were none. In our study 15.5% of patients had Charlson comorbidity score ≥2, which is significantly associated with poor prognosis (30). The most common pathological UICC stage was IIIb (22.8%) whereas in the benchmark study only 8.7% had stage IIIb tumors.
According to the current guidelines EUS and PET-CT should carried out in candidates of esophagectomy (31) and previous studies have suggested the significance and benefit of PET-CT and EUS in preoperative staging of esophageal cancer and defining therapeutic strategy (32-35). However, in our study PET-CT was performed to 14.7% and EUS to 2.6% of all patients and in MIE group the respective proportions were 42.9% and 8.2% explaining high upstaging. The use of PET-CT is improving, and actions have been made to include PET-CT as a routine practice, instead of selective use. Between the years 2017 and 2020 75.4% of our patients received neoadjuvant treatment which is in line with the suggested levels for neoadjuvant therapy (11,24). Some variation was seen in neoadjuvant treatment over time. During 1999–2004, where SCC was prevalent histology type, treatment was given according to early trials (36). However, neoadjuvant treatment rate decreased until MAGIC-trial (37) involving adenocarcinoma patients followed by CROSS-trial (38) (adenocarcinoma and SCC) when treatment gradually became the standard of care. The number of examined lymph nodes was lower than in the benchmark study. Lower lymph node yield in transhiatal approach is in line with previous reports (6). Previous studies have suggested that sample processing may affect the number of lymph nodes examined, systematic fat blocking and methylene blue staining is associated with a higher total and positive lymph node yield compared to manual nodal dissection which is limited to finding small lymph nodes (39-41). There may be some reasons for higher positive margins related to transhiatal approach. Due to the cervical anastomosis longer conduit was needed compared to intrathoracic anastomosis possibly affecting distal resection margin. Also at the time, frozen sections on resection margins were not routinely used and neoadjuvant treatment was rare (19,38).
The whole study period overall complication rate was higher compared to the benchmark study. Previously reported incidence rates of complications of any severity vary greatly (24,42-44) mostly due to information bias in observational studies, which is also likely present in the current study. Major complications (CD grade ≥ IIIa) occurred in 39.1% of patients which was more than in the benchmark study (≤30.8%), and explained by the high rate of pleural effusions requiring drainage (24). As in previous studies (42,43), the most common complication in our patient cohort was pneumonia (24.1%). Transhiatal approach and cervical anastomosis has been associated with a higher frequency of recurrent laryngeal nerve trauma and anastomotic leak (45,46). Reported anastomosis leak rates were below suggested 20% Benchmark value, except 2011–2013 20% (24,42,43). Although some studies have suggested that atrial dysrhythmia is a common complication [14.5–14.6% (21,42)] our data set showed an incidence of atrial dysrhythmia rate of 5.3%. The median hospital stay was shorter compared to benchmark study (11 vs. 12). The incidence of strictures requiring endoscopic dilatation was 21.9% and repeat dilations defined as three dilatations or more was 6.8% while in a previous population-based study the respective numbers were 16.7% and 6.6% (47). With modern preoperative staging and treatment including PET-CT, neoadjuvant treatment and MIE our 1-year survival rate (82.1%, between years 2017 and 2020) was slightly below the defined benchmark (85.5%) (24), which may be due to the learning curve (14). In our study, the incidence of pulmonary complications and positive resection margins were higher compared to the benchmark values, while the hospital readmission rate was lower. Low preoperative use of PET-CT may explain higher positive resection margins. Especially lower lymph node yield in MIE era could be explained with learning curve.
Complications of any severity, major complications, positive resection margins and hospital readmission rates have reduced with the transition to MIE approach although the data also includes learning curve. A previous Dutch study reported a learning curve of 119 cases of MIE when reaching the plateau of 8% anastomotic leakage (14). However, at the beginning of the learning curve in the Netherlands and Sweden the incidence of anastomotic leakage was higher than in our study (13,14). Length of the learning curve varies depending on the study from 25 to 175 patients (44). Learning curve has taken place when changing primary approach from transhiatal to transthoracic procedure and when changing from open to minimally invasive approach. The learning curve has been included in the study and affects outcomes. This is visible in decreased lymph node yield, increased anastomosis leaks and hospital stay despite minor tissue trauma.
Systematically reviewing our results will help us find areas for improvement and implement continuous quality improvement particularly with the aim to evaluate complex and cost-intensive procedures. Our center has room for improvement in preoperative staging strategies, overall complication rates, major complications, the number of examined lymph nodes and positive resection margins. Esophageal cancer surgery has evolved over the years with changes in patient and disease profile, oncological treatment and surgical techniques. Current practice in our center is mostly in line with current recommendations. Despite improvements in 1-year survival, some common complications have remained stable, such as anastomosis leaks. There is still room for new innovations in surgical technique of esophageal cancer surgery.
We recommend that all centers go through their own material carefully so that potential areas for improvement are identified in order to improve treatment processes. Quality control can increase motivation to improve treatment strategies.
Acknowledgments
Funding: This work was supported by grants from Instrumentarium Science Foundation (OH), Finnish State Research Funding (OH), Georg C. and Mary Ehrnrooth Foundation (OH) and the Finnish Medical Foundation. Orion Research Foundation (JHK), Thelma Mäkikyrö Foundation (JHK, HH) and Mary and Georg C. Ehrnroot Foundation (JHK, HH), The Finnish cultural Foundation (HH), Vieno and Alli Suorsa’s health care foundation (HH), Päivikki and Sakari Sohlberg Foundation (JHK), The Finnish Cancer Foundation (JHK), and Sigrid Juselius Foundation (JHK).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-520
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-520
Peer Review File: Available at https://dx.doi.org/10.21037/jtd-21-520
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-520). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Oulu University Hospital Ethics Committee and the hospital district (committee’s reference number 81/2008). The need to obtain informed consent from the study patients was waived by the Finnish National Authority for Medicolegal Affairs (VALVIRA).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers 2017;3:17048. [Crossref] [PubMed]
- Sauvanet A, Mariette C, Thomas P, et al. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg 2005;201:253-62. [Crossref] [PubMed]
- Sihvo E, Helminen O, Gunn J, et al. Long-term outcomes following minimally invasive and open esophagectomy in Finland: A population-based study. Eur J Surg Oncol 2019;45:1099-104. [Crossref] [PubMed]
- Meredith KL, Maramara T, Blinn P, et al. Comparative Perioperative Outcomes by Esophagectomy Surgical Technique. J Gastrointest Surg 2020;24:1261-8. [Crossref] [PubMed]
- Kutup A, Nentwich MF, Bollschweiler E, et al. What should be the gold standard for the surgical component in the treatment of locally advanced esophageal cancer: transthoracic versus transhiatal esophagectomy. Ann Surg 2014;260:1016-22. [Crossref] [PubMed]
- Deng J, Su Q, Ren Z, et al. Comparison of short-term outcomes between minimally invasive McKeown and Ivor Lewis esophagectomy for esophageal or junctional cancer: a systematic review and meta-analysis. Onco Targets Ther 2018;11:6057-69. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Sabra MJ, Alwatari YA, Wolfe LG, et al. Ivor Lewis vs Mckeown esophagectomy: analysis of operative outcomes from the ACS NSQIP database. Gen Thorac Cardiovasc Surg 2020;68:370-9. [Crossref] [PubMed]
- Dantoc MM, Cox MR, Eslick GD. Does minimally invasive esophagectomy (MIE) provide for comparable oncologic outcomes to open techniques? A systematic review. J Gastrointest Surg 2012;16:486-94. [Crossref] [PubMed]
- Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med 2019;380:152-62. [Crossref] [PubMed]
- Gottlieb-Vedi E, Kauppila JH, Malietzis G, et al. Long-term Survival in Esophageal Cancer After Minimally Invasive Compared to Open Esophagectomy: A Systematic Review and Meta-analysis. Ann Surg 2019;270:1005-17. [Crossref] [PubMed]
- Nilsson M, Kamiya S, Lindblad M, et al. Implementation of minimally invasive esophagectomy in a tertiary referral center for esophageal cancer. J Thorac Dis 2017;9:S817-25. [Crossref] [PubMed]
- van Workum F, Stenstra MHBC, Berkelmans GHK, et al. Learning Curve and Associated Morbidity of Minimally Invasive Esophagectomy: A Retrospective Multicenter Study. Ann Surg 2019;269:88-94. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Mariette C, Markar S, Dabakuyo-Yonli TS, et al. Health-related Quality of Life Following Hybrid Minimally Invasive Versus Open Esophagectomy for Patients With Esophageal Cancer, Analysis of a Multicenter, Open-label, Randomized Phase III Controlled Trial: The MIRO Trial. Ann Surg 2020;271:1023-9. [Crossref] [PubMed]
- Brierley RC, Gaunt D, Metcalfe C, et al. Laparoscopically assisted versus open oesophagectomy for patients with oesophageal cancer-the Randomised Oesophagectomy: Minimally Invasive or Open (ROMIO) study: protocol for a randomised controlled trial (RCT). BMJ Open 2019;9:e030907 [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Armitage JN, van der Meulen JHRoyal College of Surgeons Co-morbidity Consensus Group. Identifying co-morbidity in surgical patients using administrative data with the Royal College of Surgeons Charlson Score. Br J Surg 2010;97:772-81. [Crossref] [PubMed]
- Rice TW, Gress DM, Patil DT, et al. Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:304-17.
- Helminen O, Mrena J, Sihvo E. Benchmark values for transthoracic esophagectomy are not set as the defined "best possible"-a validation study. J Thorac Dis 2018;10:4085-93. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Schmidt HM, Gisbertz SS, Moons J, et al. Defining Benchmarks for Transthoracic Esophagectomy: A Multicenter Analysis of Total Minimally Invasive Esophagectomy in Low Risk Patients. Ann Surg 2017;266:814-21. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Helminen O, Sihvo E, Gunn J, et al. Trends and results of oesophageal cancer surgery in Finland between 2004 and 2014. Eur J Cardiothorac Surg 2020;57:107-13. [Crossref] [PubMed]
- Schlottmann F, Strassle PD, Nayyar A, et al. Postoperative outcomes of esophagectomy for cancer in elderly patients. J Surg Res 2018;229:9-14. [Crossref] [PubMed]
- Ferguson MK, Martin TR, Reeder LB, et al. Mortality after esophagectomy: risk factor analysis. World J Surg 1997;21:599-603; discussion 603-4. [Crossref] [PubMed]
- Wright CD, Kucharczuk JC, O'Brien SM, et al. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. J Thorac Cardiovasc Surg 2009;137:587-95; discussion 596. [Crossref] [PubMed]
- Yamashita K, Watanabe M, Mine S, et al. The impact of the Charlson comorbidity index on the prognosis of esophageal cancer patients who underwent esophagectomy with curative intent. Surg Today 2018;48:632-9. [Crossref] [PubMed]
- Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v50-7. [Crossref] [PubMed]
- Vomackova K, Neoral C, Aujeský R, et al. The benefit of PET/CT in the diagnosis and treatment of esophageal cancer. Rozhl Chir 2015;94:8-16. [PubMed]
- Patel N, Foley KG, Powell AG, et al. Propensity score analysis of 18-FDG PET/CT-enhanced staging in patients undergoing surgery for esophageal cancer. Eur J Nucl Med Mol Imaging 2019;46:801-9. [Crossref] [PubMed]
- Noble F, Bailey DSWCIS Upper Gastrointestinal Tumour Panel, et al. Impact of integrated PET/CT in the staging of oesophageal cancer: a UK population-based cohort study. Clin Radiol 2009;64:699-705. [Crossref] [PubMed]
- Puli SR, Reddy JB, Bechtold ML, et al. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol 2008;14:1479-90. [Crossref] [PubMed]
- Urschel JD, Vasan H, Blewett CJ. A meta-analysis of randomized controlled trials that compared neoadjuvant chemotherapy and surgery to surgery alone for resectable esophageal cancer. Am J Surg 2002;183:274-9. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Hanna GB, Amygdalos I, Ni M, et al. Improving the standard of lymph node retrieval after gastric cancer surgery. Histopathology 2013;63:316-24. [Crossref] [PubMed]
- Noda N, Sasako M, Yamaguchi N, et al. Ignoring small lymph nodes can be a major cause of staging error in gastric cancer. Br J Surg 1998;85:831-4. [Crossref] [PubMed]
- Abbassi-Ghadi N, Boshier PR, Goldin R, et al. Techniques to increase lymph node harvest from gastrointestinal cancer specimens: a systematic review and meta-analysis. Histopathology 2012;61:531-42. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- van der Werf LR, Busweiler LAD, van Sandick JW, et al. Reporting National Outcomes After Esophagectomy and Gastrectomy According to the Esophageal Complications Consensus Group (ECCG). Ann Surg 2020;271:1095-101. [Crossref] [PubMed]
- Claassen L, van Workum F, Rosman C. Learning curve and postoperative outcomes of minimally invasive esophagectomy. J Thorac Dis 2019;11:S777-85. [Crossref] [PubMed]
- van Workum F, Berkelmans GH, Klarenbeek BR, et al. McKeown or Ivor Lewis totally minimally invasive esophagectomy for cancer of the esophagus and gastroesophageal junction: systematic review and meta-analysis. J Thorac Dis 2017;9:S826-33. [Crossref] [PubMed]
- Hulscher JBF, Van Lanschot JJB. Transthoracic versus transhiatal resection for carcinoma of the esophagus. Difficult Decis Thorac Surg An Evidence-Based Approach 2007;4975:208-17. [Crossref]
- Helminen O, Kytö V, Kauppila JH, et al. Population-based study of anastomotic stricture rates after minimally invasive and open oesophagectomy for cancer. BJS Open 2019;3:634-40. [Crossref] [PubMed]




