The future of left ventricular assist devices
Historical perspective
Surgical intervention for end stage heart failure really began in the 1960s with the description of the first clinical use of the intra-aortic balloon pump, the implantation of a ventricular assist device for post-cardiotomy shock and the first successful heart transplantation (1-3). Given the less than ideal initial outcomes of transplantation, there was a real need for more durable support for the failing heart. Improvements in technology and a better understanding of biocompatibility led to significant growth in the field of left ventricular assist devices (LVAD) between the 1970s and 1990s with each iteration of technology building upon the previous generation. The first real utility of mechanical circulatory support (MCS) devices in altering the course of heart failure came from the REMATCH trial comparing optimal medical therapy to the first generation of implantable, pulsatile, permanent LVADs (4). Since that time, an exponential growth of device implantations and appropriate patient selection/management has led to over 10,000 implants with durable, implantable MCS devices in the INTERMACS registry alone (Figure 1) (5).
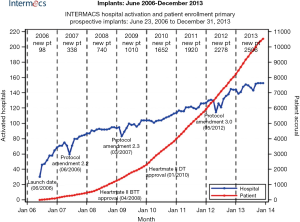
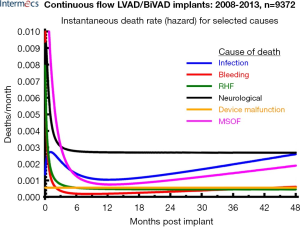
Survival, which was initially measured in days post implant, has improved to a rate of nearly 80% one year post-primary implantation. Undoubtedly, this is a result of a combination of improvements in patient selection, surgical technique and peri-operative management. Despite these advancements; however, there continues to be a set of LVAD-specific and more generalized issues that prevent MCS from becoming more uniformly adopted. Issues such as device inconvenience, the need for lifestyle modification, short battery life, need for a transcutaneous driveline, a lack of understanding of the technology, lack of appropriate timing and access to technology, need for frequent medical follow-up and device-specific complications have been barriers to a wider application of these devices. The most common cause of mortality with these devices within the first four years post implant are related to infections, bleeding, right heart failure, neurological, device malfunction and multi-system organ failure (Figure 2) (5). The future of MCS lays with a sound understanding of the past and present issues specifically related to the benefits and shortcomings of MCS technology.
Future of mechanical circulatory support (MCS)
Patient selection and outcomes
Perhaps one of the biggest accomplishments over the past decade has been the establishment of the INTERMACS database and a better understanding of the patient profile that derives the most predictable benefit from LVAD technology (Table 1) (6). In the first 6 years since its conception, we have seen a drop in the INTERMACS profile one patient from 44% to 14% while simultaneously seeing increasing survival each year. Although one could argue that healthier patients should do better; there should be no doubt that a better knowledge about patient selection and higher standards of surgical and management strategies have contributed to these gains. A host of risk scores for heart failure and LVAD implantation have been developed and validated to aid the clinician in appropriate patient selection (7-10). Although risk scores have been a great tool for discussion amongst caregivers, they can largely be subject to selection bias with aggressive selection of healthy patients and conversely sick patients skewing a given institutions outcomes despite the risk scores.
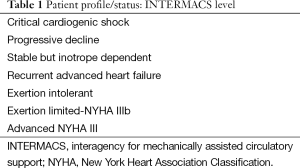
Full table
An even more pertinent question then risk on patient selection and outcomes has been whether or not we have moved into device implantation in patients that may be too well to benefit from device therapy. The ROADMAP trial is now fully enrolled with 200 patients prospective looking at optimal medical management versus MCS device initiation (11). As the results and subgroup analysis become available, we will be more fully able to answer not only the issue of survival benefit of devices in advanced heart failure, but gain insight into quality of life, perception of quality of life and freedom from rehospitalization. Unfortunately, another study looking at optimal medical therapy versus LVAD therapy, REVIVE-IT (Randomized evaluation of VAD intervention before inotropic therapy), was recently canceled due to concern over equipoise in the setting of an observed increased risk of device thrombosis in the study device.
Surgical technique
Along with the importance of appropriate patient selection, we have learned the values of meticulous surgical technique and peri-operative optimization for LVAD implantation (12). As we have progressed from invasion of multiple body cavity days of the HeartMate XVE (Thoratec Inc., Pleasanton, CA, USA) to the entirely intra-pericardial HeartWare HVAD (HeartWare Inc., Framingham, MA, USA), we have taken the surgical morbidity of maximally invasiveness and operative, raw surface bleeding largely out of the equation. Thoratec’s HeartMate III has been designed to be placed entirely intra-thoracic while the HVAD LATERAL Study™ is designed to test non-sternotomy techniques of full-support ventricular assist device implantation. The next generation of pump, HeartWare’s MVAD pushes the envelope further by being a smaller pump that can be placed via a minimally invasive procedure (13). As we continue to limit the morbidity of the surgical intervention, there is little doubt that the operation itself will become less of a limiting step in the acceptance of the technology. Additionally, we have learned that it’s not only the implantation of the pump itself but also the fixation to avoid migration, proper inflow positioning of the pump in the left ventricular cavity and avoidance of the outflow graft impinging on the right ventricle that will lead to long term success from LVAD implantation.
The importance of surgical technique has come into scrutiny with the recent rise reported by some centers in the incidence of pump thrombosis following LVAD implantation, particularly the HeartMate II (14,15). A mostly observational, prospective trial centered at implantation technique and standardization of post-operative anti-coagulation in the prevention of pump thrombosis, the PREVENT trial, is currently enrolling patients to further elucidate the validity of these recent reports. Our understanding and management of pump thrombosis has come a long way in the recent years with well outlined algorithms that argue for early pump exchange following exclusion of inflow and outflow mechanical problems (16). In addition, we have learned the usefulness of a non-sternotomy approach in reduction of morbidity and mortality when used for pump exchange (17,18).
I do believe that a great deal of pump-related complications can be avoided by meticulous attention to detail in the operation room. In addition to avoidance of above mentioned tenants, I routinely either clip or ligate the left atrial appendage as to prevent stasis in this patient population that is prone to arrhythmias. The old surgical tenant of the best way to deal with complications is by anticipation and avoidance certainly rings true in MCS.
Right ventricular support devices
In addition to surgical technique, the importance of right heart function has gained considerable attention. Risk factors and management strategies to limit right ventricular (RV) failure following LVAD implantation have been extensively described in the literature over the past five years (19-21). However, appropriate selection and prediction of the RV prior to LVAD implementation remains a diagnostic enigma (22,23). Many centers including ours, utilize a variety of intra-operative maneuvers to reduce the strain on the right ventricle. I prefer to place all my LVADs on cardiopulmonary bypass with ultra-filtration of volume, low tidal volumes with inhaled epoprostenol and coming off of bypass with the help of inotropic support for prophylactic RV protection.
The importance of RV failure post-LVAD implantation on survival is profound and as of yet, no real long term isolated right sided MCS exists. Although off-label use of HVAD for prolonged RV support has been used by many centers, the only real long term FDA approved device remains the total artificial heart. Indeed this technology has also continued to be miniaturized as the SynCardia 50 cc temporary total artificial heart for bridge to transplant has entered clinical trials. In the interim, the need for peri-operative bridging mechanical support for the RV has led to development of percutaneous strategies which currently includes the Impella RP (Abiomed, Danvers, MA, USA) and Protek Duo (CardiacAssist, Inc., Pittsburgh, PA, USA) in addition to the traditional central support offered by the CentriMag (Thoratec, Inc., Pleasanton, CA, USA) and ABIOMED BVS 5000™ (Abiomed, Danvers, MA, USA) configurations.
Type of support: partial vs. full and temporary vs. permanent
In addition to the debate of univentricular versus biventricular MCS, the concept of partial versus total circulatory support is quite intriguing. Experience with bridging patients to transplant successfully with axillary intra-aortic balloon pumps shows the utility of partial mechanical support for the failing heart (24). A more permanent, partial support solution has been the use of the CircuLite Synergy (formerly CircuLite GmbH, Germany, now HeartWare) assist device. This micro-pump, which can flow up to 3 liters/min has had mixed reviews in its ability to provide long term support for those with advanced chronic heart failure (25,26). As the literature grows, we will undoubtedly be able to more clearly define which patient population will derive the most benefit from these less invasive partial support mechanical devices.
In the acute decompensated chronic heart failure setting, the more central question is not partial versus full support but rather immediate permanent device versus temporary device as a bridge to either recovery or permanent device. The so called double bridge strategy is becoming increasingly more employed in assessing patient viability and long term benefits of permanent device implantation (27,28). It is our strategy to first rescue patients in acute cardiogenic shock with either extra-corporeal membrane oxygenator (ECMO) for biventricular support or with a CentriMag for univentricular device until a more definitive assessment can be done including assessment of psychosocial barriers to successful long term outcomes.
Patient maintenance
An important barrier in the reduction of complications in the foreseeable future will be a consensus across centers on how to best take care of patients with mechanical support devices. Currently, there are no best practice guidelines for the management of these patients and there exists considerable variability from center to center in the many aspects of caring for these complex patients. The need for a more systematic and organized approach to these patients is highlighted by the PREVENT trial and the long term balance of anticoagulation versus risk of bleeding is described from the results of the US-TRACE registry (29).
Remote monitoring of hemodynamics and pump parameters will allow a more seamless, real time communication between patients and caregivers in anticipating and dealing with potential problems. Devices such as the CardioMEMS™ (St. Jude Medical, Inc., St. Paul, MN, USA) heart sensor for pulmonary artery pressure monitoring (30) and the remote capabilities offered by the HeartAssist-5 (ReliantHeart Inc., Houston, TX, USA) (31) ventricular assist device will continue to push the border of individualized immediate care. In addition to hemodynamic parameters, the increasing role of surveillance echocardiograms is being realized. The assessment of ongoing left and right ventricular remodeling after LVAD-implantation will allow us to make more appropriate changes to not only optimize pump settings but to adjust the patient’s medical regimen as well.
Self-empowerment of patients through the internet and social media can not be underestimated and will only grow in the upcoming years. Websites, such as www.mylvad.com have enabled a culture of education not only for patients but caregivers as well. Usable forms, checklists and ability to interact with other patients will continue to drive LVAD patient-centered care forward.
The holy grail of providing untethered energy to the LVAD via a totally transcutaneous energy source or transcutaneous energy transfer (“TET”) continues to be just out of current reach. This will undoubtedly be solved within the next several years abolishing driveline infections but introducing a new sort of device-related complications.
Ongoing and upcoming trials
As we move forward with technology, it is imperative for caregivers and medical professionals to keep abreast of the current challenges and practices to minimize complications and anticipate roadblocks to overcome. The biggest hurdle in MCS is no longer patient survival, but rather reduction of morbidity and extension of quality of life. In fact, the two current trials sponsored by Thoratec, the HeartMate III trial (MOMENTUM III trial) and PREVENT trial are centered on this very topic. The HeartMate III hopes to show a reduction in pump-related adverse events while the PREVENT trial hopes to show a prevention of pump thrombosis through a standardized, clinical management pathway.
Likewise, the HeartWare sponsored trials the ENDURANCE destination therapy and supplemental trial aim at confirming the importance of blood pressure monitoring and surveillance in minimizing neurological events. The LATERAL study is designed to look at minimizing peri-operative bleeding complications and transfusions which will hopefully translate into less panel reactive antibodies for transplant eligible patients and less hospital stay for all patients. The MVAD trial will test the capabilities of the next generation of even smaller pumps in being able to provide full support while minimizing surgical morbidity.
These surgical trials in combination with the ReliantHeart’s HeartAssist-5 pump will further test the reliability, remote monitoring capabilities and ease of implantation of the next generation of LVADs. In combination with LVAD versus medical management trials of ROADMAP and REVIVE-IT, the foundation will be laid for a deeper understanding of the role of MCS in the treatment of heart failure.
Conclusions
LVADs have revolutionized the treatment options for patients suffering from advanced heart failure. As we simultaneously expand the patient population that can benefit from this technology as well as the morbidity of implantation, we will take significant strides in the treatment of advanced heart failure. We have already gained considerable gains in the therapy for this disease over the past fifty years and the future is understandably bright for MCS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Kantrowitz A, Tjonneland S, Freed PS, et al. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA 1968;203:113-8. [PubMed]
- Kilic A, Ailawadi G. Left ventricular assist devices in heart failure. Expert Rev Cardiovasc Ther 2012;10:649-56. [PubMed]
- Barnard CN. The operation. A human cardiac transplant: An interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J 1967;41:1271-4. [PubMed]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: A 10,000-patient database. J Heart Lung Transplant 2014;33:555-64. [PubMed]
- Kirklin JK, Naftel DC, Stevenson LW, et al. INTERMACS database for durable devices for circulatory support: First annual report. J Heart Lung Transplant 2008;27:1065-72. [PubMed]
- Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation 2006;113:1424-33. [PubMed]
- Lietz K, Long JW, Kfoury AG, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: Implications for patient selection. Circulation 2007;116:497-505. [PubMed]
- Cowger J, Sundareswaran K, Rogers JG, et al. Predicting survival in patients receiving continuous flow left ventricular assist devices. J Am Coll Cardiol 2013;61:313-21. [PubMed]
- Matthews JC, Pagani FD, Haft JW, et al. Model for end-stage liver disease score predicts left ventricular assist device operative transfusion requirements, morbidity, and mortality. Circulation 2010;121:214-20. [PubMed]
- Rogers JG, Boyle AJ, O’Connell JB, et al. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: design and rationale of the ROADMAP clinical trial. Am Heart J 2015;169:205-210.e20.
- Adamson RM, Mangi AA, Kormos RL, et al. Principles of HeartMate II implantation to avoid pump malposition and migration. J Card Surg 2015;30:296-9. [PubMed]
- Rojas SV, Avsar M, Hanke JS, et al. Minimally invasive ventricular assist device surgery. Artif Organs 2015;39:473-9. [PubMed]
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33-40. [PubMed]
- McCarthy FH, Kobrin D, Rame JE, et al. Increasing frequency of left ventricular assist device exchanges in the United States. Ann Thorac Surg 2015;100:1660-5. [PubMed]
- Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant 2013;32:667-70. [PubMed]
- Soleimani B, Stephenson ER, Price LC, et al. Clinical experience with sternotomy versus subcostal approach for exchange of HeartMate II left ventricular assist device. Ann Thorac Surg 2015;100:1577-80. [PubMed]
- Baldwin AC, Sandoval E, Letsou GV, et al. Surgical approach to continuous-flow left ventricular assist device explantation: A comparison of outcomes. J Thorac Cardiovasc Surg 2016;151:192-8. [PubMed]
- Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant 2015;34:1123-30. [PubMed]
- Atluri P, Goldstone AB, Fairman AS, et al. Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann Thorac Surg 2013;96:857-63; discussion 863-4. [PubMed]
- Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: Incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010;139:1316-24. [PubMed]
- Grant AD, Smedira NG, Starling RC, et al. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am Coll Cardiol 2012;60:521-8. [PubMed]
- Matthews JC, Koelling TM, Pagani FD, et al. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol 2008;51:2163-72. [PubMed]
- Estep JD, Cordero-Reyes AM, Bhimaraj A, et al. Percutaneous placement of an intra-aortic balloon pump in the left axillary/subclavian position provides safe, ambulatory long-term support as bridge to heart transplantation. JACC Heart Fail 2013;1:382-8. [PubMed]
- Mohite PN, Sabashnikov A, Simon AR, et al. Does CircuLite Synergy assist device as partial ventricular support have a place in modern management of advanced heart failure? Expert Rev Med Devices 2015;12:49-60. [PubMed]
- Meyns BP, Simon A, Klotz S, et al. Clinical benefits of partial circulatory support in New York Heart Association Class IIIB and early class IV patients. Eur J Cardiothorac Surg 2011;39:693-8. [PubMed]
- Lawson WE, Koo M. Percutaneous ventricular assist devices and ECMO in the management of acute decompensated heart failure. Clin Med Insights Cardiol 2015;9:41-8. [PubMed]
- Marasco SF, Lo C, Murphy D, et al. Extracorporeal life support bridge to ventricular assist device: The double bridge strategy. Artif Organs 2015. [Epub ahead of print]. [PubMed]
- Katz JN, Adamson RM, John R, et al. Safety of reduced anti-thrombotic strategies in HeartMate II patients: A one-year analysis of the US-TRACE Study. J Heart Lung Transplant 2015;34:1542-8. [PubMed]
- Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:935-44. [PubMed]
- Pektok E, Demirozu ZT, Arat N, et al. Remote monitoring of left ventricular assist device parameters after HeartAssist-5 implantation. Artif Organs 2013;37:820-5. [PubMed]


