Red blood cell distribution width and cardiovascular diseases
Introduction
The red blood cells (RBCs), also known as erythrocytes, are non-nucleated blood elements exhibiting a typical oval biconcave shape. Although the normal size of RBCs is usually comprised between 80 and 100 fL, a number of physiological (e.g., pregnancy, ageing or physical exercise) and pathological (e.g., iron deficiency anemia, hemolytic anemia, hereditary spherocytosis, congenital hemoglobin disorders such as thalassemia or hemoglobin variants) conditions may impair erythropoiesis and hence promote a higher degree of heterogeneity of RBC volumes (1). This process is characterized by the appearance of smaller (i.e., <60 fL) and larger (up to 120 fL) elements, which is conventionally known as anisocytosis (Figure 1) (1,2). The red blood cell distribution width (RDW) is a rather simple measure of RBC size heterogeneity, which is calculated by dividing the standard deviation (SD) of erythrocyte volumes for the mean corpuscular volume (MCV) (i.e., RDW = SD/MCV). Although result can hence be expressed either in absolute values (i.e., RDW-SD) or as a percentage (i.e., RDW-%), the latter approach is more widely used in routine laboratory practice. Since the RDW is not a direct measure of anisocytosis, but can be easily and inexpensively calculated from the MCV, the vast majority of hematological analyzers automatically provide the RDW value within the complete blood cell count (CBC). It is noteworthy, however, that the different approaches used for measuring the erythrocyte size (i.e., impedance or optical techniques), as well as the size limits and the relative height of the RBC histogram used for the calculation differ widely across commercial hematological analyzers, so that the clinical usefulness of this parameter is still plagued by a poor degree of harmonization among different manufacturers (3). Accordingly, no universal reference range can be used for this measure, since values are typically instrument-dependent and the physiologic range of values may vary between a minimum of 11% and a maximum of 15%, respectively.
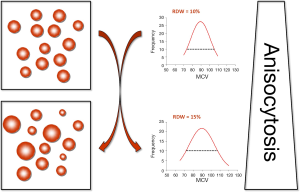
Beside RBC disorders, many acute and chronic cardiovascular diseases including acute coronary syndrome (ACS), ischemic cerebrovascular disease, peripheral artery disease (PAD), heart failure (HF), atrial fibrillation (AF) and hypertension are often associated with a high degree of anisocytosis (4). Earlier studies in the general population pinpointed the existence of an intriguing association between RDW and stroke or carotid atherosclerosis (5,6). Wen et al. (7) observed a close relationship between high RDW and ultrasound detection of advanced subclinical atherosclerosis, such as an increase of intimal-medial thickness (IMT) and the evidence of carotid plaques. Sánchez-Chaparro et al. (8) studied 217,567 Spanish working people undergoing a routine medical checkup, and reported that a high RDW is associated with metabolic syndrome (MetS), a well-known condition encompassing multiple risk factors for cardiovascular diseases.
The persistent RDW increase in cardiovascular diseases has been attributed to the effective stimulation of erythropoiesis by erythropoietin (EPO), a hormone secreted during hypoxic events, which promotes the release of enlarged RBCs from bone marrow (9). Another hypothesis is that elevated RDW may be due to a slight reduction of RBC turnover (10). More specifically, since the size of RBCs gradually reduces with ageing of the cells, a decreased rate of RBC turnover would allow smaller cells to persist for longer into the circulating (10). The growing interest in RDW, as reflected by the increasing number of scientific articles published in this topic over the past decade (2), prompted many scientists to speculate that the chronic inflammatory state which often accompanies acute and chronic cardiovascular diseases may be another powerful erythropoiesis modulator (11). In line with this hypothesis, a number of proinflammatory cytokines are effective to inhibit EPO secretion and RBC maturation, thus enhancing anisocytosis (12,13).
Besides the unquestionable clinical value in the differential diagnosis of anemias, interesting evidence recently emerged that the RDW may provide valuable information for diagnosing a variety of disorders and for establishing the short- and long-term prognosis in patients with these conditions (2,14,15). Therefore, the aim of article is to provide an overview of the current scientific literature about the epidemiologic association, the putative role and the clinical usefulness of measuring RDW in patients with cardiovascular diseases.
RDW in ACSs
One of the first study investigating the potential role of RDW for the diagnosis of ACS was published by our group, in the 2009. In brief, RDW was measured in 2,304 patients consecutively admitted to the emergency department with chest pain (14). Overall, 456 patients were finally diagnosed as having ACS, and their median RDW value was found to be significantly higher than that of non-ACS patients (15.1% vs. 13.5%; P<0.001). The area under the curve (AUC) of RDW for predicting ACS was 0.70 [95% confidence interval (CI), 0.69-0.72]. An increased RDW was hence significantly associated with the presence of ACS, exhibiting an odds ratio (OR) of 3.60 (95% CI, 2.87-4.51). In an following study including 1,971 patients admitted to the emergency department for chest pain, Cemin et al. also found that RDW was a significant predictor of acute myocardial infarction (AMI), exhibiting an AUC of 0.61 (95% CI, 0.54-0.68). The sensitivity and specificity of RDW at the 13.7% cut-off value were 0.75 and 0.52, respectively (16). Zalawadiya et al. (17) studied 7,556 participants of the National Health and Nutrition Examination Surveys 1999-2006, and observed that an increased RDW value was a powerful and independent predictor of future risk of coronary heart disease (CHD) (OR for each unit increase in RDW, 1.35; 95% CI, 1.27-1.45). A linear association between RDW and risk of AMI was recently described in 25,612 participants of the Tromsø Study in 1994-1995 (18), wherein each 1% increment in RDW was associated with a 13% increased risk [hazard ratio (HR), 1.13; 95% CI, 1.07-1.19]. Participants with RDW values above the 95th percentile had 71% higher risk of AMI compared to those with RDW in the lowest quintile (HR, 1.71; 95% CI, 1.34-2.20).
A high RDW value was found to be a significant predictor of adverse outcomes in patients with ACS, and especially of both in-hospital and long-term cardiovascular mortality. Uyarel et al. (19) studied 2,506 patients undergoing primary percutaneous coronary intervention (PCI) for ST-segment elevation myocardial infarction (STEMI), and showed that patients with elevated RDW (i.e., 16.1%) at admission had higher in-hospital mortality rate compared to those with normal RDW (7.6% vs. 3.6%; P<0.001). Gul et al. performed a prospective study including 310 patients, and reported that the RDW at admission was a significant predictor of adverse clinical outcomes in patients with non-ST elevation myocardial infarction (NSTEMI) and unstable angina pectoris (UAP) (20). In particular, the 3-year mortality rate was significantly higher in the high RDW group comparted to the low RDW group (19% vs. 6%). A significant association was also found between high admission RDW and adjusted risk of cardiovascular mortality (HR, 3.2; 95% CI, 1.3-7.78). Bekler et al. studied 251 patients diagnosed with UAP and NSTEMI (21), and showed that an elevated RDW value was associated with the number of coronary arteries narrowed (r=0.190; P=0.002) and with fragmented QRS (r=0.238; P=0.003), a well-known predictor of cardiac events and all-cause mortality. Interestingly, it was recently demonstrated that anisocytosis correlates with the severity of atherosclerosis (22), and is hence associated with the complexity of coronary artery disease (CAD). Interestingly, Isik et al. (23) showed that patients with angiographic CAD had significantly higher RDW values compared with those without CAD (14.4% vs. 12.5%; P<0.001). In multivariate analysis, RDW was found to be an independent predictor of both angiographic CAD (OR, 4.80; 95% CI, 2.41-9.57) and of high (i.e., >32) SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) score (OR, 2.28; 95% CI, 1.45-3.60). In another recent study, the RDW value was found to be associated with severity of cardiac ischemia, being higher in patients with NSTEMI compared to those with UAP (14.6% vs. 13.1%; P=0.006) (24). In NSTEMI patients, an increased RDW value (i.e., >14.9%) was associated with higher risk of bleeding (HR, 2.67; 95% CI, 1.17-6.10) (25).
A substantial association between RDW and prognosis of AMI patients was also observed by Ren et al. in 1,442 Chinese patients with stable angina pectoris (26). In particular, higher RDW values on admission were associated with an increased 1-year cardiac mortality (quartile 1: 0.51%; quartile 2: 0.56%; quartile 3: 0.86%; quartile 4: 2.27%; P<0.001) and 1-year ACS (quartile 1: 1.55%; quartile 2: 1.96%; quartile 3: 2.89%; quartile 4: 3.70%; P<0.001). In logistic regression analysis, RDW independently predicted cardiac mortality (OR, 1.54; 95% CI, 1.06-3.22) and ACS (OR, 1.86; 95% CI, 1.23-3.49) during a 1-year follow-up. More recently, Timóteo et al. studied 787 consecutive patients with ACS (27), and reported that an increased RDW value (i.e., >13.9%) was a significant predictor of death (HR, 1.17; 95% CI, 1.03-1.32).
RDW in ischemic cerebrovascular disease
There is now consolidated evidence that the RBC volume is higher in patients with stroke compared to those without, and is also a significant and independent predictor of both cardiovascular and all-cause mortality in these patients (28). Interestingly, RDW appears to be associated with the risk of stroke not only in patients with HF (29,30), AF (31) and CAD (32), but also in the general population.
Söderholm et al. studied 26,879 participants of the population-based Malmö Diet and Cancer Study (16,561 women and 10,318 men aged 45-73 years) without a history of coronary events or stroke (5), and observed that subjects in the highest quartile of RDW exhibited a significantly increased incidence of stroke (HR, 1.31; 95% CI, 1.11-1.54) and cerebral infarction (HR, 1.32; 95% CI, 1.10-1.58). Vayá et al. measured RDW in 163 patients admitted for cryptogenic stroke and in 186 matched healthy controls (33), and showed that a RDW value >14% was independently associated with the risk of cryptogenic stroke (OR, 2.54; 95% CI, 1.30-4.96). In a case-control study including 224 consecutive patients with first-ever ischemic stroke, Ramírez-Moreno and colleagues (34) reported that patients in the highest quartile of RDW (i.e., >14.6%) had a significantly higher risk of stroke compared to those in the lowest quartile (OR, 4.50; 95% CI, 2.50-8.01).
In a subsequent study, Jia et al. measured RDW in a population of 392 patients with primary ischemic stroke (35), and found that RDW was significantly associated with carotid IMT (r=0.436). Even more importantly, RDW was found to be an independent predictor of carotid artery atherosclerosis (OR of the highest vs. the lowest quartile, 3.10; 95% CI, 2.46-7.65).
Kim et al. measured RDW at emergency department admission in 847 consecutive patients with a first-ever episode of acute cerebral infarction (36), and found that this parameter was independently associated with poor functional outcome (OR, 1.22 per 1% RDW increment; 95% CI, 1.06-1.41) and all-cause death (OR, 1.39 per 1% RDW increment; 95% CI, 1.17-1.66) 3 months after stroke. More recently, Kara et al. performed a prospective cohort study including 88 patients with acute ischemic stroke (37), and reported that RDW values were higher in patients with worse Glasgow Coma Scale (GCS), Canadian Neurological Scale (CNS) and National Institutes of Health Stroke Scale (NIHSS) scores.
RDW in PAD
Several lines of evidence seemingly attest that the erythrocyte volume is modestly increased in patients with PAD, so that an elevated MCV has been considered a potential risk factor for this condition (38-40). As regards the RDW, Zalawadiya et al. studied 6,950 participants of the National Health and Nutrition Examination Survey (41), and observed an increase of PAD prevalence in parallel with increasing RDW values (4.2% in quartile 1 vs. 13.9% in quartile 4; P<0.001). The addition of RDW to the American College of Cardiology/American Heart Association-defined PAD screening criteria led to a substantial increase of diagnostic performance (i.e., AUC from 0.657 to 0.727; P<0.0001).
Demirtas et al. prospectively followed 82 consecutive patients with PAD and established disease severity according to the Fontaine classification system (42). Interestingly, the mean RDW value gradually increased across Fontaine stages, from 13.6% in patients in stage I, to 14.8% in those in stage II, up to 15.4% in patients in stage III. Another epidemiological investigation assessed the prognostic utility of RDW. In brief, Ye et al. (43) studied 13,039 consecutive outpatients with PAD and reported that those in the highest quartile of RDW (>14.5%) had a 66% greater risk of mortality compared to those in the lowest quartile (RDW <12.8%; P<0.0001). A 1% increment RDW was associated with a 10% greater risk of all-cause mortality (HR: 1.10; 95% CI, 1.08-1.12).
RDW in AF
Likewise patients with PAD, the presence of larger erythrocytes is commonplace in patients with AF (44-46). Although the etiology of the non-anemic and non-alcoholic macrocytosis remains largely unclear, it has been speculated that oxidative stress and chronic inflammation may contribute to reduce RBC survival, thus causing the appearance of a mixed population (small and large erythrocytes, corresponding to old and young elements, respectively) in the circulation (47-49).
Adamsson Eryd et al. (50) carried out a prospective study including 27,124 subjects (age 45-73 years, 62% women) with no history of AF, HF, MI or stroke, who were followed up for a mean period of 13.6 years. The incidence of AF gradually increase across quartiles of RDW, with subjects in the highest quartile exhibiting a HR of 1.33 (95% CI, 1.16-1.53) compared to those in the lowest quartile. The risk remained essentially unchanged after adjustment for potential confounding factors, and even after exclusion of patients with MI or HF (HR 1.30; 95% CI, 1.13-1.51). This association was then confirmed in a case-control study including 117 patients with non-valvular AF (103 paroxysmal and 14 chronic AF) and 60 healthy matched controls (51). More specifically, RDW levels were significantly higher in AF patients than in the control group (13.4% vs. 12.6%; P=0.01). In multivariate logistic regression analysis, RDW was found to be an independent predictor of non-valvular AF (OR 4.18; 95% CI, 2.15-8.15). Liu et al. also studied 133 consecutive patients with paroxysmal non-valvular AF and 101 patients without AF matched for sex, age and atherosclerotic risk factors (52), and reported that RDW was significantly higher in the AF group than that in controls (12.7% vs. 12.4%; P<0.05). In multivariate logistic regression analysis, the RDW value remained significantly and independently associated with paroxysmal AF (OR, 1.63; 95% CI, 1.01-2.61). Interestingly, a RDW value >12.5% exhibited 0.48 sensitivity and 0.67 specificity for the presence of paroxysmal AF.
The predictive value of RDW was then confirmed in patients with post-operative AF. Ertaş et al. retrospectively analyzed data of 132 patients with post-operative AF undergoing nonemergency coronary artery bypass grafting (CABG) (53), and showed that preoperative RDW values were significantly higher in patients who developed AF than in those who did not (13.9% vs. 13.3%; P=0.03). A preoperative RDW value >13.4% predicted the onset of AF with 0.61 sensitivity and 0.60 specificity. In another investigation, Korantzopoulos et al. studied 109 patients undergoing elective cardiac surgery (on-pump coronary artery bypass and/or valve surgery) (54), and showed that RDW was the only independent predictor of AF in multivariate logistic regression analysis (OR, 1.46; 95% CI, 1.08-1.99). A RDW value >13.3% predicted AF with 0.80 sensitivity and 0.60 specificity, respectively. Kurt et al. performed a nested case-control study including 320 non-anemic patients with AF (55), and found that RDW values were significantly associated with higher CHA2DS2-VASc score (AUC, 0.65; 95% CI, 0.59-0.71). A prospective study including 300 consecutive patients with AF followed up for a median up period of 3.2 years was carried out by Wan et al. (56). Compared to patients in the lowest quartile of RDW, those in the highest quartile exhibited a significantly higher risk of both all-cause mortality (HR, 1.02; 95% CI, 1.01-1.04) and major adverse events (MAEs) (HR, 1.01; 95% CI, 1.002-1.023). These results were confirmed by Gurses et al. who followed 299 patients with symptomatic paroxysmal or persistent AF for a median period of 24 months (57), and found that the RDW value was an independent predictor of late AF recurrence in multivariate Cox proportional hazard regression analysis (HR 1.88; 95% CI, 1.41-2.50).
RDW in HF
RDW is now regarded as an emerging marker for predicting the onset and evolution of HF (58). In patients with HF, the presence of anisocytosis may be interpreted as a homeostatic response to the disease, thus reflecting the existence of a potential link between ineffective erythropoiesis and chronic inflammation (11).
In 2011 Borné et al. published the results of the large Malmö Diet and Cancer population-study, including 26,784 subjects (aged 45-73 years, 61% women) without history of MI, stroke or HF, who were followed up for a mean period of 15 years (59). During follow-up, patients in the highest quartile of RDW exhibited a significantly increased risk of developing HF compared to those in the lowest quartile (HR, 1.47; 95% CI, 1.14-1.89). Interestingly, the strength of this association substantially decreased after adjustment for values of N-terminal pro-B-type natriuretic peptide, cystatin C and high-sensitive C-reactive protein (HR, 1.64; 95% CI, 0.90-3.00), thus suggesting that these biomarkers may have contributed to the observed relationship between RDW and incident HF. In the following European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort study including 17,533 participants followed up for a mean period of 11 years (60), Emans et al. observed a non-linear increase of HF risk across quartiles of RDW. In particular, subjects in the highest quartile of RDW exhibited a 44% higher risk of incident HF compared to those in the lowest quartile (HR, 1.44; 95% CI, 1.15-1.80). At variance with the previous study, this association remained significant after adjustment for established risk factors including C-reactive protein, creatinine, iron and ferritin (HR, 1.40; 95% CI, 1.11-1.77).
As regards the prognostic value of RDW in patients with HF, Oh et al. studied 100 patients with acute HF (61), and observed that increased RDW values significantly predicted the presence of elevated left ventricular filling pressure (AUC, 0.633; P<0.05). Nishizaki et al. retrospectively analyzed the medical records of 160 patients aged 80 years or older who died in the Department of Cardiology in Juntendo Tokyo Koto Geriatric Medical Center (62). HF was found to be the leading cause of death in this patients, and a RDW value ≥16.5% was found to be significantly associated with fatal HF (OR 2.36; 95% CI, 1.10-5.04).
Additional studies showed that RDW may provide useful clinical information in patients with HF (63-67). Interestingly, the degree of anisocytosis provides prognostic information which not only seems independent from the hemoglobin value (68), but that is even more accurate than that of the hemoglobin concentration (69). In line with previous findings, Pascual-Figal et al. measured RDW at the time of hospital discharge in 268 consecutive non-anemic patients with acutely decompensated HF (70), and reported that patients with RDW >15% had a 3-fold higher risk of developing new-onset anemia during a 6-month follow-up period (OR, 3.1; 95% CI, 1.5-5.1). In a following analysis based on the same study population (71), a change of RDW values during hospitalization predicted both all-cause deaths (HR, 1.19; 95% CI, 1.01-1.41) and cardiac-based mortality (HR, 1.31; 95% CI, 1.02-1.68) after adjustment for additional risk factors.
Recently, Huang et al. performed a meta-analysis of 17 studies totaling 18,288 patients with HF (65), and concluded that RDW on admission and discharge, as well as its variation during treatment, are prognostic markers in HF patients. In particular, each 1% increase in baseline RDW was associated with a 10% enhanced risk of all-cause mortality (OR, 1.10; 95% CI, 1.07-1.13).
RDW in essential hypertension
It has been recently demonstrated that the value of RDW is increased in hypertensive patients (72), especially in those with non-dipper hypertension (73). It is hence not surprising that anisocytosis, mainly resulting from ongoing vascular inflammation, is correlated with complications of essential hypertension, especially with abnormal left ventricle (LV) geometric pattern (74), pathological changes of selected target organs (75), and early-stage renal function (76). It has also been suggested that the placental hypoxia during preeclampsia may stimulate erythropoiesis, thus leading to increased RDW values in preeclamptic women (77). Due to these important findings, Fici et al. recently suggested that RDW may be used as a reliable biomarker for evaluating the efficacy of anti-hypertensive drugs (78).
Conclusions
There is now reliable evidence, convincingly supported by a large number of epidemiological studies, that anisocytosis is frequent in patients with a number of cardiovascular diseases. It seems also apparent that an increased RDW value may significantly (and independently) predict many unfavorable clinical outcomes including recurrence of vascular ischemia, mortality and MAEs. At variance with other conventional biomarkers used for diagnosis and prognostication of patients with cardiovascular diseases, RDW carries some clinical and practical advantages (79). In particular, although the presence of anisocytosis is not specific for (or predictive of) any particular pathological condition, an increased RDW value significantly and independently reflects a worse prognosis in patients with cardiovascular disorders, as well as in the general population. Therefore, the degree of anisocytosis would provide valuable information for the clinical and therapeutic management, since it seems reasonable to suggest that a more timely and aggressive treatment should be established in patients with increased RDW. Preliminary evidence then suggests that this parameter may be used as a surrogate marker of, or even a treatment target for, patients with dyslipidemia (80). Another substantial advantage of RDW emerges from the fact that its value is automatically generated by the modern generation of hematological analyzers. RDW assessment is rapid, easy, inexpensive, does not require specific skills or instrumentation, and is hence measurable in virtually all clinical laboratories performing routine or urgent testing. The current lack of standardization represents the major drawback, wherein RDW values generated by different hemocytometers should not be used for longitudinal monitoring of patients’ data (3).
Nevertheless, an essential and yet unresolved question remains, that is whether the association between RDW and cardiovascular diseases is causal, or rather anisocytosis may occur as a consequence of underlying metabolic derangements that are commonplace in patients with cardiovascular diseases. Indeed, erythropoiesis is substantially influenced by the concentration of many inflammatory cytokines, oxidative stress, poor nutritional status, dyslipidemia and increased RBC turnover. Most of these conditions are frequently encountered in patients with cardiovascular diseases, and it seems hence reasonable to hypothesize that RDW may be an epiphenomenon rather than an effective player in the pathogenesis of cardiovascular diseases. Regardless of its putative role, however, the considerable evidence available so far suggests that the clinical role of RDW may be broadened beyond the conventional boundaries of erythrocyte disorders, especially for supporting the diagnosis and prognostication of patients with cardiovascular diseases such ACS, ischemic cerebrovascular disease, PAD, HF and AF.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lippi G, Plebani M. Red blood cell distribution width (RDW) and human pathology. One size fits all. Clin Chem Lab Med 2014;52:1247-9. [PubMed]
- Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86-105. [PubMed]
- Lippi G, Pavesi F, Bardi M, et al. Lack of harmonization of red blood cell distribution width (RDW). Evaluation of four hematological analyzers. Clin Biochem 2014;47:1100-3. [PubMed]
- Montagnana M, Cervellin G, Meschi T, et al. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med 2011;50:635-41. [PubMed]
- Söderholm M, Borné Y, Hedblad B, et al. Red cell distribution width in relation to incidence of stroke and carotid atherosclerosis: a population-based cohort study. PLoS One 2015;10:e0124957. [PubMed]
- Lappegård J, Ellingsen TS, Vik A, et al. Red cell distribution width and carotid atherosclerosis progression. The Tromsø Study. Thromb Haemost 2015;113:649-54. [PubMed]
- Wen Y. High red blood cell distribution width is closely associated with risk of carotid artery atherosclerosis in patients with hypertension. Exp Clin Cardiol 2010;15:37-40. [PubMed]
- Sánchez-Chaparro MA, Calvo-Bonacho E, González-Quintela A, et al. Higher red blood cell distribution width is associated with the metabolic syndrome: results of the Ibermutuamur CArdiovascular RIsk assessment study. Diabetes Care 2010;33:e40. [PubMed]
- Yčas JW, Horrow JC, Horne BD. Persistent increase in red cell size distribution width after acute diseases: A biomarker of hypoxemia? Clin Chim Acta 2015;448:107-17. [PubMed]
- Patel HH, Patel HR, Higgins JM. Modulation of red blood cell population dynamics is a fundamental homeostatic response to disease. Am J Hematol 2015;90:422-8. [PubMed]
- Inuzuka R, Abe J. Red blood cell distribution width as a link between ineffective erythropoiesis and chronic inflammation in heart failure. Circ J 2015;79:974-5. [PubMed]
- Macdougall IC, Cooper A. The inflammatory response and epoetin sensitivity. Nephrol Dial Transplant 2002;17 Suppl 1:48-52. [PubMed]
- Lippi G, Targher G, Montagnana M, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med 2009;133:628-32. [PubMed]
- Lippi G, Filippozzi L, Montagnana M, et al. Clinical usefulness of measuring red blood cell distribution width on admission in patients with acute coronary syndromes. Clin Chem Lab Med 2009;47:353-7. [PubMed]
- Lippi G, Targher G, Montagnana M, et al. Relationship between red blood cell distribution width and kidney function tests in a large cohort of unselected outpatients. Scand J Clin Lab Invest 2008;68:745-8. [PubMed]
- Cemin R, Donazzan L, Lippi G, et al. Blood cells characteristics as determinants of acute myocardial infarction. Clin Chem Lab Med 2011;49:1231-6. [PubMed]
- Zalawadiya SK, Veeranna V, Niraj A, et al. Red cell distribution width and risk of coronary heart disease events. Am J Cardiol 2010;106:988-93. [PubMed]
- Skjelbakken T, Lappegård J, Ellingsen TS, et al. Red cell distribution width is associated with incident myocardial infarction in a general population: the Tromsø Study. J Am Heart Assoc 2014;3:e001109. [PubMed]
- Uyarel H, Ergelen M, Cicek G, et al. Red cell distribution width as a novel prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis 2011;22:138-44. [PubMed]
- Gul M, Uyarel H, Ergelen M, et al. The relationship between red blood cell distribution width and the clinical outcomes in non-ST elevation myocardial infarction and unstable angina pectoris: a 3-year follow-up. Coron Artery Dis 2012;23:330-6. [PubMed]
- Bekler A, Gazi E, Tenekecioglu E, et al. Assessment of the relationship between red cell distribution width and fragmented QRS in patients with non-ST elevated acute coronary syndrome. Med Sci Monit 2014;20:413-9. [PubMed]
- Sahin O, Akpek M, Sarli B, et al. Association of red blood cell distribution width levels with severity of coronary artery disease in patients with non-ST elevation myocardial infarction. Med Princ Pract 2015;24:178-83. [PubMed]
- Isik T, Uyarel H, Tanboga IH, et al. Relation of red cell distribution width with the presence, severity, and complexity of coronary artery disease. Coron Artery Dis 2012;23:51-6. [PubMed]
- Tenekecioglu E, Yilmaz M, Yontar OC, et al. Red blood cell distribution width is associated with myocardial injury in non-ST-elevation acute coronary syndrome. Clinics (Sao Paulo) 2015;70:18-23. [PubMed]
- Sánchez-Martínez M, López-Cuenca A, Marín F, et al. Red cell distribution width and additive risk prediction for major bleeding in non-ST-segment elevation acute coronary syndrome. Rev Esp Cardiol (Engl Ed) 2014;67:830-6. [PubMed]
- Ren H, Hua Q, Quan M, et al. Relationship between the red cell distribution width and the one-year outcomes in Chinese patients with stable angina pectoris. Intern Med 2013;52:1769-74. [PubMed]
- Timóteo AT, Papoila AL, Lousinha A, et al. Predictive impact on medium-term mortality of hematological parameters in Acute Coronary Syndromes: added value on top of GRACE risk score. Eur Heart J Acute Cardiovasc Care 2015;4:172-9. [PubMed]
- Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci 2009;277:103-8. [PubMed]
- Balta S, Demir M, Demirkol S, et al. Red cell distribution width is related to stroke in patients with heart failure. Clin Appl Thromb Hemost 2015;21:190. [PubMed]
- Kaya A, Isik T, Kaya Y, et al. Relationship between red cell distribution width and stroke in patients with stable chronic heart failure: a propensity score matching analysis. Clin Appl Thromb Hemost 2015;21:160-5. [PubMed]
- Saliba W, Barnett-Griness O, Elias M, et al. The association between red cell distribution width and stroke in patients with atrial fibrillation. Am J Med 2015;128:192.e11-8.
- Tonelli M, Sacks F, Arnold M, et al. Relation Between Red Blood Cell Distribution Width and Cardiovascular Event Rate in People With Coronary Disease. Circulation 2008;117:163-8. [PubMed]
- Vayá A, Hernández V, Rivera L, et al. Red blood cell distribution width in patients with cryptogenic stroke. Clin Appl Thromb Hemost 2015;21:241-5. [PubMed]
- Ramírez-Moreno JM, Gonzalez-Gomez M, Ollero-Ortiz A, et al. Relation between red blood cell distribution width and ischemic stroke: a case-control study. Int J Stroke 2013;8:E36. [PubMed]
- Jia H, Li H, Zhang Y, et al. Association between red blood cell distribution width (RDW) and carotid artery atherosclerosis (CAS) in patients with primary ischemic stroke. Arch Gerontol Geriatr 2015;61:72-5. [PubMed]
- Kim J, Kim YD, Song TJ, et al. Red blood cell distribution width is associated with poor clinical outcome in acute cerebral infarction. Thromb Haemost 2012;108:349-56. [PubMed]
- Kara H, Degirmenci S, Bayir A, et al. Red cell distribution width and neurological scoring systems in acute stroke patients. Neuropsychiatr Dis Treat 2015;11:733-9. [PubMed]
- Bareford D, Lucas GS, Caldwell NM, et al. Erythrocyte deformability in peripheral occlusive arterial disease. J Clin Pathol 1985;38:135-9. [PubMed]
- Haltmayer M, Mueller T, Luft C, et al. Erythrocyte mean corpuscular volume associated with severity of peripheral arterial disease: an angiographic evaluation. Ann Vasc Surg 2002;16:474-9. [PubMed]
- Mueller T, Haidinger D, Luft C, et al. Association between erythrocyte mean corpuscular volume and peripheral arterial disease in male subjects: a case control study. Angiology 2001;52:605-13. [PubMed]
- Zalawadiya SK, Veeranna V, Panaich SS, et al. Red cell distribution width and risk of peripheral artery disease: analysis of National Health and Nutrition Examination Survey 1999-2004. Vasc Med 2012;17:155-63. [PubMed]
- Demirtas S, Karahan O, Yazici S, et al. The relationship between complete blood count parameters and Fontaine’s Stages in patients with peripheral arterial disease. Vascular 2014;22:427-31. [PubMed]
- Ye Z, Smith C, Kullo IJ. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am J Cardiol 2011;107:1241-5. [PubMed]
- Lowe GD, Jaap AJ, Forbes CD. Relation of atrial fibrillation and high haematocrit to mortality in acute stroke. Lancet 1983;1:784-6. [PubMed]
- Takahashi N, Ashida T, Kiraku J, et al. Increase in erythrocyte volume in patients with chronic atrial fibrillation. Jpn Heart J 1997;38:387-91. [PubMed]
- Peverill RE, Harper RW, Smolich JJ. Inverse relation of haematocrit to cardiac index in mitral stenosis and atrial fibrillation. Int J Cardiol 1999;71:149-55. [PubMed]
- Kiefer CR, Snyder LM. Oxidation and erythrocyte senescence. Curr Opin Hematol 2000;7:113-6. [PubMed]
- Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005;352:1011-23. [PubMed]
- Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 2012;60:2263-70. [PubMed]
- Adamsson Eryd S, Borné Y, Melander O, et al. Red blood cell distribution width is associated with incidence of atrial fibrillation. J Intern Med 2014;275:84-92. [PubMed]
- Güngör B, Özcan KS, Erdinler İ, et al. Elevated levels of RDW is associated with non-valvular atrial fibrillation. J Thromb Thrombolysis 2014;37:404-10. [PubMed]
- Liu T, Shao Q, Miao S, et al. Red cell distribution width as a novel, inexpensive marker for paroxysmal atrial fibrillation. Int J Cardiol 2014;171:e52-3. [PubMed]
- Ertaş G, Aydin C, Sönmez O, et al. Red cell distribution width predicts new-onset atrial fibrillation after coronary artery bypass grafting. Scand Cardiovasc J 2013;47:132-5. [PubMed]
- Korantzopoulos P, Liu T. RDW as a marker of postoperative atrial fibrillation. Int J Cardiol 2015;191:109. [PubMed]
- Kurt M, Tanboga IH, Buyukkaya E, et al. Relation of red cell distribution width with CHA2DS2-VASc score in patients with nonvalvular atrial fibrillation. Clin Appl Thromb Hemost 2014;20:687-92. [PubMed]
- Wan H, Yang Y, Zhu J, et al. The relationship between elevated red cell distribution width and long-term outcomes among patients with atrial fibrillation. Clin Biochem 2015;48:762-7. [PubMed]
- Gurses KM, Yalcin MU, Kocyigit D, et al. Red blood cell distribution width predicts outcome of cryoballoon-based atrial fibrillation ablation. J Interv Card Electrophysiol 2015;42:51-8. [PubMed]
- Lippi G, Cervellin G. Risk assessment of post-infarction heart failure. Systematic review on the role of emerging biomarkers. Crit Rev Clin Lab Sci 2014;51:13-29. [PubMed]
- Borné Y, Smith JG, Melander O, et al. Red cell distribution width and risk for first hospitalization due to heart failure: a population-based cohort study. Eur J Heart Fail 2011;13:1355-61. [PubMed]
- Emans ME, Gaillard CA, Pfister R, et al. Red cell distribution width is associated with physical inactivity and heart failure, independent of established risk factors, inflammation or iron metabolism; the EPIC-Norfolk study. Int J Cardiol 2013;168:3550-5. [PubMed]
- Oh J, Kang SM, Hong N, et al. Relation between red cell distribution width with echocardiographic parameters in patients with acute heart failure. J Card Fail 2009;15:517-22. [PubMed]
- Nishizaki Y, Yamagami S, Suzuki H, et al. Red blood cell distribution width as an effective tool for detecting fatal heart failure in super-elderly patients. Intern Med 2012;51:2271-6. [PubMed]
- Felker GM, Allen LA, Pocock SJ, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol 2007;50:40-7. [PubMed]
- Shao Q, Li L, Li G, et al. Prognostic value of red blood cell distribution width in heart failure patients: a meta-analysis. Int J Cardiol 2015;179:495-9. [PubMed]
- Huang YL, Hu ZD, Liu SJ, et al. Prognostic value of red blood cell distribution width for patients with heart failure: a systematic review and meta-analysis of cohort studies. PLoS One 2014;9:e104861. [PubMed]
- Jenei ZM, Förhécz Z, Gombos T, et al. Red cell distribution width as predictive marker in CHF: testing of model performance by reclassification methods. Int J Cardiol 2014;174:783-5. [PubMed]
- Núñez J, Núñez E, Rizopoulos D, et al. Red blood cell distribution width is longitudinally associated with mortality and anemia in heart failure patients. Circ J 2014;78:410-8. [PubMed]
- Tseliou E, Terrovitis JV, Kaldara EE, et al. Red blood cell distribution width is a significant prognostic marker in advanced heart failure, independent of hemoglobin levels. Hellenic J Cardiol 2014;55:457-61. [PubMed]
- Dai Y, Konishi H, Takagi A, et al. Red cell distribution width predicts short- and long-term outcomes of acute congestive heart failure more effectively than hemoglobin. Exp Ther Med 2014;8:600-6. [PubMed]
- Pascual-Figal DA, Bonaque JC, Manzano-Fernández S, et al. Red blood cell distribution width predicts new-onset anemia in heart failure patients. Int J Cardiol 2012;160:196-200. [PubMed]
- Uemura Y, Shibata R, Takemoto K, et al. Elevation of red blood cell distribution width during hospitalization predicts mortality in patients with acute decompensated heart failure. J Cardiol 2015. [Epub ahead of print]. [PubMed]
- Tanindi A, Topal FE, Topal F, et al. Red cell distribution width in patients with prehypertension and hypertension. Blood Press 2012;21:177-81. [PubMed]
- Ozcan F, Turak O, Durak A, et al. Red cell distribution width and inflammation in patients with non-dipper hypertension. Blood Press 2013;22:80-5. [PubMed]
- Kilicaslan B, Dursun H, Aydin M, et al. The relationship between red-cell distribution width and abnormal left ventricle geometric patterns in patients with untreated essential hypertension. Hypertens Res 2014;37:560-4. [PubMed]
- Fornal M, Wizner B, Cwynar M, et al. Association of red blood cell distribution width, inflammation markers and morphological as well as rheological erythrocyte parameters with target organ damage in hypertension. Clin Hemorheol Microcirc 2014;56:325-35. [PubMed]
- Li ZZ, Chen L, Yuan H, et al. Relationship between red blood cell distribution width and early-stage renal function damage in patients with essential hypertension. J Hypertens 2014;32:2450-5; discussion 2456. [PubMed]
- Kurt RK, Aras Z, Silfeler DB, et al. Relationship of red cell distribution width with the presence and severity of preeclampsia. Clin Appl Thromb Hemost 2015;21:128-31. [PubMed]
- Fici F, Celik T, Balta S, et al. Comparative effects of nebivolol and metoprolol on red cell distribution width and neutrophil/lymphocyte ratio in patients with newly diagnosed essential hypertension. J Cardiovasc Pharmacol 2013;62:388-93. [PubMed]
- Lippi G, Mattiuzzi C. The biomarker paradigm: between diagnostic efficiency and clinical efficacy. Pol Arch Med Wewn 2015;125:282-8. [PubMed]
- Lippi G. Red blood cell distribution width and mean platelet volume: Surrogate markers for, or treatment targets in, dyslipidemia? Clin Biochem 2015;48:555-6. [PubMed]


