Dealing with surgical left ventricular assist device complications
Introduction
The percentage of the population that is elderly in the United States is projected to increase in coming years, with a likely concomitant increase in the number of patients with end-stage heart failure (1). With improvements in left ventricular assist device (LVAD) technology and outcomes, there will undoubtedly be a higher number of LVAD implants especially given that nationwide heart transplant volume has remained relatively stagnant due to donor shortages. In fact, a recent analysis of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) demonstrated that certain subsets of destination therapy LVAD patients had comparable 2-year survival rates as heart transplant recipients, raising the debate over the equivalence of these two therapies (2). Although outcomes continue to improve, there remain several significant complications associated with this treatment modality that currently limit overall survival and long-term outcomes (Table 1). In this review, we provide an overview of the management of specific complications associated with LVADs.
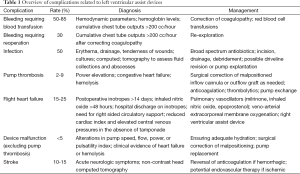
Full table
Bleeding
Bleeding is the most common complication following LVAD implantation. With earlier generation pulsatile flow pumps, rates of bleeding requiring reoperation were as high as 50% (3). In the more recent HeartMate II LVAD (Thoratec Corporation, Pleasanton, CA, USA) bridge-to-transplant trial, the rate of bleeding requiring reoperation was 31%, with 53% of patients transfused at least two units of red blood cells (4). A randomized trial comparing continuous versus pulsatile flow pumps for the treatment of advanced heart failure in patients ineligible for transplantation demonstrated that the newer generation continuous flow LVADs had a reoperation rate for bleeding of 30% post-implant, with 81% of the cohort requiring a blood transfusion for bleeding (5).
The high rates of bleeding even in contemporary series underscore the importance of an algorithmic approach to managing this complication. As with any cardiac surgical patient, it is important to distinguish bleeding from diffuse coagulopathy versus surgical bleeding. Monitoring of laboratory parameters including prothrombin time, partial thromboplastin time, platelet count, and fibrinogen levels guide the administration of platelets, fresh frozen plasma, and cryoprecipitate. In the setting of ongoing coagulopathic bleeding, some groups advocate for the use of recombinant factor VII. Factor VII should be used cautiously in patients with LVADs given the potential for serious thromboembolic events, particularly at higher doses (6). Additionally, concern remains over the potential for establishing substrate for early and late VAD thrombosis with the use of factor VII.
Meticulous attention to hemostasis in the operating room is essential as with any surgical procedure. Some surgeons use topical hemostatic agents along suture lines during LVAD implantation to reduce bleeding risk although its efficacy has not been convincingly established in prior series or randomized trials. Cannulation sites can be more prone to bleeding than in the typical cardiac surgical patient due to frequently distended and friable atria in end-stage heart failure patients. The driveline tunnel and preperitoneal pocket can also be prone to bleeding. These should be created prior to heparinization, and avoidance of inadvertent abdominal entry and electrocautery of visible vessels and muscle bleeding is important. Electrocautery or suture ligation should also be generously utilized to control raw surface or wire bleeding, respectively.
Surgical bleeding is generally suspected with cumulative chest tube outputs of over 200 mL per hour in patients with normal or corrected coagulation parameters. Increasing central venous pressures, increasing pressor requirements, and decreasing LVAD flows can also raise clinical suspicion for surgical bleeding. Early rather than delayed re-exploration is generally advised as massive transfusion can result in right heart dysfunction or failure. Retained large blood clots can also be a nidus for infection and chest washouts can help in reducing this risk.
Delayed sternal closure is one strategy that can be employed, particularly in cases of coagulopathy, hemodynamic instability, or right heart dysfunction following LVAD implantation. Earlier concerns related to delayed sternal closure focused on a perceived higher risk of infection. However, a single center series of 364 LVAD patients demonstrated that delayed sternal closure was not associated with increased risk of mortality or infection, although patients had a longer stay in the intensive care unit (7).
The gastrointestinal tract is another potential source of bleeding in the LVAD patient. Gastrointestinal bleeding occurs in approximately 20% of LVAD patients, and can be related to anticoagulation, mucosal ischemia, acquired von Willebrand’s syndrome, and arteriovenous malformations (8). The initial approach to management involves discontinuing anticoagulation and antiplatelet agents, initiating intravenous proton pump inhibitor therapy, and administering blood products as necessary although they should be given judiciously in transplant candidates due to allosensitization. Upper and lower endoscopy are performed for localization of the bleeding source, and if negative, other diagnostic modalities such as mesenteric angiography, tagged red blood cell scan, and capsule endoscopy can be pursued. These modalities are safe for use in patients supported with an LVAD. Endoscopic control of bleeding can be successful in some cases. Surgical exploration is indicated in cases of ongoing or massive bleeding not amenable to endoscopic control. Acute general surgical procedures in the LVAD patient can be challenging due to patients being anticoagulated, non-pulsatile blood flow, and the potential physical obstruction caused by the pump and driveline. In the case of refractory GI bleeding, extreme interventions including octreotide and oral contraceptive use have been described (9).
Infection
In addition to the more common infections that can affect all postsurgical patients such as urinary tract infections and pneumonia, there are specific device-related infections in patients implanted with an LVAD. These include infections of the pump pocket, driveline infections, and LVAD-associated endocarditis. The incidences of these LVAD-related infections are lower in newer generation continuous flow pumps as compared to the older generation devices (5). This may in part be due to a more compact design and smaller driveline. The most common pathogen responsible for pump pocket and driveline infections is Staphylococcus aureus, with Staphylococcus epidermidis and Enterococcus species also being common causes (10,11).
LVAD-related infections can manifest with fever, leukocytosis, purulent drainage, and tenderness. Computed tomography can be useful for assessing fluid collections or abscesses. Cultures should be sent for definitive diagnosis and for tailoring of antibiotics. The diagnosis can be challenging given the complex patient population, the frequent and prolonged hospital stays, variable symptoms and signs, and prior exposure to antibiotics. When infections do occur, they typically occur within 3 months of implantation.
Initial management consists of broad spectrum antibiotics. Surgical incision and drainage and possible debridement is required for pump pocket infections with fluid collections. Muscle or omental flaps or vacuum-assisted closure therapy may be utilized in more severe cases. Superficial and localized driveline infections may be treated with systemic antibiotics alone, although in some cases surgical debridement with driveline revision may be needed. LVAD-associated endocarditis is associated with more substantial morbidity and mortality risk. LVAD explantation may be required, particularly if associated with sepsis, septic emboli, or end-organ dysfunction.
Pump thrombosis
Pump thrombosis is defined under the wider category of device malfunction and pump failure, in cases where thrombus is documented within the pump itself or conduit and can lead to circulatory failure. Of concern is that a multi-institutional review of nearly 900 HeartMate II LVADs demonstrated an abrupt increase in the rate of pump thrombosis at 3 months post-implant starting in early 2011, from 2.2% to 8.4% (12). An analysis of the INTERMACS registry including roughly 9,000 patients revealed a decrease in freedom from device exchange or death related to thrombosis at 6-month post-implant around the same time period (13).
Risk factors for pump thrombosis can be divided into patient-related, device-related, and management-related factors. In terms of patient-related factors, any prothrombotic condition can predispose to pump thrombosis. This includes congestive heart failure, infection, malignancy, and hypercoagulable states such as factor V Leiden mutation, antiphospholipid syndrome, or protein C or S deficiency. Device-related factors include local heat generated by the pump, and outflow graft kinking or extrinsic compression. A major risk factor for pump thrombosis related to management is inadequate anticoagulation. There is variability in anticoagulation practices between providers and institutions, and the risk of pump thrombosis may increase in patients with bleeding events due to reduction or discontinuation of anticoagulation. In some cases pump speed may be reduced to improve native heart ejection and pulsatility, although such reduction is another risk factor for thrombosis.
Pump thrombosis can manifest with power elevations of the device, left or right-sided congestive heart failure, and with evidence of hemolysis. Increased lactate dehydrogenase is a marker of the latter, and in most circumstances anticoagulation will be intensified when marked or progressive elevations of lactate dehydrogenase are encountered. Imaging is useful in the diagnosis of pump thrombosis. Chest radiographs can be used to assess inflow cannula and outflow grafts, and computed tomography angiogram can also evaluate positioning of the inflow cannula and outflow graft and assess for the presence of thrombus as well. Echocardiogram is another imaging modality that is useful in assessing for left ventricular thrombus, left ventricular dilatation, mitral regurgitation, and aortic valve opening. Left and right heart catheterization can provide the usual hemodynamic parameters and in addition ventriculography and contrast through the outflow graft can evaluate filling.
The International Society for Heart and Lung Transplantation working group has published an algorithm for the management of suspected pump thrombosis that is germane to the HeartMate II device (14). Surgical intervention is required to correct malpositioned or kinked inflow cannulae or outflow grafts. In cases where imaging demonstrates good positioning, no obstruction, and with poor left ventricle unloading, admission to the intensive care unit for inotropes, diuretics, and intravenous heparin may be needed. If there is no resolution of the lack of left ventricle unloading, power elevations, and hemolysis, then antiplatelet agents such as glycoprotein IIIb/IIa inhibitors or direct thrombin inhibitors should be added. If still not resolved, then surgical candidacy for pump exchange, urgent transplantation, or explant for recovery should be evaluated, with thrombolytics considered for patients who are not candidates for surgery.
Some reports suggest a lower rate of pump thrombosis with the intrapericardial continuous flow HeartWare system (15). In addition, rates of pump exchange may be lower with the HeartWare as compared to the HeartMate II in patients with pump thrombosis. An analysis of 382 patients supported with the HeartWare found 34 pump thrombosis events in 31 patients, with medical management attempted in 30 cases with a success rate of 50% (16).
Pump exchange can be performed via a median sternotomy or through a less invasive subcostal approach for the HeartMate II. The latter can be performed either off bypass or on cardiopulmonary bypass initiated through axillary or femoral arterial cannulation and femoral venous cannulation. The off pump technique, which we advocate, with the HeartMate II involves exposing the pump through a left subcostal incision. The bend-relief is disconnected from the pump outlet, the pump power is turned off assuming hemodynamic stability, and the driveline is cut. The outflow graft and inflow are clamped, and the outflow graft is unscrewed from the titanium tube at the pump outlet. A clamp is then used to hold the collet at the pump inlet, and the pump is rotated counterclockwise until it can be removed from the inlet. Brisk bleeding noted from the inflow and outflow after transient release of the clamps confirms their patency and ensures there are no clots in the grafts. The new pump can then be inserted by reversing the sequence above.
Similarly, minimally invasive techniques, utilizing a limited anterior left thoracotomy, can be used for exchange of the HeartWare HVAD device. Once exposed the outflow graft would require exposure and enough length to facilitate a clamp. The HVAD pump can be removed from the sewing ring by engaging and loosening the screw. The original pump can then be removed and the new pump inserted. Bleeding can be controlled with either digital control or use of a large Foley catheter with balloon. The original outflow graft should be transected and reanastomosed to the new HVAD pump graft. Alternatively the graft can be released from the original pump by releasing the connector and reattaching to the new pump. Given tight clearances, this may be a complicated undertaking. Though this can be performed off-pump, we advocate the use of cardiopulmonary bypass to minimize blood loss and hemodynamic instability.
Right heart failure
There are various published definitions of right heart failure after LVAD implantation. A commonly utilized definition is the need for postoperative inotropes for more than 14 days, the need for inhaled nitric oxide for more than 48 hours, the need for right sided mechanical circulatory support, or hospital discharge on an inotrope after LVAD implantation. The presence of at least two of the following hemodynamic parameters in the absence of tamponade can also signal right heart dysfunction or failure post-LVAD implant: a cardiac index of less than 2.0 L/min/m2, mixed venous oxygen saturation less than 55%, central venous pressure greater than 16 mmHg, and mean arterial pressure less than 55 mmHg.
Although the rate of right heart failure after LVAD implantation varies according to the definition that is used, it generally affects 15-25% of LVAD patients (17). Commonly identified predictors of right heart failure after LVAD implantation include preoperative renal or hepatic dysfunction, inotrope dependency, intra-aortic balloon pump, and parameters associated with baseline low right ventricular contractility such as low right ventricular stroke work index (18-20). Multiple groups have in fact constructed risk scores for right heart failure that can be used to risk stratify patients at the time of LVAD implantation, with the ultimate hope of selecting which patients should be placed on upfront biventricular support rather than LVAD alone (18-20). Survival has been demonstrated to be improved in patients undergoing early, elective biventricular support as compared to patients who require urgent right ventricular support in a delayed fashion following LVAD implantation (21).
Prevention of right ventricular failure relies upon optimization of preload, contractility, and afterload in the perioperative period. Aggressive diuresis to maintain central venous pressures less than 15 mmHg is important. Pulmonary vasodilators may be needed to reduce elevated pulmonary artery pressures and reduce right ventricular afterload. Correction of coagulopathy and meticulous hemostasis are also important components of right heart failure prevention as these can decrease the utilization of blood products and reduce volume overloading.
Once right heart failure occurs after LVAD implantation, medical therapy includes agents such as milrinone to improve contractility and dilate the pulmonary vasculature. Inhaled nitric oxide and epoprostenol can also be used for pulmonary vasodilation and reduction of pulmonary vascular resistance. Patients who are deemed high risk for right heart failure preoperatively may be selected for upfront biventricular support. Intraoperatively, right heart failure is identified by a low cardiac index of less than 2.0 L/min/m2 and elevated central venous pressure. In these circumstances a temporary right ventricular assist device may be inserted at the time of LVAD implantation, particularly if unable to wean from cardiopulmonary bypass. Post-LVAD implant right heart failure that is refractory to medical management may require the implantation of a right ventricular assist device postoperatively. In a study of 484 patients in the HeartMate II bridge-to-transplantation trial, 6% ultimately required a right ventricular assist device after LVAD implantation (17). Peripheral veno-arterial extracorporeal membrane oxygenation can also be used to support the right ventricle as it recovers after LVAD implantation, although it is not as effective at unloading the ventricle and can be associated with thromboembolism, major bleeding, and extremity malperfusion.
Device malfunction
Device malfunction may be due to pump thrombosis or to other factors related to physical hardware. Pump thrombosis has been discussed previously. Various LVAD parameters including pump rotor speed, power, pulsatility index, and flow should be monitored in conjunction with the clinical status of the patient to provide early recognition of device malfunction. A percutaneous lead or motor failure manifests as the pump vibrating or “running rough”, not maintaining set speed, and high pulsatility index in conjunction with heart failure symptoms and a decreased blood pressure with increased pulse pressure. The manufacturer should be contacted and if the controllers are damaged, then the pump should be run on batteries. If the lead is damaged, it may be able to be repaired and if not, replacement of the pump may be required. A left ventricular suck down or suction event manifests with decreases in pump speed and flow. The patient should be adequately hydrated. In cases of inflow obstruction, there may be suction events and decreases in pump flow, pump power, and pulsatility index, and clinically there may be heart failure symptoms, hemolysis, or tamponade. Management includes ensuring adequate hydration, supporting the right ventricle, and re-exploring the chest if there is tamponade or if there is a need to adjust pump positioning. Outflow obstruction can manifest with similar LVAD parameters and clinical symptoms as inflow obstruction and may also require re-exploration to correct malpositioning.
Stroke
A study of over 5,300 continuous flow LVAD patients from the INTERMACS registry found estimated 1-month, 3-month, and 1-year stroke rates of 3%, 5%, and 11%, respectively (22). In LVAD patients presenting with an acute neurologic deficit, it is important to expeditiously evaluate the international normalized ratio (INR) and platelet count and to calculate the Glasgow Coma Scale score. A non-contrast head computed tomogram should be obtained. It is important to distinguish between hemorrhagic and ischemic stroke. In patients on antiplatelet agents who present with a hemorrhagic stroke and an INR greater than 1.4, prothrombin complex concentrate or fresh frozen plasma in addition to vitamin K, desmopressin acetate, and platelets should be administered (23). If the INR is less than 1.4, then only desmopressin acetate and platelets should be given. In cases of ischemic stroke, a computed tomography angiogram of the brain and potential endovascular therapy should be considered if the infarction is less than one-third of the cerebral hemisphere, the onset is within 8 hours, the National Institutes of Health Stroke Scale is greater than 6, and there are cortical or brainstem symptoms (23).
The rates of post-implant stroke are higher in those supported with the HeartWare device as compared to the HeartMate II. A review of 332 patients in the HeartWare pivotal bridge to transplant and continued access protocols trial demonstrated a 14.8% stroke event rate (24). A total of 7.5% and 7.8% of the study population had ischemic and hemorrhagic cerebrovascular accidents, respectively. Although the rates were higher, the majority of strokes were nonfatal. In those with an ischemic stroke, 65% had a modified Rankin score of 2 or less, 25% had no residual neurologic deficit, and 88% survived the stroke (24). Of interest are the results from the ENDURANCE supplemental trial that aims to confirm that regular monitoring and management of blood pressure is associated with a significantly lower rate of adverse neurologic events after LVAD implantation.
Conclusions
LVADs will be used in the treatment of end-stage heart failure with increasing frequency in coming years. There is a spectrum of complications associated with LVAD therapy. Although the management of these complications varies, early recognition and treatment are uniformly essential to prevent further morbidity and mortality. As new circulatory support devices and technology are introduced, the re-evaluation of these complication rates and identification of unique aspects of diagnosis and management will be prudent.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- United States Census Bureau: 65+ in the United States: 2005. Available online: http://www.census.gov/library/publications/2005/demo/p23-209.html, accessed August 23, 2015.
- Kirklin JK, Naftel DC, Pagani FD, et al. Long-term mechanical circulatory support (destination therapy): on track to compete with heart transplantation? J Thorac Cardiovasc Surg 2012;144:584-603; discussion 597-8. [PubMed]
- Kasirajan V, McCarthy PM, Hoercher KJ, et al. Clinical experience with long-term use of implantable left ventricular assist devices: indications, implantation, and outcomes. Semin Thorac Cardiovasc Surg 2000;12:229-37. [PubMed]
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007;357:885-96. [PubMed]
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [PubMed]
- Bruckner BA, DiBardino DJ, Ning Q, et al. High incidence of thromboembolic events in left ventricular assist device patients treated with recombinant activated factor VII. J Heart Lung Transplant 2009;28:785-90. [PubMed]
- Stulak JM, Romans T, Cowger J, et al. Delayed sternal closure does not increase late infection risk in patients undergoing left ventricular assist device implantation. J Heart Lung Transplant 2012;31:1115-9. [PubMed]
- Islam S, Cevik C, Madonna R, et al. Left ventricular assist devices and gastrointestinal bleeding: a narrative review of case reports and case series. Clin Cardiol 2013;36:190-200. [PubMed]
- Rennyson SL, Shah KB, Tang DG, et al. Octreotide for left ventricular assist device-related gastrointestinal hemorrhage: can we stop the bleeding? ASAIO J 2013;59:450-1. [PubMed]
- Schaffer JM, Allen JG, Weiss ES, et al. Infectious complications after pulsatile-flow and continuous-flow left ventricular assist device implantation. J Heart Lung Transplant 2011;30:164-74. [PubMed]
- Monkowski DH, Axelrod P, Fekete T, et al. Infections associated with ventricular assist devices: epidemiology and effect on prognosis after transplantation. Transpl Infect Dis 2007;9:114-20. [PubMed]
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33-40. [PubMed]
- Kirklin JK, Naftel DC, Kormos RL, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant 2014;33:12-22. [PubMed]
- Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant 2013;32:667-70. [PubMed]
- Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012;125:3191-200. [PubMed]
- Najjar SS, Slaughter MS, Pagani FD, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2014;33:23-34. [PubMed]
- Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010;139:1316-24. [PubMed]
- Pettinari M, Jacobs S, Rega F, et al. Are right ventricular risk scores useful? Eur J Cardiothorac Surg 2012;42:621-6. [PubMed]
- Fitzpatrick JR 3rd, Frederick JR, Hsu VM, et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant 2008;27:1286-92. [PubMed]
- Drakos SG, Janicki L, Horne BD, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol 2010;105:1030-5. [PubMed]
- Fitzpatrick JR 3rd, Frederick JR, Hiesinger W, et al. Early planned institution of biventricular mechanical circulatory support results in improved outcomes compared with delayed conversion of a left ventricular assist device to a biventricular assist device. J Thorac Cardiovasc Surg 2009;137:971-7. [PubMed]
- Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant 2013;32:141-56. [PubMed]
- Willey JZ, Demmer RT, Takayama H, et al. Cerebrovascular disease in the era of left ventricular assist devices with continuous flow: risk factors, diagnosis, and treatment. J Heart Lung Transplant 2014;33:878-87. [PubMed]
- Slaughter MS, Pagani FD, McGee EC, et al. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2013;32:675-83. [PubMed]


