Is sleeve lobectomy safe after induction therapy?—a systematic review and meta-analysis
Introduction
The main approach to manage non-small cell lung cancer (NSCLC) with a curative intent remains surgical resection. For centrally-located NSCLCs, pneumonectomy is indicated if several lobes or if central broncho-vascular structures are involved. Alternatively, pulmonary sleeve resection is a parenchyma-sparing surgical procedure that was proven to be valid in centrally-located NSCLCs (1,2). This approach has the advantage of providing complete tumor resection while avoiding pneumonectomy. Initially proposed for NSCLC patients with poor cardiopulmonary functions, sleeve lobectomy (SL) has now replaced pneumonectomy even in patients with excellent cardiopulmonary functions, when technically feasible. Several studies or meta-analyses have demonstrated better post-operative outcomes and better quality of life in favor of pulmonary sleeve resection in comparison with pneumonectomy, with equivalent oncological outcomes (3-6). However, SL has been associated with higher distant recurrence rates than pneumonectomy in disease with nodal involvement (N1 or N2) (7,8). Thereby, induction treatment by either chemotherapy and/or radiotherapy is currently used to reduce the size of the tumor to potentially avoid the pneumonectomy and facilitate parenchyma-sparing procedures. Induction therapy is often needed before SL, to decrease the risk of subsequent recurrence, minimize the tumor size and ultimately facilitate complete resection. However, induction therapy may cause fibrosis and treatment related changes, which make dissection of lobar bronchus or artery and reconstructive procedure more difficult and complex (6), and raise concerns about the effects of chemotherapy and/or radiotherapy on vascular and bronchial anatomy (9,10) and airway healing. The aim of this meta-analysis was to assess the impact of induction therapy on sleeve resections, focusing on (I) post-operative 30-day mortality, (II) 30-day post-operative morbidity, (III) anastomotic related complications and (IV) overall survival.
We present the following article in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-939).
Methods
Data sources and search strategies
Electronical searches were performed on the MEDLINE using OVID, EMBASE and Google Scholar databases for articles published between January 1st 2000 and December 31st 2020. Two investigators (LEC and MG) independently carried out the literature search. A search protocol was established prior to any research. The search terms used are described in Appendix 1.
References from articles were also reviewed. Disagreements were resolved by a discussion between the two investigators. If no consensus was reached, the study was removed from the pool. The search was limited to human studies with no limit in sample size. Cochrane Library databases were also reviewed to search any previous meta-analysis on the subject. Findings were executed and reported following the PRISMA statements. Data collection was then performed independently and in duplicate by the two authors.
Selection criteria
Literature search results were screened for title and abstract referring to pulmonary sleeve resection in the context of induction chemo- and/or radiotherapy. Then, only full articles published in English were kept. Our exclusion criteria were: (I) article only available in the form of abstract, book chapter, case-report or narrative review; (II) full articles not available in English. The remaining articles were reviewed and studies with available relevant data were pooled. During this step, studies designed as observational studies, randomized clinical trials or non-randomized clinical trials meeting the following criteria were included: data were separately available for patients undergoing induction therapy or surgery alone with end-points or outcomes expressed as percentages or means with standard deviation, and patient benefiting from a pneumectomy were distinguished from patients undergoing a SL or bilobectomy. Finally, the quality of each individual study was assessed using the STROBE checklist.
Data extraction
Postoperative outcomes were compared between patients undergoing induction therapy followed by surgery and patients undergoing surgery alone. Parameters of interest were postoperative mortality, postoperative morbidity, anastomosis-related complications and 5-year survival. Mortality was defined as any death during in-hospital or 30 days after surgery.
Post-operative morbidity was defined as any post-operative complications arising after surgery including anastomotic complications. Anastomotic complications were defined as any stenosis or dehiscence at the site of the anastomosis and broncho-pleural fistula (BPF) or broncho-vascular fistula. Characteristics of each population were extracted and included average age, predominant sex, number of participants in each group, type of sleeve resection performed, oncological parameters, type of disease (benign or malign), staging (for cancer patients only), number of patients undergoing pre-operative chemotherapy alone, radiotherapy alone or combined therapy. The studies characteristics (retrospective or prospective study) were reported.
Statistical analysis
We computed the pooled odds ratio (OR) for each parameter, using the Mantel-Haenszel method. If one group displayed 0 patient for a given parameter, we used the Haldane-Anscombe correction consisting in adding 0.5 to each group for computing the OR. If both groups displayed 0 patient, we excluded the study for the computation of the parameters. We computed the confidence interval (CI) on a 95% base and reported the P value of each CI. We considered a P value <0.05 to be significant. The variance used to compute the CI was obtained from the log of the pooled OR as presented by Robins et al. (11). If a substantial lack of homogeneity with a I2 values over 60% and a significant P value (see next section) was observed, a random-model (DerSimonian and Liard method) was applied. In this case, the inverse-variance-weighted method was chosen to obtain OR and 95% CI of the pooled OR, using the inter-study variance.
Heterogeneity assessment and publications bias
Q-Cochrane statistic was computed to assess the heterogeneity between studies. We chose the I2 value as the reference to quantify it. For P values <0.05, we considered that we could not exclude a lack of homogeneity in the pool of study. Our reference for interpreting the I2 value was 0% to 30%: might not be important; 30% to 60%: may represent moderate heterogeneity; 60% to 75%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity; following the Cochrane recommendation (12).
We used funnel plots to investigate potential publication bias with 95% CIs. We followed the Stern and Egger’s method plotting the log OR on x-axis and standard error (SE) on y-axis. Interpretation of funnel plots was performed visually by searching for any asymmetry or suggestive lack of studies in any side around the null OR, which could indicate a publication bias.
Statistic computations were performed with Excel and R software. Display in forest plot and funnel plot were visually generated using the package “meta” in R.
Results
Study selection
After the first screening across databases and exclusion of duplicate studies, we identified 283 potential articles. Details of the selection of the studies are provided in Figure 1. Finally, we could pool 9 studies.
Among these 9 studies, 7 were retrospective (13-19) and 2 were prospective studies (20,21). All of these articles reported the details for post-operative mortality, morbidity and the rate of anastomotic complications. Caso et al. (15) and Veronesi et al. (22) reported no patient mortality in either group and so were excluded from computation for this parameter. In the same manner, Caso et al. (15) did not witness any anastomotic complications and the study was also excluded from the pool. Seven studies (13,15-20) presented the 5-year survival rate and were included in the pooled ratio. Details of parameters and design from individual studies can be found in Table 1.
Table 1
| First author, year of publication | Design | Surgery performed | No. of patients (SA/IT) | Mortality (SA/IT) | Morbidity (SA/IT) | Anastomosis complications (SA/IT) | Five-years survival (SA/IT) |
|---|---|---|---|---|---|---|---|
| Bagan, 2009 (13) | RCC | SL | 159 (74%/26%) | 2%/2% | 25%/21% | 3%/0% | 65%/74% |
| Burfeind, 2005 (14) | RCC | SL, BP | 73 (74%/26%) | 4%/0% | 35%/42% | 0%/5.2% | NR |
| Caso, 2018 (15) | RCC | SL, double SL | 15 (73%/27%) | 0%/0% | 38%/50% | 0%/0% | 80%/100% |
| Comacchio, 2019 (16) | RCC | SL | 159 (69%/31%) | 0%/2% | 27%/35% | 18%/18% | 36%/35% |
| Gonzalez, 2013 (17) | RCC | SL | 99 (72%/28%) | 3%/4% | 49%/54% | 3%/11% | 45%/28% |
| Koryllos, 2020 (18) | RCC | SL | 501 (73%/27%) | 2%/5% | 41%/47% | 3%/8% | 47%/34% |
| Milman, 2009 (19) | RCC | SL, SBL | 64 (67%/33%) | 5%/0% | 47%/43% | 4.7%/0% | 48%/41% |
| Gómez-Caro, 2012 (20) | PC | SL | 79 (67%/33%) | 6%/0% | 30%/35% | 2%/0% | 76%/33% |
| Veronesi, 2002 (22) | PC | SL, SBL | 55(51%/49%) | 0%/0% | 43%/37% | 0%/3.7% | NR |
Numbers of patients (%) in surgery group/numbers of patients (%) in induction group. BP, bronchoplasties; SA, sleeve lobectomy alone group; IT, sleeve lobectomy with induction therapy; RCC, retrospective clinical cohort; SL, sleeve lobectomy; NR, not reported; SBL, sleeve bilobectomy; PC, prospective cohort.
Population of interest
The pooled population was composed of 1,204 patients for a mean age of 60.3±5.2 years. Among them, 930 were male (77.2%). A total of 352 patients (29.2%) were included in the induction group. Of those, 187 patients (53.1%) received a combined radio-chemotherapy induction therapy, 161 patients (45.7%) received chemotherapy alone and 1 patient (0.28%) radiotherapy alone. The 3 remaining modalities were not reported (NR). All patients presented lung cancer except five patients who presented pulmonary metastasis from renal cell cancer, schwannoma, muco-epidermoid cancer, colon cancer metastasis and inflammatory myofibroblastic tumor. The pre-operative and pre-induction tumoral stage were reported for 1,138 patients with a majority of stage II (500 patients, 43.9%), followed by stage III (421 patients, 37.0%) and stage I (172 patients, 15.1%). Of the remaining patients, 36 presented a stage IV (3.2%) and 9 a stage 0 (0.8%). Patients’ characteristics are reported in Table 2.
Table 2
| First author, year of publication | No. of patients | Mean age (SD or range) | Male patients | Cancers | Stages (0/1/2/3/4) | Induction therapy (C/R/X) | Induction criteria |
|---|---|---|---|---|---|---|---|
| Bagan, 2009 (13) | 159 | 57 (±11) | 67% | NSCLC | –/30%/38%/30%/3% | 100%/–/– | N2, T3, T4, need for size reduction |
| Burfeind, 2005 (14) | 73 | 58 (11–78) | 60% | Any malignant etiology | –/33%/27%/25%/4% | 21%/–/79% | N2, hilar M1, T4, mediastinal invasion |
| Caso, 2018 (15) | 15 | 49 (±27) | 33% | Various tumors | 20%/33%/20%/20%/7% | 1C, others: NR | NR |
| Comacchio, 2019 (16) | 159 | 66 (36–84) | 79% | NSCLC | 2%/11%/73%/11%/3% | 76%/2%/22% | N2 |
| Gonzalez, 2013 (17) | 99 | 62 (29–83) | 76% | NSCLC | 3%/25%/37%/35%/– | 43%/–/57% | Potentially resecable T1–3N2M0 |
| Koryllos, 2020 (18) | 501 | 64 (NR) | 70% | Lung cancer | –/4%/42%/50%/4% | 30%/–/70% | N2, advanced mediastinal involvement |
| Milman, 2009 (19) | 64 | 60 (±10.6) | 73% | NSCLC | –/23%/27%/44%/6% | –/–/100% | NR |
| Gómez-Caro, 2012 (20) | 79 | 64 (38–83) | 89% | NSCLC | –/24%/53%/23%/– | –/–/100% | N2 |
| Veronesi, 2002 (22) | 55 | 63 (44–76) | 85% | Lung cancer | NR | 89%/–/11% | N2, T4, SCLC |
C/R/X, chemotherapy/radiotherapy/combined chemotherapy and radiotherapy. SD, standard deviation; NR, not reported; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer.
In-hospital mortality
Five studies (13,14,16,18,20) contributed to the pooled estimate for mortality (Figure 2). We excluded 2 studies that reported the 90-day mortality (17,19). Overall mortality rate ranges from 0% to 3.8%. The OR was equal to 1.80 with a 95% CI from 0.76 to 4.25 (P value =0.19). No lack of homogeneity was observed (I2=0%, P=0.499482) across the studies.
Morbidity
Nine studies (13-20,22) were pooled for the parameter of morbidity, ranging from 23.9% to 50.5% with an estimate OR of 1.17 (95% CI: 0.90–1.52, P value =0.237) (Figure 3). No lack of homogeneity was observed (I2=0%, P=0.960317) among the studies.
Anastomotic complications
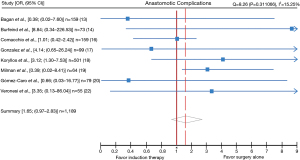
Eight studies (13,14,16-20,22) reported on anastomotic complications. The overall rate of anastomotic complications was 5.2% (63/1,204). The estimate OR was 1.65 (95% CI: 0.97–2.83, P value =0.06) (Figure 4). No lack of homogeneity was observed (I2=15.2%, P=0.311066) across studies. Anastomotic complications rates ranged between 0% and 18% in both groups. A total of 25 patients over 352 undergoing induction (7.1%) and 38 of the 852 patients in surgery alone group (4.4%) presented anastomotic complications. Bagan et al. (13) reported 3 cases of anastomotic complications (1 BPF and 2 anastomotic stenosis) (3%) in group surgery alone and none in the induction group. Burfeind et al. (14) observed only 1 case of BPF after induction therapy (5.2%). Comacchio et al. (16) reported 5 cases of BPF (4% in induction group vs. 2.7% in surgery alone), 9 cases of early anastomotic stenosis (4% in induction vs. 6.3% in surgery alone) and 15 cases of late anastomotic stenosis (10% in induction vs. 9% in surgery alone). Gómez-Caro et al. (20) presented only 1 case of BPF in the surgery alone group (1/53 vs. 0/26)). Gonzalez et al. (17) reported bronchial anastomosis complications occurred in 3 patients (10.8%) with neoadjuvant therapy (2 BPF and 1 stenosis) and in 2 without (2.8%) (1 BPF and 1 stenosis) (P=0.3). Koryllos et al. (18) observed a total of 21 anastomosis insufficiencies (8% induction vs. 2.7% surgery alone), described by the authors as necrosis or perforation of the bronchial wall. However, no detail over the nature of each of it was given. Milman et al. (19) reported 1 case of anastomotic stenosis, 1 case of broncho-pulmonary artery fistula after surgery alone (4.7%) and none in induction group. Finally, Veronesi et al. (22) observed 1 cases of late stenosis after induction therapy (3.7%). The estimated rate of BPF and early or late anastomotic stenosis (excluding the study of Koryllos due to missing informations) was 16/691 (2.3%) and 31/691 (4.5%) respectively.
Five-year survival
The 5-year survival was reported in seven studies (13,15-20) and varied from 20% to 76% in the surgery-only group and from 28% to 100% in the induction group. The pooled OR was 1.52 (95% CI: 1.15–2.00, P value =0.003) (Figure 5). No lack of homogeneity between studies have been observed (I2=51.6%, P=0.053813).
The funnel plots for the mortality, anastomosis complications and 5-year survival did not display any asymmetry. However, regarding the pool for morbidity, a suspicion of small studies or publication bias could not be excluded.
Discussion
Sleeve pulmonary lobectomy is preferred over pneumonectomy for centrally located NSCLCs, provided that a complete resection can be achieved. Several studies have shown that SL presents similar survival and recurrence rates for adjusted tumor stage and is associated with lower post-operative mortality and morbidity and better quality of life compared with pneumonectomy (1-4,6). However, recurrences remain frequent in sleeve resection procedures and neo-adjuvant radio-chemotherapy may be proposed to reduce the size of the tumor and thus enhancing chances for a complete resection and decreasing distant or local relapse rates. Conversely, induction therapy has been associated with major complications if followed by pneumonectomy (21). Pre-operative chemotherapy allows treatment by lobectomy in otherwise non-resectable lesions and patients unable to tolerate a pneumonectomy (23). However, sleeve resections feasibility after induction therapy is often questioned because the procedure requires a reliable anastomosis (24-27).
Our meta-analysis pools the results of 1,204 patients who underwent pulmonary sleeve resection with (n=352) and without induction treatment (n=852) offering an analysis of the main post-operative outcomes.
After SL, reported postoperative mortality and morbidity rates range between 2–5% and 15–47%, respectively, even if SL is combined with additional surgical procedures such as pulmonary artery, superior vena cava, or chest wall resection or performed in elderly patients for whom a lung-sparing procedure is associated with lower mortality (10,14,28,29). Mortality is often comparable between patients with or without radio-chemotherapy. In our review, all of the studies showed a comparable mortality ranging from 0% to 5.5% for the induction group and 0% to 4.7% in the surgery alone group. With an OR of 1.80, a 95% CI from 0.76 to 4.25 and corresponding P value of 0.19, we found that it was not significantly different between these two groups. In our study, mortality represents respectively 3.3% (9/272) of patients in the induction group, and 1.86% (13/699) of patients in surgery group. In either case, these rates are acceptable and comparable to the ones found in the literature.
Occurrences of general morbidities after the intervention also seem comparable between induction patients and surgery alone patients. No significant difference occurred in individual studies included in this meta-analysis. Morbidity rate ranged from 21.4% to 54% in induction group and 27% to 49% in surgery alone group. The pooled results evidenced an OR of 1.17, a 95% CI between 0.90 and 1.52 and a P value of 0.237. Reported SL or bilobectomy complication rates were 32.9 and 45.8%, respectively (30). Pooled rates from our meta-analysis are evaluated to 36.8% in the surgery group (314/852) and 40.6% (143/352) in the induction and surgery group. Again, morbidity rates were not significantly increased by induction therapy. However, induction treatment, especially radio-chemotherapy, has been the subject of controversy if applied in the context of SL. Induction-related injuries of the bronchial microvascularization may potentially predispose to airway complications, such as bronchopleural or bronchovascular fistula and bronchial stenosis. Bronchovascular fistulas may occur occasionally, but BPFs are more common, with a reported incidence of 1% to 5% after SL (1,7,10,28,29). The reported incidence of stenosis at the site of bronchial anastomosis ranges between 1% and 4% (10,28,29,31). These complications may result from inappropriate surgical procedures, such as mechanical tension at the level of the anastomosis or bronchial devascularization during dissection, or from nutritional deficiencies or advanced tumor stage (32). Induction therapy may additionally compromise bronchial healing as a result of radio-chemotherapy-induced microvascular injuries and desmoplastic tissue reactions.
Anastomotic complications rates derived from our meta-analysis ranged between 0% and 18% in induction group as in surgery alone group. We observed almost statistical differences (P=0.06) between the two groups with increased anastomotic complication rate in the induction therapy group. Risk factors inducing worse outcomes at the site of the anastomosis have been described by several studies. The main one after induction therapy is the impact of radiotherapy alone on bronchial tissue. As shown by Yamamoto et al. (9), radiation seems to negatively impact healing capacity of the tissue. Interestingly, these authors observed that whereas chemotherapy seems to have a little effect on blood flow, radiations significantly worsen healing potential of the surrounding tissue. In our meta-analysis, Comacchio et al. (16) and Koryllos et al. (18) supported the view that radiotherapy bears a potential harmful effect in the context of sleeve resection. Comacchio et al. pointed out mediastinal radiotherapy as a risk factor for bronchial complications. They observed a statistically significant difference in terms of anastomotic complication rate between patients undergoing induction chemotherapy and patients undergoing pre-operative chemo-radiotherapy or radiotherapy alone (10.8% vs. 41.6%, respectively, P=0.01), indicating that the radiotherapeutic component might be one of the causes behind this difference. Similar findings were reported by Koryllos who reported equivalent rates of anastomotic complications after no pretreatment and chemotherapy (2.7% and 2.4%, respectively), but increased rates of anastomotic complications after radiotherapy (10.4%) (P=0.002). Rea et al. (10) also found an increased incidence of BPFs after radiotherapy. More recently, Rodriguez et al. (33) investigated the impact of chemoradiotherapy compared to surgery or surgery with neoadjuvant chemotherapy in sleeve patients. They observed that neoadjuvant chemoradiations increased pulmonary airways complications. In line with these observations, some authors proposed to reconsider the pertinence of pre-operative radiotherapy since it does not result in survival differences when compared to post-operative radiotherapy alone (34) and it seems to increase the rate of anastomotic complications. Conversely, some studies speak in favor of induction therapy: pre-operative chemo-radiotherapy allows to avoid a positive resection margin, which has been identified as a predictive factor of anastomotic complications (10,35). Induction radiotherapy should be cautiously discussed when considering a SL due to the increased rate of anastomotic complications.
Anastomotic complications have often been reported to be manageable with bronchoscopy and successfully treated. The rate of BPF and early or late anastomotic stenosis was 2.3% and 4.5%, respectively. We observed a total of 12 re-operations (14-18,20), with four cases of BPFs, one case of persistent air leak, one case of anastomotic stenosis and one case of broncho-arterial fistula. The remaining re-operations were performed by Koryllos et al. (18) with no detail over the indication. Gonzalez et al. (17) and Koryllos et al. (18) performed a sleeve bilobectomy (SBL) and a middle lobe and intermediate bronchus resection respectively, with new anastomosis in order to avoid a pneumonectomy. Again, the main management of anastomotic complications remained bronchoscopy with a total of 34 bronchoscopies and led to the resolution of the insufficiency, excepted in one case that needed a completion pneumonectomy (16). In addition, 16 complications required a stenting procedure. It is noteworthy that Koryllos et al. did not perform bronchoscopy, but successfully managed 16 anastomosis insufficiencies (of 21) by intensifying antibiotics (18). Among all complications, two were fatal. The patient who presented a broncho-arterial fistula reported by Milman et al. (19) died after the completion by pneumonectomy, while a fatal BPF was reported by Comacchio et al. (16). Each one occurred early in the post-operative care.
The other point of frequent discussion is the need to cover the bronchial anastomosis. Several techniques have been developed for this purpose, including the intercostal pedicle flap, the pleural and pericardial flaps, the pericardial fat pad flap, the pedicled pericardiophrenic graft, and the omentum. This point was not analyzed in our meta-analysis. However, most authors recommend routine coverage of bronchial anastomosis of post-inductive sleeve resections. On the other hand, Storelli et al. (31) have reported on 25 patients undergoing SL after induction therapy without bronchial complications, despite the fact that no anastomosis wrapping had been performed.
Our review reported a significant difference of survival between surgery and induction groups. With a pooled OR of 1.52, surgery alone seems to favor the 5-year survival rate as compared to induction followed by surgery (P value =0.003). Indeed, when induction is associated with sleeve lobectomies, studies show similar survival (19), or even worse survival in the induction group compared to surgery alone group (20). Moreover, reduced function of remaining lungs has been observed in context of induction therapy because of its chronic effects. This impacts long-term outcomes (36), even when one considers factors enhancing long-term survival linked to induction therapy, such as a negative resection margin (37) or mediastinal downstaging to N0 (16,37). In addition, decreased 5-year survival can also be explained by the imbalance in tumor staging between induction group or surgery alone group (18), since the induction group will pool patients with more advanced disease, thus poorer prognosis.
There are several limitations to our meta-analysis. First, the design of the included studies might have induced a selection bias. Also, the studies did not display the rate of avoided pneumectomies notably avoided cases by benefiting from induction therapy. In addition, difference in outcomes between upfront pneumonectomy and SL with induction therapy (IT) was not assessed. Then, the heterogeneity of information reported in the studies did not allow us to constitute a solid pool of patients undergoing radiotherapy alone and fully investigate its specific effects. Thus, our conclusions on anastomosis complications should be interpreted with caution, due to the limited number of patients included. In the same spirit, no distinction was made between the different modalities of induction therapy. Survival analysis should be cautiously interpreted due to the absence of data regarding the individual oncological status such as tumor stage, including tumor size and lymph nodal metastasis, involvement of hilar structures. Finally, and in addition to the compulsory limitations of an aggregated meta-analysis, we could only pool a small number of studies with only two reporting data at the patient level, which limited the inference that could be made from our statistical analysis. Consequently, the results of this meta-analysis did not provide the level of risk for an individual patient, and the aim of this study was restricted to observation of outcomes after exposition.
In conclusion, SL for NSCLC can be safely performed after induction chemotherapy and/or radiochemotherapy, with similar mortality and morbidity but with an increased risk of airway complications especially after induction radiotherapy and poorer survival prognosis at 5 years.
Acknowledgments
We would like to specially thank Matthieu Zellweger for his work which has been an important contribution to the article.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-939
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-939). MG serves as an unpaid editorial board member of Journal of Thoracic Disease from Feb 2021 to Jan 2023. JYP reports grants from Chercher & Trouver, Oncoswiss and Novartis. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Terzi A, Lonardoni A, Falezza G, et al. Sleeve lobectomy for non-small cell lung cancer and carcinoids: results in 160 cases. Eur J Cardiothorac Surg 2002;21:888-93. [Crossref] [PubMed]
- Park JS, Yang HC, Kim HK, et al. Sleeve lobectomy as an alternative procedure to pneumonectomy for non-small cell lung cancer. J Thorac Oncol 2010;5:517-20. [Crossref] [PubMed]
- Li Z, Chen W, Xia M, et al. Sleeve lobectomy compared with pneumonectomy for operable centrally located non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 2019;8:775-86. [Crossref] [PubMed]
- Stallard J, Loberg A, Dunning J, et al. Is a sleeve lobectomy significantly better than a pneumonectomy? Interact Cardiovasc Thorac Surg 2010;11:660-6. [Crossref] [PubMed]
- Ma Z, Dong A, Fan J, et al. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20-8. [Crossref] [PubMed]
- Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: optimal management strategy using decision analysis techniques. Ann Thorac Surg 2003;76:1782-8. [Crossref] [PubMed]
- Tronc F, Grégoire J, Rouleau J, et al. Long-term results of sleeve lobectomy for lung cancer. Eur J Cardiothorac Surg 2000;17:550-6. [Crossref] [PubMed]
- Perentes JY, Zellweger M, Gonzalez M. Is pneumonectomy still necessary? J Thorac Dis 2018;10:6414-7. [Crossref] [PubMed]
- Yamamoto R, Tada H, Kishi A, et al. Effects of preoperative chemotherapy and radiation therapy on human bronchial blood flow. J Thorac Cardiovasc Surg 2000;119:939-45. [Crossref] [PubMed]
- Rea F, Marulli G, Schiavon M, et al. A quarter of a century experience with sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2008;34:488-92; discussion 492. [Crossref] [PubMed]
- Robins J, Greenland S, Breslow NE. A general estimator for the variance of the Mantel-Haenszel odds ratio. Am J Epidemiol 1986;124:719-23. [Crossref] [PubMed]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester: John Wiley & Sons, 2019.
- Bagan P, Berna P, Brian E, et al. Induction chemotherapy before sleeve lobectomy for lung cancer: immediate and long-term results. Ann Thorac Surg 2009;88:1732-5. [Crossref] [PubMed]
- Burfeind WR Jr, D'Amico TA, Toloza EM, et al. Low morbidity and mortality for bronchoplastic procedures with and without induction therapy. Ann Thorac Surg 2005;80:418-21; discussion 422. [Crossref] [PubMed]
- Caso R, Watson TJ, Khaitan PG, et al. Outcomes of minimally invasive sleeve resection. J Thorac Dis 2018;10:6653-9. [Crossref] [PubMed]
- Comacchio GM, Schiavon M, Azzolina D, et al. Does Induction Therapy Increase Anastomotic Complications in Bronchial Sleeve Resections? World J Surg 2019;43:1385-92. [Crossref] [PubMed]
- Gonzalez M, Litzistorf Y, Krueger T, et al. Impact of induction therapy on airway complications after sleeve lobectomy for lung cancer. Ann Thorac Surg 2013;96:247-52. [Crossref] [PubMed]
- Koryllos A, Lopez-Pastorini A, Zalepugas D, et al. Bronchus Anastomosis Healing Depending on Type of Neoadjuvant Therapy. Ann Thorac Surg 2020;109:879-86. [Crossref] [PubMed]
- Milman S, Kim AW, Warren WH, et al. The incidence of perioperative anastomotic complications after sleeve lobectomy is not increased after neoadjuvant chemoradiotherapy. Ann Thorac Surg 2009;88:945-50; discussion 950-1. [Crossref] [PubMed]
- Gómez-Caro A, Boada M, Reguart N, et al. Sleeve lobectomy after induction chemoradiotherapy. Eur J Cardiothorac Surg 2012;41:1052-8. [Crossref] [PubMed]
- Thomas M, Rübe C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol 2008;9:636-48. [Crossref] [PubMed]
- Veronesi G, Solli PG, Leo F, et al. Low morbidity of bronchoplastic procedures after chemotherapy for lung cancer. Lung Cancer 2002;36:91-7. [Crossref] [PubMed]
- Bagan P, Berna P, Pereira JC, et al. Sleeve lobectomy versus pneumonectomy: tumor characteristics and comparative analysis of feasibility and results. Ann Thorac Surg 2005;80:2046-50. [Crossref] [PubMed]
- Toyooka S, Soh J, Yamamoto H, et al. Extended sleeve lobectomy after induction chemoradiotherapy for non-small cell lung cancer. Surg Today 2015;45:1121-6. [Crossref] [PubMed]
- D'Andrilli A, Venuta F, Maurizi G, et al. Bronchial and arterial sleeve resection after induction therapy for lung cancer. Thorac Surg Clin 2014;24:411-21. [Crossref] [PubMed]
- Cusumano G, Marra A, Lococo F, et al. Is sleeve lobectomy comparable in terms of short- and long-term results with pneumonectomy after induction therapy? A multicenter analysis. Ann Thorac Surg 2014;98:975-83. [Crossref] [PubMed]
- Ohta M, Sawabata N, Maeda H, et al. Efficacy and safety of tracheobronchoplasty after induction therapy for locally advanced lung cancer. J Thorac Cardiovasc Surg 2003;125:96-100. [Crossref] [PubMed]
- Yildizeli B, Fadel E, Mussot S, et al. Morbidity, mortality, and long-term survival after sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;31:95-102. [Crossref] [PubMed]
- Fadel E, Yildizeli B, Chapelier AR, et al. Sleeve lobectomy for bronchogenic cancers: factors affecting survival. Ann Thorac Surg 2002;74:851-8; discussion 858-9. [Crossref] [PubMed]
- Thomas PA, Falcoz PE, Bernard A, et al. Bilobectomy for lung cancer: contemporary national early morbidity and mortality outcomes. Eur J Cardiothorac Surg 2016;49:e38-43; discussion e43. [Crossref] [PubMed]
- Storelli E, Tutic M, Kestenholz P, et al. Sleeve resections with unprotected bronchial anastomoses are safe even after neoadjuvant therapy. Eur J Cardiothorac Surg 2012;42:77-81. [Crossref] [PubMed]
- Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites or recurrences. Ann Thorac Surg 2004;77:1152-6; discussion 1156. [Crossref] [PubMed]
- Rodriguez M, Dezube AR, Bravo-Iniguez CE, et al. Impact of Neoadjuvant Chemoradiation on Adverse Events After Bronchial Sleeve Resection. Ann Thorac Surg 2021;112:890-6. [Crossref] [PubMed]
- Shah AA, Berry MF, Tzao C, et al. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg 2012;93:1807-12. [Crossref] [PubMed]
- Yatsuyanagi E, Hirata S, Yamazaki K, et al. Anastomotic complications after bronchoplastic procedures for nonsmall cell lung cancer. Ann Thorac Surg 2000;70:396-400. [Crossref] [PubMed]
- Nomori H, Shiraishi A, Cong Y, et al. Impact of induction chemoradiotherapy on pulmonary function after lobectomy for lung cancer. J Thorac Cardiovasc Surg 2018;155:2129-2137.e1. [Crossref] [PubMed]
- Shapiro M, Swanson SJ, Wright CD, et al. Predictors of major morbidity and mortality after pneumonectomy utilizing the Society for Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2010;90:927-34; discussion 934-5. [Crossref] [PubMed]