A comparative analysis of lung cancer patients treated with lobectomy via three-dimensional video-assisted thoracoscopic surgery versus two-dimensional resection
Introduction
Open thoracotomy and video-assisted thoracoscopic surgery (VATS) were performed to treat early stage non-small cell lung cancer, metastatic disease, and benign tumors. Compared with open thoracotomy, VATS enables a smaller incision without removal or stretching of the ribs, avoiding injuries to respiratory muscles and thus minimizing the loss of lung function (1,2). Moreover, with a smaller incision, patients will suffer less pain postoperatively and expectorate more easily, reducing the incidence of postoperative pulmonary infection and other complications (3). Since the early 1990s, traditional two-dimensional (2D) VATS has rapidly developed and has been widely applied around the world. However, a 2D image lacks depth and can result in image distortion, impaired hand-eye coordination, and decreased ability to estimate size. The visual information gained via binocular vision allows for precise intraoperative movement and can therefore affect the operation (4,5). Limitations of the 2D system such as depth perception and spatial orientation still remain a challenge even for the experienced surgeon. Surgeons learn to compensate for the these limitations by using 2D cues such as light and shade, relative size of organs, organ interposition, texture gradient, aerial perspective, and motion parallax (6). With earlier generations of 3D endoscopy surgeons indeed reported improved depth perception, however, they also reported problems with headaches and ocular fatigue (7,8). Now, endoscopic procedures can be viewed stereoscopically, the surgeon simply wears glasses to create the sense of depth. Despite the significant advantages claimed, it is unclear whether 3D VATS is superior to 2D VATS systems.
The aim of this study was to compare 3D VATS lobectomy with 2D VATS lobectomy in non-small cell lung cancer (NSCLC) patients, assessing short-term outcomes of perioperative morbidity, postoperative complications, oncologic efficacy (number of lymph nodes resected) and cost of care.
Materials and methods
Study design
This study was a two-center trial sponsored by the Combination Project of Guangdong Province and the Ministry of Education (No. 2011B090400ation 478), and Natural Science Foundation of Liaoning (No. 2014020103). The study was conducted in accordance with the Helsinki Declaration. Our institutional review board approved this study, and written informed consent was obtained from all patients.
Participants
Patients were allocated to 2D and 3D VATS groups. Computer-generated block allocation was initiated by a data manager in the VATS research group and placed in individual sealed envelopes, ensuring that both the surgeon and the thoracic research assistant interviewing potential candidates for the study were blind to the allocation code. Each envelope was opened in front of the patient on entry into the study after written informed consent was received. All patients undergoing isolated VATS radical resection (lobectomy with systematic lymph node dissection) carried out by two surgeon groups over a 12-month period were included. Patients received pre-operative chest high-resolution thin-slice enhanced CT scans and pulmonary function tests. For those suspected of lung cancer, additional upper abdomen CT, head MRI, whole-body bone scintigraphy or whole-body PET/CT examinations were done to exclude distant metastases.
Indications for VATS lobectomy included: no ipsilateral thoracotomy history; no evidence of severe pleural adhesions; resectable lesions ≤5 cm; no clinical sign of multiple N2 metastases. This series included consecutive patients whom preoperative intention was to resect with VATS procedure.
Exclusion criteria included: patients with a history of neoadjuvant chemotherapy or radiotherapy, procedures other than lobectomy, such as wedge, segmentectomy, bilobectomy, pneumonectomy, or chest wall resection.
Surgical technique
Surgery was performed by consultant thoracic surgeons who had performed at least 2,000 VATS lobectomies. All operations were performed by the same group of thoracic surgeons in our hospital, both of which have had 3D VATS available since July 2013. Our thoracic surgeons have similar learning curves for 3D VATS. Each VATS procedure was performed via three ports without rib spreading and 100% monitor vision. Surgical procedures in the 2D group were performed using a Karl Stortz system (Karl Stortz GmbH & Co. KG, Tuttlingen, Germany). Those in the 3D group were performed with a Karl Stortz 3D system (Karl Stortz GmbH & Co. KG, Tuttlingen, Germany). Surgeons wore polarized 3D lenses to view the images on a screen during 3D VATS operations. The video resolution of the 2D and 3D systems were equal in this study. A 30-degree endoscope was used. The patients were intubated with double-lumen endotracheal tubes under general anesthesia. With patients in a contralateral supine position, the upper limb of the affected side was positioned on the hand bracket. The observation incision was positioned at the level of the seventh or eighth intercostal space on the posterior axillary line, with the main manipulative incision 3 cm to the anterior axillary line as the center. An upper lobectomy at the fourth intercostal space and a lower lobectomy at the fifth, allowing two surgical tools to be introduced or withdrawn simultaneously. The harmonic scalpel was operated along with the suturing instrument and the aspirator. For the auxiliary, an auxiliary manipulative incision measuring approximately 1 cm in length was made at the same intercostal space posterior to the posterior axillary line as the observation incision. The surgeon stood in front of the patient, completing the procedures through the manipulative incision by watching the screen without using direct visualization or a rib distractor during the operation. The veins, arteries, and bronchi were separated anatomically, and the lymph nodes in stations 10 and 11 were dissected. Specimen bags were inserted to remove lung tissue, and the mediastinal lymph node dissection was subsequently performed again (on the left, stations 4, 5, 6, 7, 8, and 9; on the right, stations 2, 4, 7, 8, and 9).
Postoperative treatment
Patients in both groups received similar postoperative care. Patients were extubated at the end of the procedure if physiologically stable, then admitted to the intensive care unit, and finally discharged the next day to a general surgical ward. Data of postoperative complications were collected prospectively, and data regarding tumor size, histologic type, and TNM stage were obtained from the pathologic records. Pathological staging was performed according to the seventh edition of the TNM Classification of Malignant Tumours by the International Union Against Cancer (UICC) (9).
Outcomes
The primary outcome of this study was operative morbidity. Secondary outcomes included oncologic efficacy (number of lymph nodes resected), postoperative complications and cost of care. Postoperative complications included respiratory complications (defined as clinical manifestation of pneumonia or bronchopneumonia confirmed by computed tomographic scan); cardiovascular complications (defined as persistent arrhythmia requiring medical treatment); chylothorax (defined as the appearance of milky fluid from thoracic drains after onset of enteral nutrition); wound infections; and other complications. Postoperative mortality was defined as death from any cause.
Care cost analysis
Care costs were reported as averages, and all categories were direct costs to the hospital. Indirect costs such as management salaries, insurance, utilities, and building depreciation were excluded because it was assumed they would be similar between groups. Direct hospital cost data were collected with the center’s finance group and separated into nine distinct categories.
Statistical analysis
We used power analysis and sample-size software to calculate the sample size. Previous studies reported significantly shorter performance times using 3D systems than using 2D systems (6,10,11). Assuming that 10% of patients would be lost to follow-up and using a statistical power of 80%, we estimated that 140 patients were needed for each part of the study. To reduce the proportion of loss of follow-up, we included 150 patients for each group. The Pearson’s chi-squared test or Fisher exact tests were used to compare categorical data and the t-test or Mann-Whitney U-test for continuous data. All analyses were performed with the statistical package SPSS (SPSS 17.0). A P value of less than 0.05 was considered statistically significant.
Results
A total of 300 patients with NSCLC who underwent VATS lobectomy with systematic lymph node dissection during the 12-month study period were included in the analysis. One hundred and twenty-two patients (not meeting inclusion criteria or declined to participate) were excluded from the analysis (Figures 1,2).
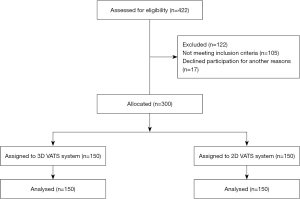
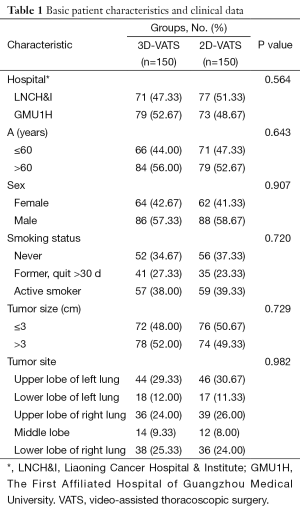
Table 1 displays the demographic characteristics and preoperative examination data for patients in the two groups. No statistically significant differences were observed (hospital, age, sex, smoking status, tumor size and tumor site).
Full table
Table 2 summarizes the findings in the two study groups. Operative mortality did not differ significantly between the two cohorts (0 of 150 in the 3D VATS group vs. 0 of 150 in the 2D VATS group). No significant differences existed between 3D and 2D VATS systems for estimated blood loss (P=0.798), conversion to open surgery (P=0.751), number of times bleeding occurred (P=0.684), number of lymph nodes resected (P=0.168), drainage duration (P=0.413), hospital stay (P=0.213) and postoperative complications (P=0.882). Postoperative complications occurred in 56 patients. The mean operative time in the 3D-VATS group (145 minutes) was significantly lower in the 2D group (176 minutes) in the 2D-VATS group (P=0.006).
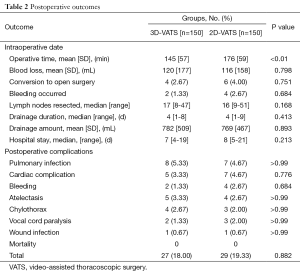
Full table
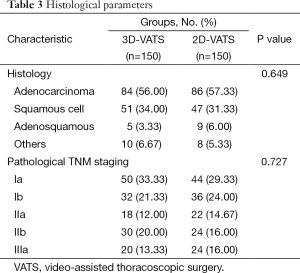
Table 3 lists the pathological diagnoses of the excised lesions. The histological types of the lesions resected and pathological TNM staging in the two groups were similar, with no significant differences.
Full table
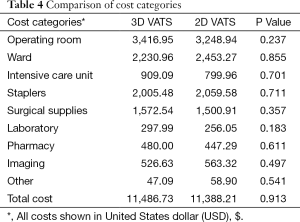
Differences in the nine cost categories are illustrated in Table 4. The operating room, ward room and staplers were the three main drivers of cost for all modalities. No statistically significant differences were found in each cost categories and total cost (P=0.913).
Full table
Discussion
In recent years, VATS has been associated with highly satisfactory results. An increasing body of evidence suggests that perioperative outcomes of this minimally invasive technique are better than those of conventional open thoracotomy. Several studies have reported reduced incidences of arrhythmia, pneumonia, pain, and inflammatory markers (12-15). It is important for general thoracic surgeons to understand the relationship between tumors and surrounding organs during surgery; however, many anatomical variations are possible in the thorax, which can complicate this goal. The lack of depth perception and spatial orientation when using traditional 2D imaging is a recognized limitation of minimally invasive surgery in comparison with open surgery (16). VATS has been proven to be beneficial when it comes to morbidity and patients’ post-operative quality of life (17). To improve operative time and surgical performance, 2D vision uses monocular cues to compensate for the lack of depth perception. They include motion parallax through movement of the VATS, relative position and size of instruments and anatomic structures, shading of light and dark, and texture grading (18,19). Conversely, 3D vision offers the advantage of improved depth perception and accuracy comparable to open surgeries (20). Visual performance and motor skills are a function of depth perception allowing improved discrimination and recognition of targeted organs and their partsJeny (21). The separate input from two viewpoints allows for summation on a cortical level and perceived improvements in resolution with 3D imaging (22). Acuity has been improved by 10% using binocular vision (23). Although 3D visualization is intuitively considered an important and contributing factor for improved performance during laparoscopic surgery, publications comparing 2D and 3D vision in the last two decades have reported contradictory results (4,8,19,24-28). The 3D imaging system with stereoscopic vision addresses many of the disadvantages of 2D imaging. The lack of depth perception (with 2D) imaging is a clear handicap during the initial learning curve. The reduction in dexterity with the currently available instruments remains a drawback of traditional minimally invasive surgery. While the 3D system affords many advantages, in its current iteration it presents a smaller field of view and a wider scope diameter than 2D systems. The limitations of 3D visualization include its sporadic availability, and the need for extra eyewear.
Up to now, just one study on 3D VATS has been reported (29). They stated that the use of 3D VATS system reduced the surgical time (by 17%). Our surgical time was similar to this smaller sample size (only 18 patients) study. 3D VATS was preferred by the operating surgeons for lung tumor resection. Mostly because the depth perception provided by the 3D imaging system, aided visualization of critical vascular relationships and multiple tissue layers, such as the bronchi, mediastinal structures, esophagus and thoracic duct. Converting from the 3D to the 2D system was not necessary during any of the operations in our study. Although we did not objectively assess adverse effects in the surgeons, no surgeons reported nausea or headaches. Based on the short-term results of the 150 patients who underwent the 3D-VATS technique, we believe that 3D VATS and 2D VATS lobectomy are both safe procedures with low operative mortalities. Although bleeding occurred in two patients, it was well controlled endoscopically without requiring blood transfusion. Our results show that lung resection with 3D VATS system was associated with significantly shorten operative time than with 2D VATS, but there was no significant decrease in blood loss, duration of chest tube drainage, length of hospital stay, postoperative and complications. The cost of care between 2D VATS and 3D VATS are similar.
Although thoracic surgeons having equivalent equipment and similar surgical skills performed VATS, our results should be interpreted cautiously because of the selection of the relatively less complicated patients for VATS at an early phase. All surgeons reported that they had better depth perception using the 3D system. 3D VATS can provide better sense of depth to facilitate precise operation and, in turn, shorten the operation time. We observed a decreasing time of operation within the 3D VATS group after experienced surgeons gained more 3D VATS experience. Our preliminary data supports the use of 3D-VATS as an alternative to the traditional 2D system. In summary, there is no evidence that 3D VATS is less safe than 2D VATS for resection of NSCLC. Thus, 3D-VATS systems should improve minimally invasive surgery, and enable more complex resections to be performed in the future. It would be reasonable for surgeons to investigate using 3D to perform VATS lobectomy, as it may confer advantages for some surgeons.
Acknowledgements
Funding: This study was supported by the Combination Project of Guangdong Province and the Ministry of Education (No. 2011B090400ation 478), and Natural Science Foundation of Liaoning (No. 2014020103). The funders had no role in study design, data collection and analysis, decision to publish, or prepare of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [PubMed]
- Liang C, Wen H, Guo Y, et al. Severe intraoperative complications during VATS Lobectomy compared with thoracotomy lobectomy for early stage non-small cell lung cancer. J Thorac Dis 2013;5:513-7. [PubMed]
- Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23. [PubMed]
- Chan AC, Chung SC, Yim AP, et al. Comparison of two-dimensional vs three-dimensional camera systems in laparoscopic surgery. Surg Endosc 1997;11:438-40. [PubMed]
- Taffinder N, Smith SG, Huber J, et al. The effect of a second-generation 3D endoscope on the laparoscopic precision of novices and experienced surgeons. Surg Endosc 1999;13:1087-92. [PubMed]
- Storz P, Buess GF, Kunert W, et al. 3D HD versus 2D HD: surgical task efficiency in standardised phantom tasks. Surg Endosc 2012;26:1454-60. [PubMed]
- Becker H, Melzer A, Schurr MO, et al. 3-D video techniques in endoscopic surgery. Endosc Surg Allied Technol 1993;1:40-6. [PubMed]
- Hanna GB, Shimi SM, Cuschieri A. Randomised study of influence of two-dimensional versus three-dimensional imaging on performance of laparoscopic cholecystectomy. Lancet 1998;351:248-51. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Tanagho YS, Andriole GL, Paradis AG, et al. 2D versus 3D visualization: impact on laparoscopic proficiency using the fundamentals of laparoscopic surgery skill set. J Laparoendosc Adv Surg Tech A 2012;22:865-70. [PubMed]
- Cicione A, Autorino R, Breda A, et al. Three-dimensional vs standard laparoscopy: comparative assessment using a validated program for laparoscopic urologic skills. Urology 2013;82:1444-50. [PubMed]
- Kirby TJ, Rice TW. Thoracoscopic lobectomy. Ann Thorac Surg 1993;56:784-6. [PubMed]
- Walker WS, Carnochan FM, Pugh GC. Thoracoscopic pulmonary lobectomy. Early operative experience and preliminary clinical results. J Thorac Cardiovasc Surg 1993;106:1111-7. [PubMed]
- Muraoka M, Oka T, Akamine S, et al. Video-assisted thoracic surgery lobectomy reduces the morbidity after surgery for stage I non-small cell lung cancer. Jpn J Thorac Cardiovasc Surg 2006;54:49-55. [PubMed]
- Yim AP, Wan S, Lee TW, et al. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 2000;70:243-7. [PubMed]
- Honeck P, Wendt-Nordahl G, Rassweiler J, et al. Three-dimensional laparoscopic imaging improves surgical performance on standardized ex-vivo laparoscopic tasks. J Endourol 2012;26:1085-8. [PubMed]
- Kiran RP, Kirat HT, Ozturk E, et al. Does the learning curve during laparoscopic colectomy adversely affect costs? Surg Endosc 2010;24:2718-22. [PubMed]
- Hanna GB, Shimi SM, Cuschieri A. Task performance in endoscopic surgery is influenced by location of the image display. Ann Surg 1998;227:481-4. [PubMed]
- Falk V, Mintz D, Grünenfelder J, et al. Influence of three-dimensional vision on surgical telemanipulator performance. Surg Endosc 2001;15:1282-8. [PubMed]
- Yamauchi Y, Shinohara K. Effect of binocular stereopsis on surgical manipulation performance and fatigue when using a stereoscopic endoscope. Stud Health Technol Inform 2005;111:611-4. [PubMed]
- Blake R, Levinson E. Spatial properties of binocular neurones in the human visual system. Exp Brain Res 1977;27:221-32. [PubMed]
- Wenzl R, Lehner R, Vry U, et al. Three-dimensional video-endoscopy: clinical use in gynaecological laparoscopy. Lancet 1994;344:1621-2. [PubMed]
- Rabin J. Two eyes are better than one: binocular enhancement in the contrast domain. Ophthalmic Physiol Opt 1995;15:45-8. [PubMed]
- Dion YM, Gaillard F. Visual integration of data and basic motor skills under laparoscopy. Influence of 2-D and 3-D video-camera systems. Surg Endosc 1997;11:995-1000. [PubMed]
- Herron DM, Lantis JC 2nd, Maykel J, et al. The 3-D monitor and head-mounted display. A quantitative evaluation of advanced laparoscopic viewing technologies. Surg Endosc 1999;13:751-5. [PubMed]
- Mueller MD, Camartin C, Dreher E, et al. Three-dimensional laparoscopy. Gadget or progress? A randomized trial on the efficacy of three-dimensional laparoscopy. Surg Endosc 1999;13:469-72. [PubMed]
- Patel HR, Ribal MJ, Arya M, et al. Is it worth revisiting laparoscopic three-dimensional visualization? A validated assessment. Urology 2007;70:47-9. [PubMed]
- Webber AL, Wood J. Amblyopia: prevalence, natural history, functional effects and treatment. Clin Exp Optom 2005;88:365-75. [PubMed]
- Bagan P, De Dominicis F, Hernigou J, et al. Complete thoracoscopic lobectomy for cancer: comparative study of three-dimensional high-definition with two-dimensional high-definition video systems †. Interact Cardiovasc Thorac Surg 2015;20:820-3. [PubMed]


