
Low CD4 T cell count predicts radiological progression in severe and critically ill COVID-19 patients: a case control study
Introduction
Coronavirus disease-19 (COVID-19) is the disease in humans caused by infection of the novel β coronavirus 2019-nCoV/SARS-CoV-2 (1). Which ranges from asymptomatic or mild illness to severe respiratory tract infections, such as those seen in severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) (2,3). Patients may progress rapidly, posing enormous burden on the public health and medical systems. As of May 24th, 2021, 166,860,081 cases have been confirmed globally and 3,459,996 deaths reported (4). Reported mortality ranges from 2% to 12% and could be as high as 52.4% in patients who develop ARDS (5).
The ability to predict disease aggravation is pivotal, especially when therapeutic options are limited. Like other viral pneumonia, computed tomographic (CT) is a main tool for assessing disease course and severity in COVID-19 (6-9). While prior studies have described clinical features and lung abnormalities in the course of COVID-19 (3,10-14), literature on factors predicting pulmonary progression remains scarce. In this study, we evaluated the potential clinical and radiological factors predicting radiological progression in severe and critically ill COVID-19 cases.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-20-1848).
Methods
Study design, participants, and data collection
In this case control study, we retrospectively collected consecutive severe and critically ill patients hospitalized for coronavirus pneumonia in the Renmin Hospital of Wuhan University (Wuhan, China) between Feb 6th 2020 and Feb 21st 2020. Those who hospitalized for less than 1 week or lacking follow-up CT were excluded. The hospital served as a designated center for management COVID-19 cases. The diagnosis and severity of COVID-19 were according to the Novel Coronavirus Pneumonia Diagnostic and Treatment Guideline issued by Chinese National Health Commission (version 7) (see Table S1) (15). Patients were categorized into two groups: (II) progression group (increased area of lung change in follow up chest CT); (II) non-progression group (improvement or no obvious change in follow up CT). Electronic medical and nursing records were reviewed for extracting data. Data of patients with complete demographic, clinical, laboratory, and radiological data were collected using standardized form. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Renmin Hospital’s institutional ethics board (No. WDRY2020-K048) and individual consent for this retrospective analysis was waived.
Laboratory and imaging methods
SARS-CoV-2 infections were confirmed using real-time reverse transcriptase-polymerase chain reaction assays (RT-PCR) via throat swab or sputum sample. Complete blood count, coagulation profile, biochemical parameters, myocardial enzymes, CD4 and CD8 T cell counts, C-reactive protein, and procalcitonin were collected routinely during hospitalization. Initial and follow up chest CT scan were done for patients upon admission and about 5
Statistical analysis
Continuous data are expressed as mean ± SD or median (25th
Results
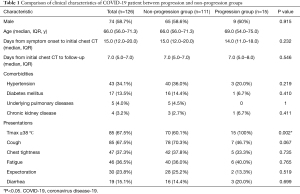
During the study period, a total of 162 severe or critically ill patients with COVID-19 were identified. 36 were excluded due to hospitalization for less than 1 week (n=7) or lacking follow-up CT (n=29) (see Figure 1). Of the enrolled 126 patients, 97 (77.0%) and 29 (23.0%) cases were severe and critically ill upon admission, respectively. Median time from disease onset to initial chest CT scan was 15.0 (IQR, 12.0–20.0) days. Median interval to follow-up CT was 7.0 (IQR, 5.0–7.0) days. On reexamination, 15 (11.9%) cases presented with progression (progression group) and 111 (88.1%) cases had no progression (non-progression group) in CT images. Nine of the severe cases and six of the critically ill cases had CT progression. Percentage of CT progression did not significantly differ between severe and critically ill patients (9.3% vs. 20.7%, P=0.096).
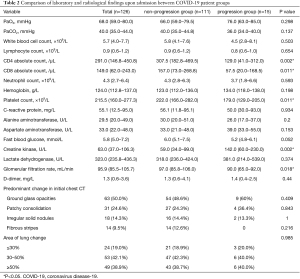
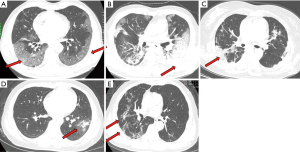
Baseline clinical characteristics of all cases and patients in each group are summarized in Table 1. Seventy (55.6%) patients had at least one underlying disease. The most common were hypertension (34.1%) and diabetes mellitus (13.5%). The most common symptoms at onset were peak body temperature >38 °C (67.5%), cough (67.5%), chest tightness (37.3%), and fatigue (36.5%). Comparison of laboratory examinations and chest CT features between progression and non-progression group are summarized in Table 2. Lung abnormalities of COVID-19 in HRCT are presented in Figure 2. The most common manifestation on HRCT was ground glass opacity (50.0%), followed by patchy consolidation (24.6%), irregular solid nodules (14.3%), and fibrous stripes (9.5%). Majority of the patients had abnormalities over 30% of the lung area. Pulmonary change of 30
Full table
Full table
Compared with non-progression group, patients who progressed were more likely to have peak body temperature >38 °C (100% vs. 60.1%, P=0.002), less likely to present with cough (46.7% vs. 70.3%, P=0.067), had lower platelet count [179.0 (129.0
The following categorical variable were entered in logistic regression analysis (see Table 3): symptom of cough, fever ≥38.0 °C, platelet count <125×109/L, absolute CD4 T cell count <200/µL, absolute CD8 cell count <220/µL, GFR <90 mL/min, CK >198 U/L, and blood glucose ≥7.0 mmol/L. On both univariable and multivariable analysis, absolute CD4 T cell count <200/µL was the only factor significantly associated with progression in lung involvement (P=0.004 and P=0.015, respectively).

Full table
Discussion
In this study, we described the clinical and radiological features of severe and critically ill COVID-19 cases, and demonstrated that CD4 T cell count <200/µL was significantly related to the progression of chest CT abnormalities in those patient groups.
It is worth noting that lung changes are essentially affected by disease course. Peak stage of lung involvement is approximately 10 days (9
Although not statistically significant, we found that ground glass opacities and patchy consolidation was more frequently seen in the progression group. In addition, no patients with the predominant pattern of fibrous stripes aggravated. The latter finding is consistent with the fact that in COVID-19, like other viral pneumonia, fibrotic changes are seen during remission stage and may remain after recovery (6,16,17).
Major gap remains in the understanding of pathogenicity of novel coronavirus disease. However, a number of similarities exists between SARS-CoV, SARS-CoV-2, and other members of human coronaviruses. In all cases, the immune system plays crucial role in pathologies (18). Prior studies in infections caused by MERS, SARS, and SARS-CoV-2 demonstrated that marked lymphopenia including a dramatic loss of CD4 T cell were observed in severe cases and correlated with disease severity (19-23). Severe SARS patients have a delayed development of adaptive immune response, which is the main reason for SARS patients to develop life-threatening illness compared with other mild HCoVs (24,25). The decrease in CD4 T cell may be a result of T-cell infection, impaired T cell functions, and apoptosis caused by coronaviruses (26). Conversely, those with low level of T cells show weakened responses to viruses, which may in turn lead to pneumonia progression (27,28).
Our finding that CD4 T cell count <200/μL predicts pulmonary progression is consistent with prior research findings in HCoVs, and supports the speculation that immune deficiency is associated with disease deterioration. It highlights the importance of evaluating cellular immunity in the management of COVID-19 patients, as well as searching for new therapeutic options using immunoenhancers. Currently, several drugs including remdesivir, hydroxychloroquine, and chloroquine are administered in multiple countries, or under clinical trials. Nonetheless, no specific immuneoenhancer has been recommended. The effectiveness of those used extensively during SARS and MERS outbreak, including interferons, intravenous gammaglobulin (IVIG), and thymosin is yet to be proven in COVID-19.
This study has a few limitations. First, this was a retrospective study done in a single center, which served as a center for severe and critically ill patients. Whether the same finding applies in mild to moderate cases is worth looking into. Second, other factors that may influence the extent of pulmonary involvement, such as medication upon admission or bacterial co-infections, were not included in the analysis. Well-designed prospective studies will help to elucidate the correlation between other possible parameters and radiological changes in the future. Third, clinical parameters such as symptoms and time to hospital discharge were not recorded during follow-up. Further research about the relationship between chest CT progression and clinical status is needed.
In conclusion, our study demonstrated that absolute CD4 T cell count <200 μL/L predicts pulmonary progression in severe and critically ill COVID-19 patients, highlighting the importance of evaluating cellular immunity and close monitoring of those with low CD4 T cell counts.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-20-1848
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jtd-20-1848
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1848). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Renmin Hospital’s institutional ethics board (No. WDRY2020-K048) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270-3. [Crossref] [PubMed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. [Crossref] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- WHO. Weekly Operational Update on COVID-19 2021. Available online: https://www.who.int/publications/m/item/weekly-operational-update-on-covid-19 [24-may-2021]
- Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934-43. [Crossref] [PubMed]
- Koo HJ, Lim S, Choe J, et al. Radiographic and CT Features of Viral Pneumonia. Radiographics 2018;38:719-39. [Crossref] [PubMed]
- Li K, Wu J, Wu F, et al. The Clinical and Chest CT Features Associated With Severe and Critical COVID-19 Pneumonia. Invest Radiol 2020;55:327-31. [Crossref] [PubMed]
- Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 2020;30:3306-9. [Crossref] [PubMed]
- Han X, Cao Y, Jiang N, et al. Novel Coronavirus Disease 2019 (COVID-19) Pneumonia Progression Course in 17 Discharged Patients: Comparison of Clinical and Thin-Section Computed Tomography Features During Recovery. Clin Infect Dis 2020;71:723-31. [Crossref] [PubMed]
- Huang Y, Cheng W, Zhao N, et al. CT screening for early diagnosis of SARS-CoV-2 infection. Lancet Infect Dis 2020;20:1010-1. [Crossref] [PubMed]
- Dai WC, Zhang HW, Yu J, et al. CT Imaging and Differential Diagnosis of COVID-19. Can Assoc Radiol J 2020;71:195-200. [Crossref] [PubMed]
- Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20:425-34. [Crossref] [PubMed]
- Long C, Xu H, Shen Q, et al. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol 2020;126:108961 [Crossref] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [Crossref] [PubMed]
- National Health Commission. Novel Coronavirus Pneumonia Diagnosis and Treatment Guideline (7th trial version). [cited 2020 May 5]. Available online: www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf
- Pan F, Ye T, Sun P, et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020;295:715-21. [Crossref] [PubMed]
- Shaw B, Daskareh M, Gholamrezanezhad A. The lingering manifestations of COVID-19 during and after convalescence: update on long-term pulmonary consequences of coronavirus disease 2019 (COVID-19). Radiol Med 2021;126:40-6. [Crossref] [PubMed]
- Fung TS, Liu DX. Human Coronavirus: Host-Pathogen Interaction. Annu Rev Microbiol 2019;73:529-57. [Crossref] [PubMed]
- Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 2013;13:752-61. [Crossref] [PubMed]
- Li T, Qiu Z, Han Y, et al. Rapid loss of both CD4+ and CD8+ T lymphocyte subsets during the acute phase of severe acute respiratory syndrome. Chin Med J (Engl) 2003;116:985-7. [PubMed]
- Li T, Qiu Z, Zhang L, et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis 2004;189:648-51. [Crossref] [PubMed]
- Calvet J, Gratacós J, Amengual MJ, et al. CD4 and CD8 Lymphocyte Counts as Surrogate Early Markers for Progression in SARS-CoV-2 Pneumonia: A Prospective Study. Viruses 2020;12:1277. [Crossref] [PubMed]
- Zheng HY, Zhang M, Yang CX, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol 2020;17:541-3. [Crossref] [PubMed]
- Cameron MJ, Bermejo-Martin JF, Danesh A, et al. Human immunopathogenesis of severe acute respiratory syndrome (SARS). Virus Res 2008;133:13-9. [Crossref] [PubMed]
- Channappanavar R, Zhao J, Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res 2014;59:118-28. [Crossref] [PubMed]
- de Wit E, van Doremalen N, Falzarano D, et al. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 2016;14:523-34. [Crossref] [PubMed]
- Chu H, Zhou J, Wong BH, et al. Middle East Respiratory Syndrome Coronavirus Efficiently Infects Human Primary T Lymphocytes and Activates the Extrinsic and Intrinsic Apoptosis Pathways. J Infect Dis 2016;213:904-14. [Crossref] [PubMed]
- Zhou X, Jiang W, Liu Z, et al. Virus Infection and Death Receptor-Mediated Apoptosis. Viruses 2017;9:316. [Crossref] [PubMed]



