Network meta-analyses on in-stent restenosis treatment: dealing with complexity to clarify efficacy and safety
Major advances have occurred during the last decade in the prevention of in-stent restenosis (ISR). Notably, drug-eluting stents (DES) drastically reduced the incidence of ISR as compared with that seen with bare-metal stents (BMS) (1). However, DES-ISR still occurs especially when these devices are used in adverse clinical and anatomic scenarios (1). In addition, BMS are frequently used in selected patient subsets, including those unable to maintain a prolonged dual antiplatelet regimen and those at high bleeding risk (1). Therefore, nowadays treatment of ISR still represents a real challenge in every day clinical practice (2-4). Although the acute results obtained by repeated interventions are largely favorable the long-term outcome of these patients is frequently shadowed by clinical recurrences (2-4). Of concern, the underlying anatomic substrate of DES-ISR appears to be particularly complex and prone to recurrent ISR (2). Furthermore, recent evidence suggest that ISR presentation, formerly considered a benign phenomenon, is frequently associated with unstable symptoms, including a significant number of patients fulfilling current criteria for myocardial infarction (2). Many randomized clinical trials have compared different therapeutic strategies in patients with ISR (2-4). These include plain balloon angioplasty (BA), cutting balloon angioplasty, BMS, ablative devices, brachytherapy, DES and drug-coated balloons (DCB). Recent clinical practice guidelines suggest that both DES and DCB are effective (recommendation/evidence IA) for patients suffering from ISR (5). Nevertheless, the therapy of choice for these patients currently remains unsettled. Indeed, most randomized trials used surrogate late angiographic parameters (including percent diameter stenosis, binary restenosis, minimal lumen diameter and late lumen loss) as a measure of efficacy (2-4). This was a reasonable strategy to ensure an adequate enrollment of the required number of patients presenting with this relatively rare condition within a short time frame. Indeed, these trials provided major evidence on relative efficacy of these interventions. However, most randomized studies eventually enrolled a limited number of patients and, therefore, additional evidence is still warranted in order to establish the relative clinical efficacy and safety of these competing interventions.
Rigorous, methodologically sound, and carefully-performed network meta-analyses are powered to unravel additional information from the existing studies further informing the clinical decision-making process.
Current study
Very recently Lee et al. (6) performed an interesting Bayesian network meta-analysis of all available randomized clinical trials comparing BA, DES and DCB in patients with ISR. Eventually, a total of 2,059 patients from 11 randomized clinical trials were included in the final analysis [808 patients (39%) treated with DES, 694 (34%) with DCB, and 557 (27%) with BA]. Three trials compared DES with DCB, four compared DCB with BA and one study, with three arms, compared DES with DCB and BA. Four trials exclusively enrolled patients with BMS-ISR, five exclusively patients with DES-ISR whereas two studies included patients with either DES-ISR or BMS-ISR. Actually, many of the randomized trials included in this meta-analysis were part of systematic ongoing multicentric strategies addressing the treatment of patients with ISR [the PEPCAD (three trials), ISAR-DESIRE (two trials) and RIBS (two trials) programs] (6).
As expected, trials that used DES obtained larger minimal lumen diameter and lower residual diameter stenosis immediately after the procedure than the corresponding DCB and BA arms. The primary outcome measure of this study was the rate of target lesion revascularization (TLR) at late follow-up [presented as OR with 95% credible intervals (CrI)] although target vessel revascularization was considered when TLR results were not available. Using a random-effects model the risk of TLR at late follow-up was significantly lower in patients treated with DCB (OR 0.22, 95% CrI: 0.1-0.42) or DES (OR 0.24, 95% CrI: 0.11-0.47) than in those treated with BA. However, the risk of TLR was similar for DCB and DES. Likewise, the risk of binary angiographic restenosis was significantly lower in the DCB and DES groups than in the BA group. Interestingly, the risk of myocardial infarction and all-cause mortality was lowest in patients treated with DCB. Finally, the risk of major adverse events—mainly driven by TLR—was also lower in the DCB and DES groups compared with the BA group. In addition, the probability of being ranked as the best treatment regarding TLR was 59.9% for DCB followed by 40.1% for DES. Alternatively, the probability of being ranked as the best therapy considering freedom from myocardial infarction was 63% for DCB followed by 35.3% for BA. Authors concluded that DCB and DES are markedly better than BA in preventing TLR. In addition, of the two active drug-therapies, DCB showed a trend to a lower risk of myocardial infarction compared with DES.
This represents a methodologically sound, comprehensive, network meta-analysis comparing safety and efficacy of BA, DES and DCB in patients with ISR. Some issues however, deserve further discussion. The results were consistent in different sensitivity analyses that included: (I) a fixed-effects model for statistical assessment, (II) the analysis of events occurring during the first year only (instead of those seen at last follow-up available), (III) including only trials with DES-ISR or BMS-ISR, and (IV) accounting for the different duration of dual antiplatelet therapy in the diverse trials using independent analyses. Reassuringly, the pooled effect estimates provided by direct and indirect comparisons were also very consistent.
The comparison of DCB with DES regarding myocardial infraction showed a trend in favour of the DCB treatment (OR 2.0, 95% CrI: 0.89-6.1), whereas the comparison of DCB with BA regarding all caused mortality showed a trend in favour of DCB (OR 2.5, 95% CrI: 0.86-7.7). Although this would suggest that treatment with DES might be associated with a higher incidence of procedural related myocardial infarction (likely resulting from side-branch occlusion), stent thrombosis or actually occur during the treatment of recurrent ISR, detailed results on the cause and timing of myocardial infarction were not available. In fact, the risk of stent thrombosis was no different in the three treatment groups. Notably, the long-term follow-up of two recent randomized trials comparing DCB with DES also suggested a safety advantage with the use of DCB (7-9). Nevertheless, further studies, with longer clinical follow-up, are warranted to definitively address this intriguing possibility.
On the other hand, before extrapolating these results to everyday clinical practice we should keep in mind that most randomized clinical trials exclude very complex ISR cases (small vessels, total occlusions, very diffuse lesions, left main stent location) and, as a result, the generalizability of current findings to these complex anatomic scenarios is probably not justified (2-4).
Finally, of the included trials, only RIBS V (10) had an arm treated with a second-generation everolimus eluting stent whereas the remaining trials included in this meta-analysis used first-generation DES. This is important as recent studies strongly suggest the value of second-generation DES in this challenging setting (2,10,11).
Incremental value of “network” meta-analysis
The publication of meta-analyses has increased exponentially in recent years. Systematic reviews examining the comparative effectiveness among competing interventions take into account all available evidence. This primarily stems from head to head comparison studies that provide direct evidence. However, the number of head to head studies tends to be limited, especially when multiple competing interventions are available. Therefore, there is also a major need to ascertain the evidence resulting from indirect comparisons of each intervention against a common comparator (12-15). This indirect evidence complements that provided by the direct comparisons but its analysis is challenging and requires a rigorous methodology to ensure validity. These indirect comparisons rely on several assumptions and may suffer from potential biases (12-15). The risk of bias in pairwise comparisons is well known and different tools are available to address its effects and potential implications. However, the potential risk of bias is greater and more elusive when the evidence is gathered from multiple direct and indirect comparisons of competing interventions (12-13).
Recently, an extension of the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement focussing precisely on the methodological aspects of the “network” meta-analysis, has been published (12). A modified 32-item PRISMA extension check list was developed to address all relevant issues that should be reported in network meta-analysis. A key element is the “network graph” that consists of nodes (points representing the competing interventions) and edges (lines connecting the nodes that have been directly compared). Sizes of nodes and thickness of edges illustrate the number of patients and studies analyzed and, therefore, visually depict the amount of available evidence (12). The meaning and implications of the geometry of the resulting network graph should be discussed and clarified. Sometimes lumping of interventions is required. However, lumping requires a clear rationale and should only include interventions that are closely related and provide similar treatment effects. Inconsistency addresses the problem of differences between the treatment effects provided by direct and indirect comparisons. Poorly connected networks depend excessively on indirect comparisons and are less reliable than networks where most treatments have been compared against each other therefore increasing the strength of the generated evidence (12).
Results of relevant studies should be described using a tabulated presentation of relevant baseline characteristics. Importantly, the PICOs (Population, Intervention, Comparators, Outcome) criteria must be observed in the presentation of the results. These baseline characteristics are potential effect modifiers. Notably, a balanced distribution of most relevant potential effect modifiers increases the plausibility of obtaining reliable findings from indirect comparisons. Transitivity refers to the existence of comparable distribution of patient characteristics across the studies. When treatment networks contain closed loops of interventions it is possible to analyze the agreement between direct and indirect estimates of intervention effects. Forest plot summarizing treatment effects should be presented in a clear and comprehensive manner (12).
Network meta-analyses may be performed with a Bayesian (assuming an expected prior probability distribution) or frequentist approach. Bayesian approaches are commonly utilized as they ensure more flexibility of the statistical models. Carefully constructed Bayesian models may address the problem of low events rates, but analysis of studies with a low event rate should be interpreted with caution (12-15).
Finally, network meta-analyses provide the attractive additional feature to readily summarize the available evidence, namely relative rankings on effectiveness among the competing interventions. In general, these rankings should be only offered as secondary outcome measures. The central stage should be reserved to the actual effects estimates with the corresponding 95% confidence or credible intervals for the primary outcome measure. Last but not least, a general interpretation of the results in the context of prior evidence and the implications for future research should be provided in network meta-analyses (12-15).
A careful scrutiny of the elegant study of Lee et al. (6) unravels a robust methodology with detailed description of most of the relevant methodological issues described above even though the study was published months before the extended statement of the PRISMA recommendations (12).
Too many meta-analyses on ISR?
A large number of previous meta-analyses has focused on ISR treatment (Table 1) (6,15-33). Some of them were very early studies whereas other concentrated in evaluating selected therapies. Some initial meta-analyses were performed to gain further insights on the role of brachytherapy compared with conventional interventions. Other meta-analyses concentrated in assessing the results of first-generation DES. Most recent analyses tried to elucidate the relative value of DCB (Table 1). Anyhow, this would appear to be an excessive number of meta-analyses and, in fact, some of them represent nearly simultaneous analyses of the same trials, therefore yielding redundant results. Actually, any novel late breaking clinical trial provides the temptation for performing a new meta-analysis. In general, this temptation should be resisted unless the information provided by the new meta-analysis is expected to be of real value to advance the field. Otherwise, planning a brand new randomized clinical trial should be preferred to address gaps in knowledge. Notably, “patient-level” meta-analyses allow for additional insights yet they demand much work requiring true collaboration among different investigators and, unfortunately, they are scarce. Before a meta-analysis is performed the rationale of the review should be clarified in the context of what is already known. Overall, a strong suppression of neointimal hyperplasia proved to be required to prevent ISR recurrences (6,15-33). Indeed, early studies confirmed the superiority of brachytherapy over classical mechanical strategies. More recently, the superior role of pharmacoactive interventions, namely DES and DCB, over isolated mechanical interventions, became established (Table 1).
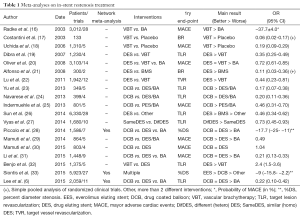
Full table
As compared with most of these previous reports, the current study by Lee et al. (6) includes a larger number of recent trials and, more importantly, the Bayesian network approach selected allowed for adequate direct and indirect comparisons among the studied therapies. This provided important novel insights on safety and efficacy.
Very recently we collaborated in yet another network meta-analysis (33). This aimed to synthesize both direct and indirect evidence from relevant trials in patients with any type of ISR comparing a wide array of coronary interventions. Importantly, in this network meta-analysis the results from second-generation everolimus-DES (provided by the recent RIBS V and IV randomized clinical trials), could be included. A total of 27 trials including 5,923 patients were deemed eligible and the primary outcome measure was percent diameter stenosis at late follow-up. Everolimus-DES emerged as the most effective treatment for percent diameter stenosis, with a difference of −9.0% (95% CI: −15.8 to −2.2) vs. DCB, −9.4% (95% CI: −17.4 to −1.4) vs. sirolimus-DES, −10.2% (95% CI: −18.4 to −2.0) vs. paclitaxel-DES, −19.2% (95% CI: −28.2 to −10.4) vs. brachytherapy, −23.4% (95% CI: −36.2 to −10.8) vs. BMS, −24.2% (95% CI: −32.2 to −16.4) vs. BA, and −31.8% (95% CI: −44.8 to −18.6) vs. rotational atherectomy. Everolimus-DES were ranked as the most effective strategy and DCB were ranked as the second most effective treatment without significant differences from sirolimus-DES or paclitaxel-DES.
Conclusions
No clear consensus exists for the treatment of ISR and this explains the variability seen in real world clinical practice. Randomized clinical trials and meta-analyses are consolidated as key elements of the evidence based medicine to inform clinical practice. Network meta-analyses are particularly useful to address evidence gaps by fully exploiting all the available scientific information. The lack of head to head studies comparing treatments of interest, the absence of comparisons powered for most hard clinical outcomes and, finally, the need for unravelling further insights into the relative effectiveness and harm of the different treatment modalities available, remain powerful drivers in this never ending research. The network meta-analyses by Lee et al. (6) and by Siontis et al. (33) provide unique and complementary insights for the treatment of patients with ISR. Both DES and DCB are very attractive in this setting. However, the particular efficacy of second-generation everolimus-DES in this adverse anatomic scenario (demonstrated in the RIBS V and VI studies) should be keep in mind during the decision making process used in every day clinical practice. Further studies, however, should confirm the very long-term efficacy of new-generation DES in patients with ISR and also establish whether comparable results may be obtained with other new-generation DES.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Editorial commissioned by the Section Editor Yue Liu (Department of Cardiology, the First Affiliated Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- García del Blanco B, Hernández Hernández F, Rumoroso Cuevas JR, et al. Spanish Cardiac Catheterization and Coronary Intervention Registry. 23rd official report of the Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology (1990-2013). Rev Esp Cardiol (Engl Ed) 2014;67:1013-23. [PubMed]
- Alfonso F, Byrne RA, Rivero F, et al. Current treatment of in-stent restenosis. J Am Coll Cardiol 2014;63:2659-73. [PubMed]
- Dangas GD, Claessen BE, Caixeta A, et al. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol 2010;56:1897-907. [PubMed]
- Byrne RA, Joner M, Alfonso F, et al. Treatment of in-stent restenosis. In: Bahatt DL, editor. Cardiovascular Intervention. A companion to Braunwald’s Heart Disease. Philadelphia: Elsevier, 2015.
- Authors/Task Force members, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [PubMed]
- Lee JM, Park J, Kang J, et al. Comparison among drug-eluting balloon, drug-eluting stent, and plain balloon angioplasty for the treatment of in-stent restenosis: a network meta-analysis of 11 randomized, controlled trials. JACC Cardiovasc Interv 2015;8:382-94. [PubMed]
- Kufner S, Cassese S, Valeskini M, et al. Long-Term Efficacy and Safety of Paclitaxel-Eluting Balloon for the Treatment of Drug-Eluting Stent Restenosis: 3-Year Results of a Randomized Controlled Trial. JACC Cardiovasc Interv 2015;8:877-84. [PubMed]
- Xu B, Gao R, Wang J, et al. A prospective, multicenter, randomized trial of paclitaxel-coated balloon versus paclitaxel-eluting stent for the treatment of drug-eluting stent in-stent restenosis: results from the PEPCAD China ISR trial. JACC Cardiovasc Interv 2014;7:204-11. [PubMed]
- Alfonso F, Cuesta J. Long-Term Results of Drug-Coated Balloons for Drug-Eluting In-Stent Restenosis: Gaining Perspective. JACC Cardiovasc Interv 2015;8:885-8. [PubMed]
- Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, et al. A randomized comparison of drug-eluting balloon versus everolimus-eluting stent in patients with bare-metal stent-in-stent restenosis: the RIBS V Clinical Trial (Restenosis Intra-stent of Bare Metal Stents: paclitaxel-eluting balloon vs. everolimus-eluting stent). J Am Coll Cardiol 2014;63:1378-86. [PubMed]
- Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, et al. A Prospective Randomized Trial of Drug-Eluting Balloons Versus Everolimus-Eluting Stents in Patients With In-Stent Restenosis of Drug-Eluting Stents: The RIBS IV Randomized Clinical Trial. J Am Coll Cardiol 2015;66:23-33. [PubMed]
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777-84. [PubMed]
- Cornell JE. The PRISMA extension for network meta-analysis: bringing clarity and guidance to the reporting of systematic reviews incorporating network meta-analyses. Ann Intern Med 2015;162:797-8. [PubMed]
- Caldwell DM. An overview of conducting systematic reviews with network meta-analysis. Syst Rev 2014;3:109. [PubMed]
- Stewart LA, Clarke M, Rovers M, et al. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA 2015;313:1657-65. [PubMed]
- Radke PW, Kaiser A, Frost C, et al. Outcome after treatment of coronary in-stent restenosis; results from a systematic review using meta-analysis techniques. Eur Heart J 2003;24:266-73. [PubMed]
- Costantini CO, Lansky AJ, Mintz GS, et al. Intravascular brachytherapy for native coronary ostial in-stent restenotic lesions. J Am Coll Cardiol 2003;41:1725-31. [PubMed]
- Uchida T, Bakhai A, Almonacid A, et al. A meta-analysis of randomized controlled trials of intracoronary gamma- and beta-radiation therapy for in-stent restenosis. Heart Vessels 2006;21:368-74. [PubMed]
- Dibra A, Kastrati A, Alfonso F, et al. Effectiveness of drug-eluting stents in patients with bare-metal in-stent restenosis: meta-analysis of randomized trials. J Am Coll Cardiol 2007;49:616-23. [PubMed]
- Oliver LN, Buttner PG, Hobson H, et al. A meta-analysis of randomised controlled trials assessing drug-eluting stents and vascular brachytherapy in the treatment of coronary artery in-stent restenosis. Int J Cardiol 2008;126:216-23. [PubMed]
- Alfonso F, Pérez-Vizcayno MJ, Hernandez R, et al. Sirolimus-eluting stents versus bare-metal stents in patients with in-stent restenosis: results of a pooled analysis of two randomized studies. Catheter Cardiovasc Interv 2008;72:459-67. [PubMed]
- Lu YG, Chen YM, Li L, et al. Drug-eluting stents vs. intracoronary brachytherapy for in-stent restenosis: a meta-analysis. Clin Cardiol 2011;34:344-51. [PubMed]
- Yu CM, Kwong JS, Sanderson JE. Drug-eluting balloons for coronary artery disease: a meta-analysis of randomized controlled trials. Int J Cardiol 2013;168:197-206. [PubMed]
- Navarese EP, Austin D, Gurbel PA, et al. Drug-coated balloons in treatment of in-stent restenosis: a meta-analysis of randomised controlled trials. Clin Res Cardiol 2013;102:279-87. [PubMed]
- Indermuehle A, Bahl R, Lansky AJ, et al. Drug-eluting balloon angioplasty for in-stent restenosis: a systematic review and meta-analysis of randomised controlled trials. Heart 2013;99:327-33. [PubMed]
- Sun Y, Li L, Su Q, et al. Comparative efficacy and safety of drug-eluting stent and conventional therapies in coronary heart disease patients with in-stent restenosis: a meta-analysis. Cell Biochem Biophys 2014;68:211-29. [PubMed]
- Vyas A, Schweizer M, Malhotra A, et al. Meta-analysis of same versus different stent for drug-eluting stent restenosis. Am J Cardiol 2014;113:601-6. [PubMed]
- Piccolo R, Galasso G, Piscione F, et al. Meta-analysis of randomized trials comparing the effectiveness of different strategies for the treatment of drug-eluting stent restenosis. Am J Cardiol 2014;114:1339-46. [PubMed]
- Mamuti W, Jiamali A, Rao F, et al. Drug-coated balloon angioplasty for drug-eluting stent restenosis: insight from randomized controlled trials. Ann Med 2014;46:679-83. [PubMed]
- Mamuti W, Ablimit A, Kelimu W, et al. Comparison of drug-eluting balloon versus drug-eluting stent in patients with in-stent restenosis: insight from randomized controlled trials. Int J Cardiol 2015;179:424-9. [PubMed]
- Li J, Liu WL, Yi X, et al. Paclitaxel-coated balloons for the treatment of patients with in-stent restenosis: A meta-analysis of angiographic and clinical data. Exp Ther Med 2015;9:2285-2292. [PubMed]
- Benjo A, Cardoso RN, Collins T, et al. Vascular brachytherapy versus drug-eluting stents in the treatment of in-stent restenosis: A meta-analysis of long-term outcomes. Catheter Cardiovasc Interv 2015. [Epub ahead of print]. [PubMed]
- Siontis GC, Stefanini GG, Mavridis D, et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet 2015;386:655-64. [PubMed]


