
Compression depth of 30 mm has similar efficacy and fewer complications versus 50 mm during mechanical chest compression with miniaturized chest compressor in a porcine model of cardiac arrest
Introduction
Sudden cardiac arrest (CA) is a common cause of death and gains public concern widely. It has been well documented that cardiopulmonary resuscitation (CPR) is the vital effective therapeutic method for victims of CA. Chest compression is the key determinant of CPR, which increases intrathoracic pressure (ITP) and directly squeezes the heart, resultantly generating oxygen delivery and blood flow to the brain and the myocardium. Both animal (1,2) and clinical studies (3,4) have shown that the quality of chest compression is the main prerequisite for a favorable outcome. Deeper chest compression is associated with increased cardiac output, a higher likelihood of successful defibrillation, and more favorable short-term survival.
However, ever since CPR was developed in the 1960s, iatrogenic injuries associated with chest compressions have been largely reported and widely discussed, thereby driving concerns that chest compressions, especially those that are too deep, increase the risk of injury (5-7). The common complications of chest compressions are bone fractures, such as rib or sternal fractures. More importantly, chest compressions may give rise to intra-thoracic and/or intra-abdominal lacerations and hemorrhage, which if happen are usually life-threatening (7-10). Furthermore, excessively deep compressions contribute to lung and airway injuries during ventilation when continuous chest compressions combined with regular ventilation are performed in the CA patient whose advanced airway has been established. Thus, the 2020 American Heart Association (AHA) guidelines highlight “enough” compression depth (at least 50 mm), meanwhile avoiding compression-associated complications (<60 mm) for manual CPR (11). However, whether the same level of compression depth is optimal for mechanical CPR remains to be determined.
Taking advantage of providing high-quality external chest compressions continuously, mechanical chest compression devices are recommended in specific circumstances, like ambulance/helicopter transport, percutaneous transluminal coronary intervention (PCI), where delivering high-quality chest compressions cannot be achieved (12). Our previous study has addressed that mechanical chest compression using a miniaturized chest compressor (MCC) (Resuscitation International Inc., Scottsdale, AZ, USA) improved hemodynamic efficacy and survival outcomes with less injury. Because of its unique design of torso restraint and a piston mechanism, an MCC increases ITP and requires a relatively shallow compression depth (13). In the current study, we aimed to explore the efficacy as well as potential complications of both 30 and 50 mm compression depths with an MCC in a porcine model of CA and CPR. Our hypothesis is that a relatively shallow compression depth (30 mm) has similar hemodynamic efficacy but fewer complications when compared to the standard compression depth (50 mm) recommended by the current AHA guidelines.
We present the following article in accordance with the ARRIVE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-812).
Methods
All animal procedures were approved by the Institutional Animal Care and Use Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (P1809). All pigs were taken care according to the Guideline for the Care and Use of Laboratory. Animals prepared by the Institute of Laboratory Animal Resources and National Institutes of Health. Animal preparation and establishment of the porcine model of CA and CPR were performed according to our previous studies (14).
Animal preparation
In the current study, we used a total of 16 domestic male pigs (38±2 kg). All pigs were kept under standard conditions with ad libitum access to food and water and were maintained at a 12 h day and night cycle. Before surgery, the pigs were fasted overnight except for water. Sequential intramuscular injection with 20 mg/kg ketamine and intravascular injection 30 mg/kg sodium pentobarbital were used to anesthetize the pigs. Another dose of 8 mg/kg sodium pentobarbital was administered in the conditions that the animals awakened or at an interval of 1 h or when appropriate to maintain anesthesia. Animals were orally intubated and ventilated with a VELA ventilator (CareFusion, San Diego, CA, USA) with peak flow of 40 L/min, a tidal volume of 10 mL/kg, and FiO2 of 21%. An infrared capnometer (NPB-70, Nellcor Puritan Bennett Inc., Pleasanton, CA, USA) was used to recorded end-tidal carbon dioxide (ETCO2) continuously. ETCO2 was maintained between 35–40 mmHg via adjusting ventilation frequency before CA. Electrocardiograph (ECG) was recorded using three electrodes, being applied to the shaved skin of left upper limbs, right lower limb and the proximal right. Via the right femoral artery, a 7 F Swan-Ganz catheter (Edwards Lifesciences Corp, Irvine, CA, USA) was placed into the thoracic aorta for arterial blood withdrawal, monitoring arterial pressure (AP). A 7 F Pentalumen Thermodilution-tipped catheter (Abbott Critical Care, Salt Lake City, UT, USA) was placed into the right atrium for recording right atrial pressure (RAP) and core body temperature measurement. For inducing ventricular fibrillation (VF), a 5 F pacing catheter (EP Technologies Inc., Mountain View, CA, USA) was placed into the right ventricle through the right femoral artery, as confirmed by transthoracic ECG. A flow probe (Ultrasonic Blood Flow Meter, T420, Transonic Systems Inc, Ithaca, NY, USA) was used to continuously record carotid blood flow (CBF). Using characteristic pressure morphology and fluoroscopy, the position of all catheters was confirmed. Saline with crystalline bovine heparin (2.5 IU/mL) was used to flush all catheters intermittently. A 5 F Millar catheter was inserted into the esophagus 35 cm distanced from the incisor teeth for recording ITP. The compressor piston was located in the midline at the lower part of sternal (the 5th interspace). A cooling/warming blanket (Blanket ROL, Cincinnati Sub-Zero Products, Cincinnati, OH, USA) was used to maintain body temperature at 37.5±0.5 °C during the whole experiment.
Experimental procedures
Baseline hemodynamics, CBF, coronary perfusion pressure (CPP), ETCO2 and ITP measurements were obtained 15 min before inducing VF. The pigs were then randomly assigned into the shallow group (compression depth =30 mm, n=8) or the standard group (compression depth =50 mm, n=8) with a sealed envelope. VF was induced with a guidewire through the abovementioned right jugular catheter into the right ventricle. VF was successfully induced by a stepwise increase to a 1 mA alternating current (AC). Ventilation was immediately disconnected when successfully inducing VF. The right jugular catheter was draw out to prevent injuries during CPR. Precordial chest compression by a pneumatically driven MCC (Sunlife Science Inc., Suzhou, China), in combination with ventilation (tidal volume 10 mL/kg weight, frequency 10 bpm, FiO2 100%), was started after 7 min of untreated VF. Chest compression rate was maintained at 100/min. Compression depth was adjusted at 30±2 mm in the shallow group and 50±2 mm in the standard group. At 2 min of CPR, epinephrine (20 µg/kg) was administrated by intravenous injection. At 5 min of CPR, animals were defibrillated with a single 120 J biphasic electrical shock with a Zoll E-Series defibrillator (Zoll Medical Corporation, Chelmsford, MA). Animals with the return of a regular rhythm with a mean aortic pressure (MAP) >50 mmHg (≥5 min) were identified as restoration of spontaneous circulation (ROSC). Additional bolus of epinephrine was delivered every 3 min after the first administration.
Another 120 J shock would be delivered in the case of recurrent VF within 30 min after ROSC. All pigs were continuously monitored for 6 h, and FiO2 was stepwise adjusted (1.0 for the 1st 10 min, 0.5 for the 2nd 10 min, and 0.21 thereafter). Rib fractures and lung injuries were assessed by a computerized tomography (CT) scan at 6 h after ROSC. After the CT scan, the pigs were euthanized with 150 mg/kg pentobarbital. Necropsy was routinely performed to inspect for CPR-associated possible injuries.
General measurements
The CODAS/WINDAQ hardware/software system (Computer Acquisition System, Cambridge, MA) was used to continuously recorded data on hemodynamics, ETCO2, CBF, and ECG. The value difference between time-coincident aortic diastolic pressure and RAP was defined as CPP. Real-time ITP was also recorded continuously during CPR. Arterial blood gas, as well as hemoglobin and lactate concentration, was analyzed with a Stat Profile pHOx Plus L analyzer (Model PHOXplusL, Nova Biomedical Corporation, Waltham, MA, USA) at baseline and every hour after ROSC.
CT scan protocol and quantitative computed tomography (QCT)
Chest QCT scans were performed at 6 h to inspect for compression-associated rib fractures and lung injuries, as indicated by ground-glass opacification (GGO) as well as intense parenchymal opacification (IPO). The pre-protocol scan plan was as follows: 120 kVp, 110 mAs, pitch of 0.95, collimation width of 128×0.6 mm, 0.5 s/r rotation time, 0.75 mm slice width, no intervals, field of view 255 mm × 255 mm, and B70s kernel for reconstruction. The whole procedure of the QCT took about 4.9 s.
Lung injuries QCT scores, including GGO and IPO, were assessed independently by 2 experienced thoracic radiologists according to established protocol (15,16). Settings optimized for lung evaluation were window level −600 HU, window width 1,200 Hounsfield units (HU). Every image was evaluated in 5 levels: 1 cm distanced from the right hemidiaphragm roof, aortic arch, and 3 other levels equally between the 2 levels. Furthermore, 4 quadrants of each image at the 5 levels were generated by a horizontal line across the middle line of the animal body (16). Definitions were shown as following: IPO: CT values >−100 HU, GGO: CT values between −500 and −100 HU, and poorly aerated segments, normal: CT values between −900 and −501 HU. Next, Lung injuries were quantified in each quadrant per level, defined as following: 0 (normal), 1 (<5% abnormal value), 2 (5–25% abnormal value), 3 (26–49% abnormal value), 4 (50–75% abnormal value), and 5 (>75% abnormal value) (16). Lastly, the 4-quadrants scores at each level were pooled, generating the sum-scores, thus the sum-scores ranged from 0 to 20 for each level. The total lung score was the sum of the five-level scores (16).
Statistical analysis
Data were presented as mean ± SD with normally distributed data, which had been confirmed for normality of distribution by Kolmogorov-Smirnov test. If not, data were presented as a median with inter-quartile range (IQR: 25th, 75th percentiles). The student’s t-test was used for comparisons of normally distributed data and the Mann-Whitney U test was used for comparisons of non-normal data. Categorical variables were compared by the Fisher’s exact test. P<0.05 was considered as significant difference.
Results
Baseline characteristics
Identical baseline characteristics between the two groups were confirmed by comparing their baseline physiological and hemodynamic parameters, as indicated by no significant differences in body weight, heart rate, RAP, mean AP, ETCO2, CBF, arterial PaO2, and lactate (Table 1).
Table 1
| Variables | Shallow group (n=8) | Standard group (n=8) |
|---|---|---|
| Body weight (kg) | 37±2 | 39±1 |
| Heart rate (beats/min) | 103±9 | 108±10 |
| Mean aortic pressure (mmHg) | 104±8 | 113±9 |
| Right atrium pressure (mmHg) | 3.8±0.5 | 3.1±0.6 |
| End-tidal carbon dioxide (mmHg) | 39.2±1.9 | 37.7±2.2 |
| Carotid blood flow (mL/min) | 205±28 | 199±23 |
| PaO2 (mmHg) | 98±18 | 104±12 |
| Arterial lactate (mmol/L) | 0.9±0.7 | 1.4±0.6 |
Values are presented as mean ± SD. Shallow, the shallow group with chest compression depth of 30 mm; Standard, the standard group with chest compression depth of 50 mm.
Intra-arrest CPR Efficacy and short-term outcome
To address that relatively shallow compression depth (30 mm) has similar hemodynamic intra-arrest CPR efficacy and short-term outcome when compared to the standard compression depth (50 mm), we compared hemodynamic parameters and ROSC rate between the two groups. There were no significant differences in CPP, ETCO2, and CBF between both groups throughout the CPR period (Figures 1-3). In detailed, CPP in the both groups was maintained ≥20 mmHg at the beginning of CPR and achieved a highest value at the 4rd min of CPR, indicating a high-quality CPR and more likely to gain successful ROSC. After the administration of epinephrine, the CPP of all animals increased while ETCO2 and CBF decreased during CPR. Even so, CPP was kept constantly ≥25 mmHg in the both groups Furthermore, we found similar trend of CBF in the both groups during the CPR period in which CBP decreased with time and was relatively constant after 3 min of CPR. CBF was also kept at an ideal level (≥30 mL/min), indicating high-quality CPR and good neurological outcome. A significantly lower intrathoracic positive pressure (ITPP) and systolic artery pressure (SAP) were measured in the shallow group at the first min of CPR; however, we didn’t find significant differences in those values between the 2 groups for the left 4 min. No differences in intrathoracic negative pressure (ITNP) were observed in the both groups during CPR (Figure 4 and Table 2). In addition, the shock times, epinephrine dose, and CPR duration time were similar between the standard group and the shallow group (Table 2). Finally, all animals were successfully resuscitated in both groups.
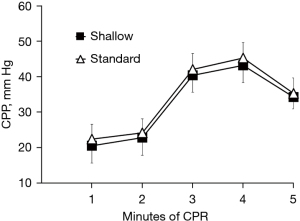
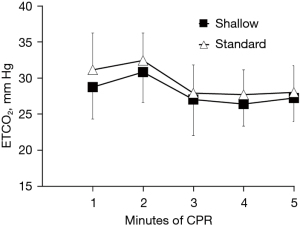
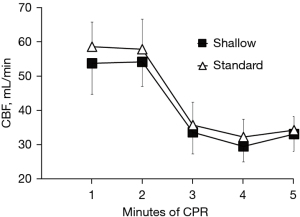
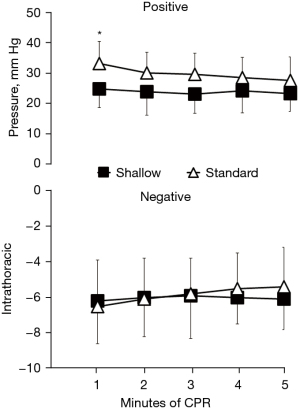
Table 2
| Variables | Shallow group | Standard group |
|---|---|---|
| Hemodynamics at 1 min of CPR | ||
| Systolic AP (mmHg) | 60±22* | 75±23 |
| Diastolic AP (mmHg) | 22±5 | 24±6 |
| Systolic RAP (mmHg) | 38±11 | 44±14 |
| Diastolic RAP (mmHg) | 4±2 | 5±2 |
| Hemodynamics at 5 min of CPR | ||
| Systolic AP (mmHg) | 73±20 | 79±18 |
| Diastolic AP (mmHg) | 41±9 | 39±7 |
| Systolic RAP (mmHg) | 51±13 | 56±8 |
| Diastolic RAP (mmHg) | 6±1 | 8±2 |
| Duration of cardiopulmonary resuscitation (min) | 5 [5–5] | 5 [5–5] |
| Number of defibrillation | 2.4 [2–3] | 2.6 [2–3] |
| Epinephrine administration (mg) | 0.74±0.04 | 0.78±0.02 |
| Number of rib fracture | 1 [0–1]* | 4 [3–4] |
Values are presented as mean ± SD or median plus interquartile range. *, P <0.05
Event-related complications
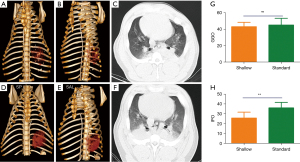
Considering similar efficacy and short-term outcome between 30 mm compression depth and 50 mm compression depth, we also concerned whether relative shallow compression depth has lower complications than the standard compression. We utilized chest QCTs to evaluate difference in the lung injury and rib fractures between the two groups. Chest QCTs demonstrated that lung complications, as indicated by the presence of GGO and IPO, were found in both the shallow and standard groups (Figure 5). However, the shallow group had significant lower IPO QCT scores compared with the standard group, indicating less serious physical injury in the shallow group. No significant difference in GGO QCT scores, which is a manifestation of pulmonary edema, was found between the two groups after resuscitation (Figure 5), indicating relatively high but similar pulmonary edema caused by different compression depth. In addition, rib fractures were found in both groups. However, animals in the shallow group were less likely to suffer from rib fracture and serious lung injury due to compression as compared to those in the standard group (Figure 5 and Table 2).
Discussion
In the current study, we sought to explore the efficacy and potential complications of different compression depths (30 vs. 50 mm) during mechanical chest compression with a Weil Mini chest compressor in the porcine model of CA and CPR. We found no significant differences in hemodynamics and short-term outcomes between the animals that underwent a shallow compression depth (30 mm) and a standard depth (50 mm). Furthermore, in spite of significantly lower SAP and ITPP values in the shallow group animals at the first min of CPR, when compared with those in the standard group, ITNP, CPP, ETCO2, or CBF were similar between the two groups. Thus, similar ROSC outcomes from CA and CPR were observed. In addition, we found the CPP of all animals was significantly increased while ETCO2 and CBF decreased during CPR after administration of epinephrine. However, animals in the shallow group were less likely to suffer from rib fractures and serious lung injury due to compression as compared to those in the standard group.
Sufficient and “enough” chest compression depth has been well recognized to increase the likelihood of ROSC for CA victims. Nevertheless, the mechanism by which CPR generates forward blood flow has not been fully elucidated and widely discussed since CPR became mainstream. Kouwenhoven et al. demonstrated that the direct squeezing of the ventricles between the sternum and the thoracic vertebra accounts for the generation of forwarding blood flow during external chest compression, which is known as the “cardiac pump mechanism” (5). On the other hand, Rudikoff et al. developed the “thoracic pump mechanism” theory and characterized that the ITP generating from CPR is responsible for the forward blood flow (17). Currently, it is accepted that both mechanisms are relevant, but each plays an essential role at different times during CPR (18-20). Our previous study has been characterized that the mechanism of chest compressions with MCC is based on both the cardiac and thoracic pump theories (13). In the current study, the MCC directly squeezed the bony thorax by a torso restraint and the heart by a piston; thus, the two mechanisms were involved.
The recent AHA guidelines do not recommend use mechanical compression devices routinely in CA patients. However, taking advantage of high-quality external chest compressions provided continuously and safely, mechanical chest compression devices are recommended in specific circumstances, like ambulance/helicopter transport, PCI, where high-quality chest compressions delivered cannot be achieved (21). However, even though a compression depth of above 50 mm is recommended for manual CPR by 2020 AHA guideline, whether that is a suitable requirement for mechanical CPR remains to be demonstrated. Due to the above-mentioned mechanisms of chest compress with MCC, in the present study we hypothesized that a relatively shallow compression depth (30 mm) would have similar hemodynamic efficacy and short-term outcomes but fewer complications when compared to the standard compression depth (50 mm) recommended by the current AHA guideline. As expected, similar hemodynamic efficacy of CPR and ROSC outcomes were observed in both groups. Furthermore, while significantly lower ITPP and SAP values were detected in the shallow group at the first min of CPR; we didn’t find significant differences in these values between the both groups for the next 4 min of CPR. It is noteworthy that the relationship of compression force and ITPP is nonlinear since chest wall compliance will change over time during low-flow states. Similarly, Koeken et al. have shown that pushing harder is not always better, as an ‘optimal’ force and depth may exist because of the changing compliances and resistances in the chest wall and intrathoracic vasculature (22). More importantly, our finding demonstrated that a relatively shallow compression depth using an MCC had a similar ITNP as the standard compression depth. Similar ITNPs achieved in both groups may result from quick and adequate chest recoil, which is achieved by the actively withdrawn of the piston and the residual tension of the torso restraint. The ITNP generated during recoil of the chest could increase the amount of blood return to the heart, a key determinant of efficacy of CPR and survival after CA (23-25). This may be a plausible mechanism of action that explains the equivalent CPP and CBF measurements, and resultantly the beneficial effects on short-term outcomes with ROSC.
In the current study, we utilized QCT to assess the CPR-associated complications and severity of lung injuries. Our previous study successfully characterized that the non-invasive property of chest QCT could efficaciously differentiate various lung lesions, allowing for quantitative and rapid assessment of chest and lung injuries (IPO and GGO scores) following CPR (15). As expected, GGO and IPO were detected in all animals at ROSC 6 h. IPO is considered to be the manifestation of physical injury following CPR and is characterized by remarkable increased lung opacity on the QCT images. On the other hand, GGO indicates lung edema, which is more commonly found in the condition of hemodynamic failure, toxin exposure, or thromboembolism event. Either higher GGO or IPO indicates a lower ROSC rate and poor outcome following CA and CPR (26-28). In the present study, we found that IPO increased with compression depth while GGO was constant. In contrast, our previous study found that the QCT score was significantly higher while there were no differences in IPO between both groups. The explanation for these divergent results undoubtedly lies in the different degree of ischemia and injury mechanism (5 vs. 10 min duration of VF in the previous study, and 30 vs. 50 mm compression depth with 7 min duration of VF in the current study). In this regard, we reasoned that deeper compression is more likely to cause physical injuries such as rib fractures, pneumorrhagia, and atelectasis, but do not increase pulmonary edema or myocardial dysfunction.
Furthermore, rib fractures were more likely to occur in the standard group. It has been well documented that manual chest compression could give rise to secondary cardiopulmonary or other injuries, commonly including bone fractures (rib sternal), pneumothoraxes, and visceral organ injury (liver, spleen or heart). The mean force required to produce a compression depth of 50–60 mm is 50–80 kg (29,30). Notably, the deeper compression depth is tightly connected to more possibility of secondary iatrogenic injuries. The possibility that complications increase when deeper compression is delivered has been reported, which showed a 3-fold rise in significant chest wall injuries and prolonged stays in the ICU subsequent to at least 50 mm compression depth as recommended by 2010 AHA guideline (8). Furthermore, a few studies have also reported mechanical device could elicit to clinically significant injuries, thus gaining the concern that mechanical compression devices could also lead to the increment of injury. Our finding adds to this and highlights the potential for injury under standard compression depth using MCC. Of note, only 30 mm instead of other compression depth was assessed in our study. Our pilot study found that 30 mm compression depth was deep enough to rescue the animals in this porcine model. Our study aimed to address the relative shallow compression depth has similar hemodynamic efficacy but fewer complications when compared to the standard compression depth. This 30 mm compression depth is not an ideal one when extrapolating this finding to other setting, like human since from different chest wall thickness and compliance. Thus, in terms of safety and efficacy, it is reasonable to extrapolate that chest compression depth together with other indicators, rather than a uniform chest compression depth, should be introduced to guide high-quality CPR in the future (31-33).
Consistent with our results, previous studies have also characterized that a remarkable reduction of ETCO2, concurrent with increased CPP, was observed after the administration of epinephrine (34,35). One possible explanation may be a reduction of CO2 elimination through expiration is attributed to pulmonary vasculature constriction because of epinephrine-associated adrenergic effect, subsequently leading to increased aberrant shunting and ventilation-perfusion mismatch. Also, we cannot rule out the possibility of epinephrine mediated α-1 agonist effect, which reduces lung perfusion. It is also important to note that CBF, which indicates cerebral perfusion, was reduced following the administration of epinephrine in our study. In keeping with our findings, Burnett et al. administered epinephrine during active compression-decompression CPR with an impedance threshold device (ACD-CPR + ITD) and recorded significantly increased markers of macrocirculation, significantly decreased CBF, and no improvement in cerebral tissue perfusion (34). This might be the rationale for why the use of epinephrine, particularly large-dose epinephrine, is not associated with a benefit toward survival with a favorable neurological outcome after CA, and may even potentially cause harm.
The main limitation of this study is that healthy animals without underlying disease were included, which is not consistent with clinical conditions. However, we focused solely on evaluating the efficacy and potential complications of different compression depths with MCC during CPR. Secondly, we didn’t evaluate longer CPR time in this study since we found hemodynamic parameters were maintained at a good level even at the 5th min of CPR before defibrillation. Longer CPR introduce the same potential bias to the both groups. Thirdly, we didn’t assess other hemodynamic parameters, like cerebral SpO2 and 24 h outcomes. It would be better to measure all of them. Our study aimed to evaluate different compression depth-related hemodynamic efficacy and complication during CPR. We found both 30 and 50 mm compression depth had favorable hemodynamic efficacy. Subsequently, all animals were successfully resuscitated and survived to 6 h due to this favorable hemodynamic efficacy. However, whether this salutary effect could be maintained for longer time and resultant good neurological outcome is unknown and is in need of further investigation. Lastly, in our study, we chose to use the number of pigs based on a previous study by Rojas-Salvador [2020]. This is another approach that is often used to determine a sample size in animal studies. In the study of Rojas-Salvador et al., 6 pigs were adopted in each group to verify statistical differences (36). In our study, we used 8 pigs in each group to achieve a similar statistical power. More importantly, our findings support and highlight the perspective of integrating other indicators such as ETCO2, hemodynamic-directed CPR, and recoil velocities with chest compression depth to titrate chest compression delivery to CA. Further research should be conducted to examine these results in a clinical setting.
Conclusions
Relatively shallow compression depth (30 mm) has similar hemodynamic efficacy but fewer complications when compared to the standard compression depth (50 mm). Animals in the shallow group were less likely to suffer from rib fractures and serious lung injury due to compression as compared to those in the standard group. In terms of safety and efficacy, it is reasonable to extrapolate that chest compression depth together with other indicators, rather than a uniform chest compression depth, should be introduced to guide high-quality CPR in the future.
Acknowledgments
Funding: This work was supported by grants from the Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515011433) and the Traditional Chinese Medicine Bureau of Guangdong Province (No. 20211073).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-812).
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-812
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-812). All authors report grants from the Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515011433) and the Traditional Chinese Medicine Bureau of Guangdong Province (No. 20211073).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were performed under a project license (P1809) granted by the Institutional Animal Care and Use Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University. Animals prepared by the Institute of Laboratory Animal Resources and National Institutes of Health.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bellamy RF, DeGuzman LR, Pedersen DC. Coronary blood flow during cardiopulmonary resuscitation in swine. Circulation 1984;69:174-80. [Crossref] [PubMed]
- Babbs CF, Voorhees WD, Fitzgerald KR, et al. Relationship of blood pressure and flow during CPR to chest compression amplitude: evidence for an effective compression threshold. Ann Emerg Med 1983;12:527-32. [Crossref] [PubMed]
- Edelson DP, Abella BS, Kramer-Johansen J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation 2006;71:137-45. [Crossref] [PubMed]
- Kampmeier TG, Lukas RP, Steffler C, et al. Chest compression depth after change in CPR guidelines--improved but not sufficient. Resuscitation 2014;85:503-8. [Crossref] [PubMed]
- Kouwenhoven WB, Jude JR, Knickerbocker GG. Closed-chest cardiac massage. JAMA 1960;173:1064-7. [Crossref] [PubMed]
- BARINGER JR. External cardiac massage. N Engl J Med 1961;265:62-5. [Crossref] [PubMed]
- Harris AW, Kudenchuk PJ. Cardiopulmonary resuscitation: the science behind the hands. Heart 2018;104:1056-61. [Crossref] [PubMed]
- Kim EY, Yang HJ, Sung YM, et al. Multidetector CT findings of skeletal chest injuries secondary to cardiopulmonary resuscitation. Resuscitation 2011;82:1285-8. [Crossref] [PubMed]
- Deliliga A, Chatzinikolaou F, Koutsoukis D, et al. Cardiopulmonary resuscitation (CPR) complications encountered in forensic autopsy cases. BMC Emerg Med 2019;19:23. [Crossref] [PubMed]
- Buschmann CT, Tsokos M. Frequent and rare complications of resuscitation attempts. Intensive Care Med 2009;35:397-404. [Crossref] [PubMed]
- Magid DJ, Aziz K, Cheng A, et al. Part 2: Evidence Evaluation and Guidelines Development: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020;142:S358-65. [Crossref] [PubMed]
- Poole K, Couper K, Smyth MA, et al. Mechanical CPR: Who? When? How? Crit Care 2018;22:140. [Crossref] [PubMed]
- Chen W, Weng Y, Wu X, et al. The effects of a newly developed miniaturized mechanical chest compressor on outcomes of cardiopulmonary resuscitation in a porcine model. Crit Care Med 2012;40:3007-12. [Crossref] [PubMed]
- Yang Z, Zheng H, Lin L, et al. Alterations in Respiratory Mechanics and Neural Respiratory Drive After Restoration of Spontaneous Circulation in a Porcine Model Subjected to Different Downtimes of Cardiac Arrest. J Am Heart Assoc 2019;8:e012441 [Crossref] [PubMed]
- Liu Z, Liu Q, Wu G, et al. Quantitative CT assessment of lung injury after successful cardiopulmonary resuscitation in a porcine cardiac arrest model of different downtimes. Quant Imaging Med Surg 2018;8:946-56. [Crossref] [PubMed]
- Burnham EL, Hyzy RC, Paine R 3rd, et al. Chest CT features are associated with poorer quality of life in acute lung injury survivors. Crit Care Med 2013;41:445-56. [Crossref] [PubMed]
- Rudikoff MT, Maughan WL, Effron M, et al. Mechanisms of blood flow during cardiopulmonary resuscitation. Circulation 1980;61:345-52. [Crossref] [PubMed]
- Liu Y, Tian Z, Yu C, et al. Transesophageal echocardiography to assess mitral valve movement and flow during long term cardiopulmonary resuscitation: How cardiac effects fade with time. Int J Cardiol 2016;223:693-8. [Crossref] [PubMed]
- Ma MH, Hwang JJ, Lai LP, et al. Transesophageal echocardiographic assessment of mitral valve position and pulmonary venous flow during cardiopulmonary resuscitation in humans. Circulation 1995;92:854-61. [Crossref] [PubMed]
- Sigurdsson G, Yannopoulos D, McKnite SH, et al. Cardiorespiratory interactions and blood flow generation during cardiac arrest and other states of low blood flow. Curr Opin Crit Care 2003;9:183-8. [Crossref] [PubMed]
- Brooks SC, Hassan N, Bigham BL, et al. Mechanical versus manual chest compressions for cardiac arrest. Cochrane Database Syst Rev 2014;CD007260 [Crossref] [PubMed]
- Koeken Y, Aelen P, Noordergraaf GJ, et al. The influence of nonlinear intra-thoracic vascular behaviour and compression characteristics on cardiac output during CPR. Resuscitation 2011;82:538-44. [Crossref] [PubMed]
- Aufderheide TP, Frascone RJ, Wayne MA, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet 2011;377:301-11. [Crossref] [PubMed]
- Aufderheide TP, Nichol G, Rea TD, et al. A trial of an impedance threshold device in out-of-hospital cardiac arrest. N Engl J Med 2011;365:798-806. [Crossref] [PubMed]
- Plaisance P, Lurie KG, Payen D. Inspiratory impedance during active compression-decompression cardiopulmonary resuscitation: a randomized evaluation in patients in cardiac arrest. Circulation 2000;101:989-94. [Crossref] [PubMed]
- Kang DH, Kim J, Rhee JE, et al. The risk factors and prognostic implication of acute pulmonary edema in resuscitated cardiac arrest patients. Clin Exp Emerg Med 2015;2:110-6. [Crossref] [PubMed]
- Wang S, Wu JY, Guo ZJ, et al. Effect of rescue breathing during cardiopulmonary resuscitation on lung function after restoration of spontaneous circulation in a porcine model of prolonged cardiac arrest. Crit Care Med 2013;41:102-10. [Crossref] [PubMed]
- Markstaller K, Rudolph A, Karmrodt J, et al. Effect of chest compressions only during experimental basic life support on alveolar collapse and recruitment. Resuscitation 2008;79:125-32. [Crossref] [PubMed]
- Tomlinson AE, Nysaether J, Kramer-Johansen J, et al. Compression force-depth relationship during out-of-hospital cardiopulmonary resuscitation. Resuscitation 2007;72:364-70. [Crossref] [PubMed]
- Handley AJ. Press hard - but perhaps not too hard. Resuscitation 2014;85:153-4. [Crossref] [PubMed]
- Lee CU, Hwang JE, Kim J, et al. A new chest compression depth indicator would increase compression depth without increasing overcompression risk. Am J Emerg Med 2015;33:1755-9. [Crossref] [PubMed]
- Lautz AJ, Morgan RW, Karlsson M, et al. Hemodynamic-Directed Cardiopulmonary Resuscitation Improves Neurologic Outcomes and Mitochondrial Function in the Heart and Brain. Crit Care Med 2019;47:e241-9. [Crossref] [PubMed]
- González-Otero DM, Russell JK, Ruiz JM, et al. Association of chest compression and recoil velocities with depth and rate in manual cardiopulmonary resuscitation. Resuscitation 2019;142:119-26. [Crossref] [PubMed]
- Burnett AM, Segal N, Salzman JG, et al. Potential negative effects of epinephrine on carotid blood flow and ETCO2 during active compression-decompression CPR utilizing an impedance threshold device. Resuscitation 2012;83:1021-4. [Crossref] [PubMed]
- Sandroni C, De Santis P, D'Arrigo S. Capnography during cardiac arrest. Resuscitation 2018;132:73-7. [Crossref] [PubMed]
- Rojas-Salvador C, Moore JC, Salverda B, et al. Effect of controlled sequential elevation timing of the head and thorax during cardiopulmonary resuscitation on cerebral perfusion pressures in a porcine model of cardiac arrest. Resuscitation 2020;149:162-9. [Crossref] [PubMed]


