Chronic outpatient management of patients with a left ventricular assist device
Introduction
The use of mechanical circulatory support (MCS) as treatment for advanced heart failure (HF) has grown exponentially over the past 15 years. The continuous flow left ventricular assist device (CF-LVAD) has become the most used form of MCS in advanced HF, especially since approval of use as destination therapy (DT) (1) and with the lack of organ availability. Long-term survival has improved and diligent outpatient management is thus particularly critical to achieve optimal outcomes. Most current guidelines for outpatient care are based on single randomized or non-randomized studies or expert opinion (levels of evidence B and C) (2). Nonetheless, these recommendations are important in optimizing survival, outcomes, and quality of life for patients. This review will discuss important outpatient management strategies for patients with HF and a left ventricular assist device (LVAD).
Heart failure (HF) management
It is important to remember that the LVAD is placed as part of the treatment for HF. The LVAD does not cure HF and only supports the left ventricle. Therefore, HF can and will still occur after LVAD implant and should be managed in accordance with the most recent guidelines for MCS care and the American College of Cardiology Foundation/American Heart Association guidelines for Heart Failure (2,3). This includes the use of angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), beta blockers, and aldosterone inhibitors to counteract the neurohormonal upregulation that occurs in HF and is known to cause unfavorable myocardial remodeling. Additionally, diuretics should be used for volume management and vasodilators (hydralazine and nitrate combination) for further afterload reduction and in the setting of elevated pulmonary vascular resistance, as needed. A caveat is that a patient may not tolerate the same HF medical therapy that was tolerated pre-LVAD implant, and the technical challenges associated with accurate measurement of blood pressure (BP) can make provision of the conventional constellation of neurohormonal blockade somewhat more difficult. Therefore, medications should be instituted and adjusted carefully based on symptoms and tolerance.
From a HF perspective, caring for a HF patient with an LVAD is similar to caring for a HF patient without an LVAD. The clinical exam findings of HF include elevated jugular veins, lower extremity edema, increased shortness of breath, orthopnea, paroxysmal nocturnal dyspnea, abdominal fullness, nausea, and early satiety, to name a few. A comprehensive cardiac exam, discussion of HF symptoms and functional status and review of pump parameters and alarm history should occur at each outpatient visit. LVAD parameters that may indicate right ventricular (RV) failure or HF decompensation include decreased pump flow and/or low pulsatility (4). A careful history and assessment may identify subtle changes that are indicative of an impending HF decompensation. Immediate action on the part of the provider may prevent a hospital admission, similar to a HF patient without an LVAD. Weight and pump parameter monitoring, following a low sodium diet, and symptom reporting are important home surveillance strategies that should be included in patient education. It is also typical for patients to continue to need loop diuretics after LVAD implant, although the dose is often much lower. These strategies are helpful in identifying potential LVAD issues or HF decompensation early allowing for immediate intervention.
Device management
Strategies to set pump speed are aimed at optimizing cardiac output and pump flows without causing RV failure, left ventricular (LV) suction events, or aortic insufficiency (AI) from fusion of leaflets (5). The initial speed at the time of implant is often different than the speed required after the patient’s status has changed following rehabilitation. Furthermore, the optimal fixed speed may change as the duration of LVAD support increases. Long-term variables such as RV function, AI, and ventricular reverse remodeling will affect the optimal pump speed. Thus, evaluation of whether the current pump speed is still optimal should occur at most visits.
Echocardiogram is an important imaging tool used to guide speed optimization and evaluate LVAD function. It should be performed at routine intervals after implant to assess for appropriate pump function, optimal LV decompression, presence of/severity of AI, and RV function (2,4). The 2013 International Society of Heart and Lung Transplantation (ISHLT) guidelines for MCS recommend an echo every 6 months for the first 2 years after LVAD implant and then annually (2). An echocardiogram may be helpful in the setting of a change in clinical status or symptoms, such as with low output symptoms, cardiac arrhythmias, or abnormal device parameters indicating suboptimal pump function. The images may help guide testing and treatment decisions.
When reviewing an echocardiogram, it is important to note the size of the LV to assess for appropriate decompression while avoiding over-decompression, which puts strain on the RV and may result in suction events. It is helpful to note the position of the septum, as a rightward shift indicates suboptimal LV decompression or fluid overload and a leftward shift suggests LV over-decompression or RV failure. Either situation may suggest a speed adjustment. Visualization of the inflow and outflow cannulas should include Doppler interrogation to assess for any turbulent or accelerated flow, which could suggest thrombus either within the pump or in the inflow or outflow cannula.
Right heart catheterization (RHC) is an important diagnostic tool in the outpatient setting that is used to guide management, much like prior to implant. Per the 2013 ISHLT MCS guidelines, RHC should be done post implant in the setting of persistent or recurrent HF symptoms after MCS placement. It is also recommended at routine intervals for patients who are listed for heart transplant to monitor pulmonary artery pressures, as pulmonary hypertension (HTN) can lead to early graft dysfunction or failure following heart transplantation (2,6). As previously noted, patients with LVAD support still have issues with HF. Therefore, there is the potential for frequent medication adjustments to improve volume status and afterload reduction in the outpatient setting. Oftentimes these efforts are successful, but there are instances when diuresis and afterload reduction are not successful in removing volume and improving symptoms, or efforts may result in renal dysfunction or worsening symptoms. In this situation, or when the etiology of the patient’s symptoms are not clear, a RHC should be considered.
Hypertension (HTN)
HTN is a risk factor for complications and poor outcomes in the setting of a CF-LVAD (2,4). The CF-LVAD is an afterload sensitive device, and increased afterload will result in decreased flow through the pump. Decreased pump flow will contribute to low cardiac output and symptoms of HF and is thought to increase the risk of device thrombus due to a relative increase in blood stasis with the pump. Furthermore, HTN may contribute to the development and progression of AI, vascular disease, and ischemia-related ventricular arrhythmias (VA). HTN is a well-known risk factor for ischemic and non-traumatic hemorrhagic stroke in an individual without an LVAD. In a patient with a CF-LVAD who is anticoagulated and has HTN, the risk of hemorrhagic stroke is even more significant, with an incidence of 0.05-0.09 events per patient year (6-8) and is one of the leading causes of death after implant (9).
HTN management in the outpatient setting is imperative in order to decrease adverse events and complications in patients with a CF-LVAD. The most recent ISHLT guidelines for MCS recommend a goal mean arterial pressure (MAP)
Accurate noninvasive BP monitoring is challenging in patients with CF-LVAD. CF-LVADs produce low pulsatile flow, which results in lower systolic BP and pulse pressure. The lack of pulsatility results in difficulty assessing BP with a traditional BP cuff. The gold standard for BP management in the setting of a CF-LVAD is an invasive arterial line (2,10). This is not feasible in the outpatient setting, and studies have shown that automated BP cuffs are only successful 50% of the time in obtaining a BP reading, due to the reduced pulsatility and pulse pressure with a CF-LVAD (11). Since HTN management is such an important aspect of long term LVAD management, investigations into the use of Doppler measurement of BP with a sphygmomanometer as well as newer cuff technologies have been described. Separate investigations have demonstrated that Doppler BP measurement most closely reflects arterial line systolic BP measurements, but may closely represent the MAP in situations of low pulse pressure (12,13). The Terumo Elemano BP monitor uses a slow-cuff deflation technology that has been shown to correlate with arterial line measurements. The advantage to this device is that is require less technical expertise in obtaining a BP than the use of Doppler measurement (12,13). It is important to determine the most accurate method of BP measurement in each patient based on the amount of pulsatility and pulse pressure that the patient has with CF-LVAD support and to target a MAP
Arrhythmias
Both atrial arrhythmias (AA) and VA are common post CF-LVAD placement with a 20% incidence of AA and 30% incidence of VA (14,15). AA are most common in the first 60 days after implant due to hemodynamic changes and the inflammatory response within the myocardium secondary to the surgical procedure, but late AA may also occur. AA have not been shown to impact survival post LVAD, but have been associated with decreased functional status and quality of life, and has been suggested to increase risk of HF hospitalization and mortality (14,16). VA are common after LVAD. This is hypothesized to be due to the apical scarring around the inflow cannula and in the event of suction events due to over-decompression of the LV. Therefore, the presence of an LVAD does not decrease the risk of VA burden. In some instances, it may increase the burden (17).
In the outpatient setting, it is important to assess for signs and symptoms of arrhythmias. AA may manifest as decreased activity tolerance, worsening fatigue, volume retention, or increased dyspnea; essentially symptoms of decompensated HF. The LVAD parameters may reveal a decrease in pulsatility or flow. In a patient with a history of AA, it is important to keep AA on the list of differentials when a patient presents with new or worsening symptoms or a change in LVAD parameters. VA often present as lightheaded/dizzy episodes, syncope, presyncope, or intermittent palpitations and often result in decreased pulsatility on the LVAD parameters. In both AA and VA, the duration of low pulsatility may vary, depending on the duration of the arrhythmia. Useful tools in diagnosing AA or VA in a patient with an LVAD are careful history and physical exam, assessment of LVAD parameters and history, 12-lead electrocardiogram, and interrogation of the internal cardioverter defibrillator (ICD). In the presence of an LVAD, arrhythmias are often tolerated by the patient due to the presence of LV unloading and less work requirement of the native LV. Prolonged ventricular fibrillation has even been reported (18). However, in the setting RV dysfunction, AA or VA may not be as well tolerated (14,15,17).
Due to evidence that the presence of an LVAD improves tolerance to VA, the utility of ICD post LVAD implant has been explored in the literature. The current ISHLT guidelines recommend reactivating the ICD post LVAD implant in patients who had an ICD in place prior to implant. The guidelines suggest considering ICD placement in patients who did not have an ICD prior to implant (2). Other investigators have demonstrated that VA are not associated with a worse prognosis, VA in the setting of an LVAD does not result in sudden cardiac death, ICD therapy is not associated with long-term survival benefit after CF-LVAD, and patients may experience inappropriate shocks from the ICD (15,17,19,20). This evolving area of knowledge should be considered within the context of the individual patient and the current guidelines.
Aortic insufficiency (AI)
The impact of LVAD on the aortic valve (AV) was noted long before the continuous flow era (21) and was recently reviewed (22). AI is a common complication after prolonged LVAD support and is a result of the AV adapting to the continuous flow physiology of the LVAD and lack of systemic pulsatility that the AV is accustomed to. This can result in AV and annulus remodeling and subsequent regurgitation. The development and progression of AI should be monitored through routine echocardiography (2,5,23).
AI causes an ineffective circulatory loop as a portion of the LVAD flow moves through the AV into the LV and then regurgitates back through the device. This results in inefficient forward flow into the systemic circulation and can result in inadequate LVAD flows, poor perfusion of end organs, and increased LV diastolic pressures (23). The long term prognosis and rate of progression of AI is not entirely clear (23) and is still being studied, but it is clear that significant AI can lead to negative hemodynamic effects and patient symptoms. Therefore, it is important to minimize the amount of AI by assuring optimal pump speed and optimizing BP management in the outpatient setting.
In order to minimize AI, the speed of the LVAD should balance adequate unloading of the LV, intermittent AV opening, and maintaining a midline septum without shift. HTN management is also critical in minimizing AI. HTN increases the transvalvular gradient, which leads to structural changes in the AV that worsen AI. HTN management will assist in controlling the degree of AI (2,23), and principles for BP management that were discussed earlier should be followed.
While there are no defined treatment algorithms for AI following LVAD placement, surgical intervention may be considered when AI is severe, refractory to medical or device management, and is causing symptoms (23). Surgical options include replacement of the AV with a bioprosthetic valve, AV repair, AV closure, or percutaneous AV closure or replacement. All of these options carry procedural and operative risks as well as the risk of AI recurrence and should be discussed thoroughly with the patient, cardiologist, and surgeon (23,24).
Anticoagulation and managing complications
Anticoagulation and antiplatelet therapy is administered after LVAD due to the potential for device thrombus formation and embolic events. Patients require long-term anticoagulation with warfarin with a goal international normalized ratio (INR) targeted for the LVAD and patient comorbidities. Additionally, antiplatelet therapy is indicated, most commonly in the form of aspirin, with the dose targeted to the specific LVAD (2,6). Other antiplatelet agents (clopidogrel, dipyridamole) may be considered in the event of a true aspirin allergy or if dual antiplatelet therapy is indicated. The newer anticoagulants (i.e., rivaroxaban, dabigatran) are not recommended in place of warfarin due to the inability to reverse these agents in the setting of a significant bleeding event, at the time of cardiac transplantation, or other emergent surgical procedure.
Bleeding complications are an obvious potential complication in the setting of chronic anticoagulation and antiplatelet therapy with long term LVAD support. In major LVAD trials, there was a 40-80% chance of bleeding requiring transfusion following LVAD and 15-30% chance of bleeding requiring surgery (6,25). There are a few hypothesis as to why this increased risk of bleeding occurs. First, acquired von Willebrand syndrome with a reduction in high molecular weight von Willebrand factor multimers occurs early after LVAD implant. There are also changes in the coagulation system that are induced by the LVAD, including reduced levels of factor XI and XII, both of which play an important role in normal hemostasis (5,26). Additionally, the lack of pulsatility of the CF-LVAD and increased shear stress on the vasculature are felt to also contribute to bleeding risk (4).
The most common site of bleeding is in the gastrointestinal (GI) tract (2,6,26). Studies have demonstrated up to a 40% incidence of GI bleeding following LVAD implant (26). Bleeding can occur anywhere in the GI tract and tends to be recurrent. Arteriovenous malformations are an angiodysplasia that occur as a result of the nonpulsatile LVAD and are often the culprit sites of bleeding found with endoscopy. Unfortunately, the source of bleeding in the GI tract is often not able to be identified, and the etiology is attributed to the acquired bleeding diatheses that are well documented in this population (2,5,26).
Balancing the simultaneous risks of bleeding and thrombosis pose a particular challenge in CF-VAD patients (27). This is especially true when approaching anticoagulation in patients with post implant bleeding complications. LVAD programs often follow a system that involves discontinuing anticoagulation and transfusing with blood if hemoglobin levels are critical. In some cases, reversing anticoagulation is necessary. It is important to identify and treat the area of bleeding, if possible. Once the source is identified and treated, blood has been replaced, hemostasis and hemodynamic stability are achieved, anticoagulation is often resumed on a case-by-case basis. The severity of the bleeding episode, number of recurrences, cause of bleeding, age of the patient, and risk of thrombosis should all be taken into consideration. Patients are often restarted on aspirin or warfarin alone and then are followed closely in the outpatient setting for recurrent bleeding, hemoglobin trends, and anticoagulation management. The second agent may or may not be added back at some point, depending on the individual’s clinical course. In some instances, the risk of recurrent bleeding and severity of previous episodes warrants discontinuing anticoagulation and antiplatelet therapy altogether. This decision should be made carefully, individually, and with much discussion with the patient and caregivers regarding the risks and benefits of stopping these medications. Outcomes following reduction or discontinuation of anticoagulation have been reported (28). The recent TRACE study suggests that patients for whom anticoagulation or antiplatelet agents have been stopped, have relatively low thrombotic event rates (29).
The development of hemolysis and subsequent pump thrombosis are potential complications that should be monitored and screened for in the outpatient setting. The exact mechanism of clinical hemolysis during MCS is not entirely understood. It is thought that the CF-LVADs may contribute to red blood cell fragmentation through increased shear stress associated with continuous flow physiology. Additionally, some have proposed that there are changes in host coagulation and immunity that may increase hemolysis risk (30-32).
Prevention of and screening for hemolysis has become of utmost importance in the last 2 years due to the increase in reporting of LVAD thrombosis and recent data suggesting that lactate dehydrogenase (LDH) is a sensitive and specific serum marker for hemolysis and early marker of thrombus deposition that should be followed in the outpatient setting (33-36). An LDH elevation of 2-2.5 times the upper limit of normal is considered to be suggestive of hemolysis (33,35). Other clinical signs of hemolysis include unexplained symptoms of HF, dark urine, elevated plasma-free hemoglobin, bilirubin, transaminases, and creatinine. These should be assessed at each outpatient visit. LVAD parameters should be monitored on a daily basis by the patient and a review of the device history should occur at the clinic visit. If a slow trend increase in power consumption is noted or frequent spikes in power, device thrombus should be suspected and further investigation is warranted.
Imaging techniques that may be helpful in diagnosing hemolysis or suspected pump thrombus are chest x-ray or chest computerized tomography (CT) and ramp echocardiogram (37). X-ray or CT assesses inflow cannula positioning and possible mechanical etiology for hemolysis. Ramp echo involves assessing the amount of LV decompression with increasing VAD speed, although loading conditions are critical (38) and differ by type of pump (39). Suboptimal LV decompression raises further suspicion for thrombus in the pump, which prohibits adequate LV unloading (40) It is important to consider the entire clinical picture when evaluating for hemolysis and/or suspected device thrombosis and to act quickly if either condition is confirmed. Intensification of anticoagulation and/or antiplatelet therapy should be considered. This often includes hospital admission for intravenous anticoagulation therapy and serial monitoring of hemolysis markers. In the event that pump thrombus is confirmed and there is hemodynamic compromise, LVAD pump exchange should be considered. Recent publications have advocated for early pump exchange (35), but the entire clinical picture, patient comorbidities and surgical risk should be considered.
In order to prevent bleeding, hemolysis, or thrombotic complications after LVAD implant, precise anticoagulation and antiplatelet therapy management in warranted (2,4). Therapies should be tailored to each individual patient depending on device type, patient comorbidities, and previous history of bleeding, thrombotic events, and hemolysis. Figure 1 illustrates a potential protocol for addressing common challenges faced when following long-term anticoagulation in a patient with a CF-LVAD.
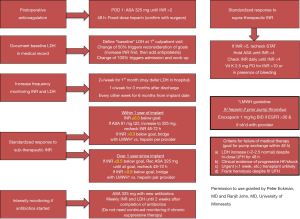
Chronic outpatient medical management of a patient with an LVAD is complex with the possibility of numerous confounding variables. The following table (Table 1) outlines common issues encountered and suggested assessment and management strategies to consider.
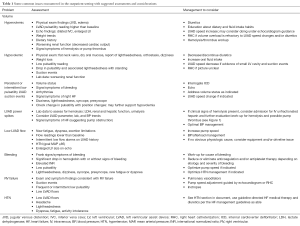
Full table
Driveline care and management
As long as the LVAD has direct communication from inside to outside the body, there remain challenges with driveline site care and maintenance of sealed surface at the exit site to prevent infection. Infection prevention begins prior to surgery with antibiotic prophylaxis and standard post-operative skin preparation. Recent data has also noted the advantage of “burying the velour” (41). Infection prevention continues throughout the life of the LVAD with appropriate wound care and close monitoring of the driveline exit site (DLES).
Driveline stabilization and support are essential to promote healing of the DLES post-operatively. Surgical interventions may be employed at the time of implant to assist with driveline stabilization and support. Securement during the early post-operative period is essential, as this allows for tissue ingrowth around the driveline, which prevents outside contamination entering the body. A pursestring suture or anchoring stitch may be employed at or below the exit site and should stay in place until the exit site is healed or until the patient is not able to tolerate the sutures (2,42,43). If the sutures are maintained over a period of time, the driveline should be assessed to assure the suture is not cutting through the outer coating due to repeated pressure or trauma.
External securement devices help stabilize the driveline over time after the anchoring sutures are removed. This can be achieved with the use of an abdominal binder or stabilization belt, which the patient is able to wash and wear. Adhesive stabilization systems such as Hollister tube holders, Centurion anchors, or Statlock device allow for more flexible support and securement of the driveline to the body and reduce trauma directly at the exit site (42,44). An adhesive system may last a week or more before needing to be changed, depending on the patient’s activity level and dermal properties. It is important to discuss and reinforce the long-term plan for driveline securement and support, as trauma to the exit site remains the leading predictor of DLES infections (2,42,45-47).
Dressing change frequency and techniques at the exit site vary among LVAD programs, and are usually completed by the support person or LVAD patient. The most common practice is for sterile technique and use of chlorhexidine gluconate (CHG) solution as the cleaning agent, with hydrogen peroxide or povidone-iodine as alternatives (2,42). Many centers continue to recommend daily dressing changes, although new practices utilizing CHG or silver impregnated dressings over the site are allowing some patients to decrease frequency to as little as once per week (2,42,44). One small, single-center study found comparable outcomes with two different approaches (48). Frequency may also be related to the center’s shower policy. In general, the DLES dressing should be changed after showering or if it becomes wet (42). In these cases, more frequent showering or activities resulting in excessive perspiration may necessitate more frequent dressing changes.
The ability of the patient and caregivers to clean and maintain the integrity of the skin around the DLES is imperative in promoting healing and preventing infection. Education regarding technique (oftentimes sterile) and skin cleaning protocol are crucial. Prior to hospital discharge after implant, great care should be taken to teach the patient and caregivers with the same supplies that will be available at home. Repetition is key, as applying sterile gloves and maintaining sterility during the procedure are habits that take multiple sessions to master. The person who will be doing the dressing change should be deemed competent in these skills prior to the patient being discharged from the hospital.
During outpatient visits, there are several aspects of the DLES and care process that should be assessed. The site itself should be assessed for evidence of healing and skin adhesion to the driveline, as well as ruling out any indication of infection or irritation to the site (2). Observation of the patient or support person completing the dressing change allows for opportunity to assess ongoing competence and reinforce any techniques as needed. The LVAD team should also review whether the patient has adequate dressing supplies at home and identify any barriers to obtaining supplies. Items such as sterile gloves or sterile drapes are not always covered by insurance, and patients may have financial difficulties obtaining the needed supplies. As more LVAD services move to fully inclusive dressing systems or kits, the difficulty in obtaining individual items may become less of an issue, although cost based on frequency of dressing changes may remain a factor.
Infection
LVAD infections, particularly those that originate at the DLES, remain the most commonly seen adverse event over time (1,45). The primary intervention for prevention of infection is meticulous care of the DLES. Chronic use of prophylactic antibiotics has not been shown to reduce the incidence of infection over time (43). Risk factors for infection include diabetes, obesity, and renal disease, all common co-morbidities in this population (45,49). One multicenter study also showed depression to be a statistically significant predictor of VAD infection, although it did not seem to affect survival (49). The surgical technique at time of implant can also impact long-term infection risk. Patients who had velour exposed on the external driveline had nearly 50% greater risk of DLES infection compared to those who had the velour portion completely covered in the subcutaneous tunnel (41). It is crucial to manage as many modifiable risk factors and comorbidities as able to prevent infection in this population.
Despite the best efforts in DLES management, driveline infections often occur over time. Early signs may include erythema, edema or pain at the exit site, and purulent drainage may be present. If the infection is isolated to the DLES alone, systemic signs such as fever, leukocytosis or elevated inflammatory markers may be absent (50). Routine assessment for suspected driveline infection include a complete blood count with differential, wound cultures if drainage is present, and blood cultures to assess for systemic involvement (2,43). Imaging should be considered to evaluate for the presence of abscess in the setting of copious drainage from the DLES. Ultrasonography or CT scan can be used to evaluate fluid collections around the driveline or pump, although artifact from the LVAD may cause difficulty in visualizing the area directly around the pump (43,45). Despite these imaging techniques, infections directly around the pump can be difficult to diagnose, as direct fluid collection for culture is not easily done without surgical intervention.
The most common pathogens in DLES infections are bacterial, with staphylococcus and pseudomonas species being predominant (42,45,49). Although fungal infections are less common, they are associated with much higher morbidity (45). Patients who develop driveline infections are seldom able to eradicate the infection while the device remains in place. Patients who develop bacteremia are at greater risk for recurrent bacteremia and have poorer outcomes (45). Involving an infectious disease specialist is helpful in long-term management of LVAD infections. Chronic suppressive antibiotic therapy may be required, with progression to parenteral therapy over time as resistant organisms may develop (2,46,51). Surgical interventions such as debridement, drainage, driveline revision, placement of antimicrobial beads in the pump pocket, or vacuum-assisted therapy may be considered in addition to antimicrobial therapy (45,50). Often, removal of the LVAD via heart transplantation or device exchange may be the only truly effective treatment, but is not without its own risks (45,46,49,50).
Education
Learning about driveline care is only one part of the LVAD experience that patients and their caregivers must master prior to discharge and maintain over time. They must also demonstrate competence with understanding the function and care of the VAD and associated equipment. Most centers provide written materials and DVDs for the patients to review (52). Patients also receive information on LVAD equipment care, post-operative care, and medications. The amount of information given during this time can be intimidating and overwhelming. In the outpatient setting, ongoing assessment of the patient and caregivers’ learning abilities, barriers to learning, and retention of information should occur (53). The education goes beyond learning about the equipment itself, and incorporates self-care skills and home surveillance strategies (2,52,53).
Widmar et al. showed that patient and caregivers rated emergency management, alarm troubleshooting, sterile technique and dressing changes as the most difficult tasks (52). These are all topics that can be reviewed frequently during early return visits based on patient and caregiver needs. Routine review of tasks should be incorporated in long-term follow-up (2). This is particularly important in the DT patients, as many continue on LVAD support for years before the need for emergency management or alarm troubleshooting skills arises. Verbal education with return demonstration of routine tasks is the most common way this review is completed (52). Local community health providers, such as emergency services, first responders and cardiac rehabilitation specialists should also receive some general education on LVAD equipment and potential medical concerns that are seen in the community (2).
Adjusting to a new way of living
Prior to discharge, time should be spent discussing how the patient and caregiver can prepare for returning home and developing a sense of normalcy. There are generally two main adjustment periods after discharge: early and late adjustment, as described by Casida et al. (54). In the early adjustment period, patients and caregivers must learn to adapt to basic functions of everyday living to accommodate the LVAD. This includes adjustments to habits related to sleep, hygiene and clothing; changes in the home environment; and psychosocial adjustments such as creating new routines, managing stress and potential changes in interpersonal relationships. Patients may also be concerned about being a physical or financial burden on their caregiver (53). Sexual function may be altered (55). Discussion with and support from the LVAD team during early clinic visits are essential as patients are working through this early adjustment period. Ongoing contact with a social worker, home health services, and LVAD support groups can supplement the medical team. In the late adjustment period, patients report more self-confidence in their proficiency with routines, abilities to care for themselves and a greater acceptance of the LVAD as part of them (54). Adjustment to home life can take weeks to months and is different for each patient.
While caregivers move through similar adjustment periods, their experiences are slightly different. Most caregivers have experience with caregiving and associated coping mechanisms developed during the patient’s decline with HF. During the early phase, caregivers often experience feelings of self-doubt and anxiety, as they feel responsible for a new set of health care needs (53,56,57). It can be beneficial for the LVAD team to talk to the caregiver separately from the patient, to gain a better sense of the concerns for themselves as well as the patient. It is important to remember that caregivers may have their own physical, emotional or medical concerns that can affect their ability to provide adequate support (58). As caregivers acclimate to living at home, they are often able to adapt to a sense of acceptance. Ongoing assessment of the caregiver at outpatient interactions is needed to assure that the caregivers have opportunities for respite, in order to avoid burnout (44,56,59,60). Although caregiving may remain a substantial part of their lives, most caregivers report they would “do it again”, as they recognize the patient may not have survived without the LVAD implant (56,57).
End of life
Any patient who has an LVAD as DT will have to face issues related to end of life. Conversations about advanced directives, palliative and end of life care should commence prior to LVAD implant, regardless of the intent of device placement (2). If the LVAD is being placed with the intent as DT, it is a Centers for Medicare and Medicaid Services requirement that palliative care conversations occur as part of the informed consent process (61). The palliative care team can help the patient and caregivers with preparedness planning to help determine that the patient’s goals of care at the time of implant and in the event of critical complications. This allows for understanding and documentation of the patient’s wishes prior to a crisis event (58,62,63).
End-of-life discussions and decisions can be complicated for patients, families, and the LVAD team. There are personal, cultural, religious, and ethical issues that must be taken into consideration (58,63). Resources outside the direct LVAD team including palliative care, ethics committees, social work, local support systems, and/or counseling services may be helpful. It is important to understand that discontinuation of LVAD therapy is ethically and legally permissible and is not considered a form of physician-assisted suicide or euthanasia (58,62,63) and may be in line with the patient’s goals of care. Thus, the importance of having these conversations early is essential.
If the decision is made to discontinue LVAD therapy, palliative and hospice services can be invaluable for symptom management as well as caregiver and family support. A protocol or checklist may help the team provide care and deactivation of the LVAD in a seamless manner (58), while decreasing the incidence of anxiety producing alarms from the LVAD. Support and education for hospice providers can help decrease the stress surrounding this event.
Conclusions
As the use of MSC has increased in the past 15 years for the treatment of advanced HF, patient survival and quality of life have improved. Consistent, comprehensive, multidisciplinary outpatient care contributes to positive outcomes. While further research is needed to solidify outpatient management guidelines, there are outpatient management strategies that have proven beneficial in this patient population. There are medical and psychosocial aspects to care that must be addressed long-term for successful outcomes and patient quality of life.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant 2014;33:555-64. [PubMed]
- Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157-87. [PubMed]
- Writing Committee Members, Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240-327. [PubMed]
- Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant 2010;29:S1-39. [PubMed]
- Cowger J, Romano MA, Stulak J, et al. Left ventricular assist device management in patients chronically supported for advanced heart failure. Curr Opin Cardiol 2011;26:149-54. [PubMed]
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [PubMed]
- Boyle AJ, Jorde UP, Sun B, et al. Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol 2014;63:880-8. [PubMed]
- Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012;125:3191-200. [PubMed]
- Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol 2009;54:312-21. [PubMed]
- Markham DW, Fu Q, Palmer MD, et al. Sympathetic neural and hemodynamic responses to upright tilt in patients with pulsatile and nonpulsatile left ventricular assist devices. Circ Heart Fail 2013;6:293-9. [PubMed]
- Bennett MK, Roberts CA, Dordunoo D, et al. Ideal methodology to assess systemic blood pressure in patients with continuous-flow left ventricular assist devices. J Heart Lung Transplant 2010;29:593-4. [PubMed]
- Lanier GM, Orlanes K, Hayashi Y, et al. Validity and reliability of a novel slow cuff-deflation system for noninvasive blood pressure monitoring in patients with continuous-flow left ventricular assist device. Circ Heart Fail 2013;6:1005-12. [PubMed]
- Wasson LT, Yuzefpolskaya M, Wakabayashi M, et al. Hypertension: an unstudied potential risk factor for adverse outcomes during continuous flow ventricular assist device support. Heart Fail Rev 2015;20:317-22. [PubMed]
- Brisco MA, Sundareswaran KS, Milano CA, et al. Incidence, risk, and consequences of atrial arrhythmias in patients with continuous-flow left ventricular assist devices. J Card Surg 2014;29:572-80. [PubMed]
- Enriquez AD, Calenda B, Miller MA, et al. The role of implantable cardioverter-defibrillators in patients with continuous flow left ventricular assist devices. Circ Arrhythm Electrophysiol 2013;6:668-74. [PubMed]
- Enriquez AD, Calenda B, Gandhi PU, et al. Clinical impact of atrial fibrillation in patients with the HeartMate II left ventricular assist device. J Am Coll Cardiol 2014;64:1883-90. [PubMed]
- Lee W, Tay A, Subbiah RN, et al. Impact of Implantable Cardioverter Defibrillators on Survival of Patients with Centrifugal Left Ventricular Assist Devices. Pacing Clin Electrophysiol 2015;38:925-33. [PubMed]
- Boilson BA, Durham LA, Park SJ. Ventricular fibrillation in an ambulatory patient supported by a left ventricular assist device: highlighting the ICD controversy. ASAIO J 2012;58:170-3. [PubMed]
- Garan AR, Yuzefpolskaya M, Colombo PC, et al. Ventricular arrhythmias and implantable cardioverter-defibrillator therapy in patients with continuous-flow left ventricular assist devices: need for primary prevention? J Am Coll Cardiol 2013;61:2542-50. [PubMed]
- Ambardekar AV, Allen LA, Lindenfeld J, et al. Implantable cardioverter-defibrillator shocks in patients with a left ventricular assist device. J Heart Lung Transplant 2010;29:771-6. [PubMed]
- Rose AG, Park SJ, Bank AJ, et al. Partial aortic valve fusion induced by left ventricular assist device. Ann Thorac Surg 2000;70:1270-4. [PubMed]
- John R, Mantz K, Eckman P, et al. Aortic valve pathophysiology during left ventricular assist device support. J Heart Lung Transplant 2010;29:1321-9. [PubMed]
- Holtz J, Teuteberg J. Management of aortic insufficiency in the continuous flow left ventricular assist device population. Curr Heart Fail Rep 2014;11:103-10. [PubMed]
- Adamson RM, Dembitsky WP, Baradarian S, et al. Aortic valve closure associated with HeartMate left ventricular device support: technical considerations and long-term results. J Heart Lung Transplant 2011;30:576-82. [PubMed]
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007;357:885-96. [PubMed]
- Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol 2010;56:1207-13. [PubMed]
- Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation 2012;125:3038-47. [PubMed]
- Whitson BA, Eckman P, Kamdar F, et al. Hemolysis, pump thrombus, and neurologic events in continuous-flow left ventricular assist device recipients. Ann Thorac Surg 2014;97:2097-103. [PubMed]
- Katz JN, Adamson RM, John R, et al. Safety of reduced anti-thrombotic strategies in HeartMate II patients: A one-year analysis of the US-TRACE Study. J Heart Lung Transplant 2015;34:1542-8. [PubMed]
- Heilmann C, Geisen U, Benk C, et al. Haemolysis in patients with ventricular assist devices: major differences between systems. Eur J Cardiothorac Surg 2009;36:580-4. [PubMed]
- Slaughter MS, Sobieski MA, Gallagher C, et al. Fibrinolytic activation during long-term support with the HeartMate II left ventricular assist device. ASAIO J 2008;54:115-9. [PubMed]
- Katz JN, Jensen BC, Chang PP, et al. A multicenter analysis of clinical hemolysis in patients supported with durable, long-term left ventricular assist device therapy. J Heart Lung Transplant 2015;34:701-9. [PubMed]
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33-40. [PubMed]
- Kirklin JK, Naftel DC, Kormos RL, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant 2014;33:12-22. [PubMed]
- Uriel N, Han J, Morrison KA, et al. Device thrombosis in HeartMate II continuous-flow left ventricular assist devices: a multifactorial phenomenon. J Heart Lung Transplant 2014;33:51-9. [PubMed]
- Shah P, Mehta VM, Cowger JA, et al. Diagnosis of hemolysis and device thrombosis with lactate dehydrogenase during left ventricular assist device support. J Heart Lung Transplant 2014;33:102-4. [PubMed]
- Uriel N, Morrison KA, Garan AR, et al. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol 2012;60:1764-75. [PubMed]
- Adatya S, Holley CT, Roy SS, et al. Echocardiographic Ramp test for continuous-flow left ventricular assist devices: do loading conditions matter? JACC Heart Fail 2015;3:291-9. [PubMed]
- Uriel N, Levin AP, Sayer GT, et al. Left Ventricular Decompression During Speed Optimization Ramps in Patients Supported by Continuous-Flow Left Ventricular Assist Devices: Device-Specific Performance Characteristics and Impact on Diagnostic Algorithms. J Card Fail 2015;21:785-91. [PubMed]
- Kato TS, Colombo PC, Nahumi N, et al. Value of serial echo-guided ramp studies in a patient with suspicion of device thrombosis after left ventricular assist device implantation. Echocardiography 2014;31:E5-9. [PubMed]
- Dean D, Kallel F, Ewald GA, et al. Reduction in driveline infection rates: Results from the HeartMate II Multicenter Driveline Silicone Skin Interface (SSI) Registry. J Heart Lung Transplant 2015;34:781-9. [PubMed]
- Cannon A, Elliott T, Ballew C, et al. Variability in infection control measures for the percutaneous lead among programs implanting long-term ventricular assist devices in the United States. Prog Transplant 2012;22:351-9. [PubMed]
- Stulak JM, Maltais S, Cowger J, et al. Prevention of percutaneous driveline infection after left ventricular assist device implantation: prophylactic antibiotics are not necessary. ASAIO J 2013;59:570-4. [PubMed]
- Baronetto A, Centofanti P, Attisani M, et al. A simple device to secure ventricular assist device driveline and prevent exit-site infection. Interact Cardiovasc Thorac Surg 2014;18:415-7. [PubMed]
- Adzic A, Patel SR, Maybaum S. Impact of adverse events on ventricular assist device outcomes. Curr Heart Fail Rep 2013;10:89-100. [PubMed]
- Nienaber JJ, Kusne S, Riaz T, et al. Clinical manifestations and management of left ventricular assist device-associated infections. Clin Infect Dis 2013;57:1438-48. [PubMed]
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc 2007;138:739-45, 747-60. [PubMed]
- Hozayen SM, Soliman AM, Eckman PM. Comparison of two ventricular assist device dressing change protocols. J Heart Lung Transplant 2012;31:108-9. [PubMed]
- Gordon RJ, Weinberg AD, Pagani FD, et al. Prospective, multicenter study of ventricular assist device infections. Circulation 2013;127:691-702. [PubMed]
- Koval CE, Rakita R; AST Infectious Diseases Community of Practice. Ventricular assist device related infections and solid organ transplantation. Am J Transplant 2013;13 Suppl 4:348-54. [PubMed]
- Nienaber J, Wilhelm MP, Sohail MR. Current concepts in the diagnosis and management of left ventricular assist device infections. Expert Rev Anti Infect Ther 2013;11:201-10. [PubMed]
- Widmar SB, Dietrich MS, Minnick AF. How self-care education in ventricular assist device programs is organized and provided: a national study. Heart Lung 2014;43:25-31. [PubMed]
- Kato N, Jaarsma T, Ben Gal T. Learning self-care after left ventricular assist device implantation. Curr Heart Fail Rep 2014;11:290-8. [PubMed]
- Casida JM, Marcuccilli L, Peters RM, et al. Lifestyle adjustments of adults with long-term implantable left ventricular assist devices: a phenomenologic inquiry. Heart Lung 2011;40:511-20. [PubMed]
- Eckman PM, Dhungel V, Mandras S, et al. Sexual function after left ventricular assist device. J Am Coll Cardiol 2013;61:2021-2. [PubMed]
- Kitko LA, Hupcey JE, Gilchrist JH, et al. Caring for a spouse with end-stage heart failure through implantation of a left ventricular assist device as destination therapy. Heart Lung 2013;42:195-201. [PubMed]
- Marcuccilli L, Casida JJ, Bakas T, et al. Family caregivers' inside perspectives: caring for an adult with a left ventricular assist device as a destination therapy. Prog Transplant 2014;24:332-40. [PubMed]
- Swetz KM, Ottenberg AL, Freeman MR, et al. Palliative care and end-of-life issues in patients treated with left ventricular assist devices as destination therapy. Curr Heart Fail Rep 2011;8:212-8. [PubMed]
- Baker K, Flattery M, Salyer J, et al. Caregiving for patients requiring left ventricular assistance device support. Heart Lung 2010;39:196-200. [PubMed]
- Egerod I, Overgaard D. Taking a back seat: support and self-preservation in close relatives of patients with left ventricular assist device. Eur J Cardiovasc Nurs 2012;11:380-7. [PubMed]
- Centers for Medicare & Medicaid Services. Proposed Decision Memo for Ventricular Assist Devices for Bridge-to-Transplant and Destination Therapy (CAG-00432R). 2013. Available online: https://www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=268
- Ben Gal T, Jaarsma T. Self-care and communication issues at the end of life of recipients of a left-ventricular assist device as destination therapy. Curr Opin Support Palliat Care 2013;7:29-35. [PubMed]
- Brush S, Budge D, Alharethi R, et al. End-of-life decision making and implementation in recipients of a destination left ventricular assist device. J Heart Lung Transplant 2010;29:1337-41. [PubMed]


