Impact of anemia on long-term ischemic events and bleeding events in patients undergoing percutaneous coronary intervention: a system review and meta-analysis
Introduction
The presence of anemia is associated with worse clinical outcomes in patients with (1-5) or without (6-8) cardiovascular disease. The Atherosclerosis Risk in Communities (ARIC) study (9) and the Women’s Ischemia Syndrome Evaluation (WISE) study (10) have identified anemia as an independent predictor for adverse cardiovascular outcomes. Similar results have been described in patients undergoing percutaneous coronary intervention (PCI) (11-13). Previous studies have shown that anemia was a risk factor for mortality (14,15) and major adverse cardiac events (MACE) (16) in patients undergoing PCI. Anemia has also been reported to be associated with ischemic events (17) and major bleeding events (11,18) in patients undergoing PCI. Improved anemia is associated with favorable outcomes in patients undergoing PCI (19) or with cardiovascular diseases (20). In addition, the non-recovery of anemia is a biomarker of poor outcome (21).
However, data focused on the ischemic events and bleeding events are still limited. Furthermore, considering that many studies (13,16,17,22) were retrospective analysis without pre-specified design, these studies should be considered hypothesis generating, and thus not adequately powered to reach the conclusion that anemia increase the risk of ischemic or bleeding events.
In order to provide the latest and most convincing evidence, we systematically reviewed the current available literature to investigate whether anemia increase incidence of long-term ischemic events and long-term bleeding events in patients undergoing PCI. The secondary objective was to evaluate the effect of anemia on long-term mortality and MACE.
Materials and methods
The systematic review and meta-analysis was conducted and reported in adherence to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Literature search and selection criteria
PubMed and Embase were searched for records reporting the impact of anemia on long-term outcomes in patients undergoing PCI. The search strategy is shown in Table 1. An English language restriction was imposed. The last search was run on October 29, 2015. Two independent investigators carried out the initial search, deleted duplicate records, screened the titles and abstracts for relevance, and identified as excluded or requiring further assessment. Then we reviewed the full-text articles for inclusion. We also manually checked the references of the retrieved articles and previous reviews to identify additional eligible studies.
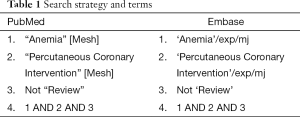
Full table
Studies meeting the following inclusion criteria were included: (I) population: patients undergoing PCI; (II) intervention: anemic patients; (III) comparison: non-anemic patients; (IV) outcome: long-term ischemic events and bleeding events; (V) design: case-control studies.
Data extraction and quality assessment
Data extraction was performed by Xiaoyan Wang and confirmed independently by Miaohan Qiu. The following information was extracted from each study: first author, year of publication, patient characteristics, No. of patients, study design, outcomes, definition of anemia. Extracted data were entered into a standardized Excel file. Discrepancies were resolved by discussion between the two investigators. The primary outcome was long-term ischemic events (including composite ischemic events, reinfarction, TVR. Secondary outcomes included long-term bleeding events, long-term mortality and long-term MACE. The methodologic quality of each study was evaluated using Newcastle-Ottawa Scale.
Statistical analysis
Differences were expressed as odds ratio (OR) with 95% confidence intervals (CIs). Heterogeneity across studies was tested by using the I2 statistic, which was a quantitative measure of inconsistency across studies. Studies with an I2 statistic of 25-50% were considered to have low heterogeneity, those with an I2 statistic of 50-75% were considered to have moderate heterogeneity and those with an I2 statistic of >75% were considered to have a high degree of heterogeneity. An I2 value greater than 50% indicates significant heterogeneity (23). The Mantel-Haenszel method with random effects model was used to calculate pooled ORs and 95% CIs. Post hoc analysis of RCTs was considered as equivalent to observational studies.
The presence of publication bias was evaluated by using the Begg et al. (24) and Egger et al. (25) tests. A P value <0.05 was judged as statistically significant, except where otherwise specified. All statistical analyses were performed using Stata 12.0 (Stata Corporation, College Station, TX, USA) and RevMan 5.2 (The Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Study identification and selection
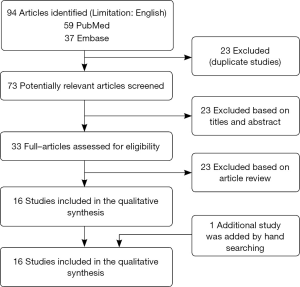
A total of 96 records were identified by the initial database search. Twenty three records were excluded for duplicates, and 40 records were excluded based on the titles and abstracts. The remaining 33 full-text articles were assessed for eligibility, and 17 (19,26-41) of them were excluded, among which five studies (19,28,31,34,36) didn’t divide the cohort into anemia and no anemia, seven studies (26,30,32,35,38,40,41) didn’t provide data of incidence of adverse events in anemic or non-anemic patients, four studies (27,29,33,39) didn’t provide data of adverse events after 1-year or more, one study (37) defined anemia as post-PCI anemia which is inconsistent with our definition. In addition, one study (42) was added by handle searching. Finally, 17 studies were included in the meta-analysis. The selection process is shown in Figure 1.
Study characteristics
The main characteristics of included studies are described in Table 2. These studies were published between 2004 and 2015. The sample size ranged from 312 to 13,032 (a total of 68,528 patients: 17,123 anemic patients and 51,405 non-anemic patients). The follow-up times range from 12 to 46.7 months. Among the 17 studies, five (11,14,18,46,48) were conducted in North America, six (16,22,42,43,45,50) in Asia, three (12,44,47) in Europe. Three (13,17,50) studies were multicenter studies. All studies were published in English.
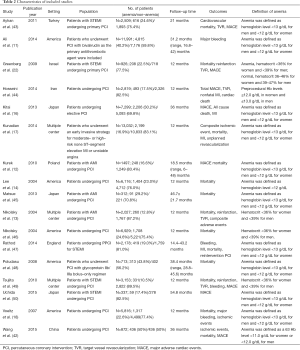
Full table
Considering ischemic events, two studies reported data regarding composite outcome of ischemic events. Eight studies provided data for target vessel revascularization (TVR) and reinfarction, which were major elements in ischemic events. Among the eight studies (13,16,17,22,43,44,47,49), five studies (13,22,43,44,49) reported the incidence of TVR after PCI, eight studies (13,16,17,22,43,44,47,49) reported incidence of reinfarction after PCI. For bleeding events, four studies (11,18,47,49) reported the incidence of bleeding events. Fifteen studies (12-14,16-18,22,42-49) reported incidence of mortality and nine studies (12,16,22,42-44,47,49,50) reported incidence of MACE. Among the 17 studies, eight studies (11,16,22,43-45,47,50) provided both the number and the incidence of adverse events, four studies (12,13,46,49) provided the incidence of adverse events. Voeltz et al. (18) provided the incidence of mortality and the number of bleeding events. Kunadian et al. (17) provided RR for adverse events (ischemic events, revascularization, reinfarction, mortality), Lee et al. (14), Poludasu et al. (48) and Wang et al. (42) provided HR for mortality.
Quality assessment
Risk-of-bias assessment of the included studies is presented in Table 3. All studies included have a total score of more than five, among which three study (14,17,48) was eight, five studies (12,16,42,43,45) was seven, five studies (13,18,22,44,49) was six, four studies (11,46,47,50) was five.

Full table
Primary outcome: long-term ischemic events
Long-term composite ischemic events
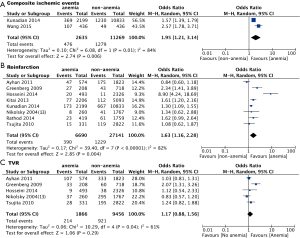
Wang et al. (42) and Kunadian et al. (17) provided data for ischemic events. Considering the substantial heterogeneity among these studies, a random effect model was used to combine the results. The pooled analysis of the two studies using a random effect model show that anemic patients are at higher risk for ischemic events (OR: 1.95, 95% CI, 1.21-3.14, P<0.01) (Figure 2A). There’s a high degree of heterogeneity between the two studies (I2=84%, P=0.01) (Figure 2A).
Long-term reinfarction
Eight studies provided data for reinfarction. The pooled analysis using a random effect model show that anemic patients are at significantly higher risk for long-term reinfarction (OR: 1.63, 95% CI, 1.16-2.28, P<0.01) (Figure 2B), with a high degree of heterogeneity for the included studies (I2=82%, P<0.01) (Figure 2B).
Long-term TVR
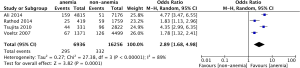
Five studies provided data for TVR. The pooled analysis using a random effect model shows that there is a trend that anemic patients are at higher risk for long-term revascularization (OR: 1.77, 95% CI, 0.88-1.56), but the difference was not statistically significant (P=0.29) (Figure 2C). There is significant heterogeneity among those included studies (I2=61%, P=0.04) (Figure 2C).
Long-term bleeding events
Four studies reported the incidence of long-term bleeding events. The pooled analysis using a random effect model shows that anemic patients are at a significantly higher incidence of bleeding events (OR: 2.89, 95% CI, 1.68-4.98, P<0.001) (Figure 3). There is a high degree of heterogeneity among the included studies (I2=89%, P<0.01) (Figure 3).
Secondary outcomes: long-term mortality and long-term MACE
Long-term mortality
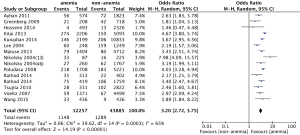
Fifteen studies provided data for long-term mortality. The pooled analysis using a random effect model shows that anemic patients are at high risk for long-term mortality (OR: 3.20, 95% CI, 2.72-3.75, P<0.01) (Figure 4). There is a moderate heterogeneity among those included studies (I2=65%, P<0.01) (Figure 4).
Long-term MACE
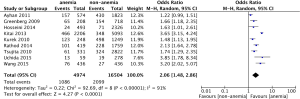
Nine studies provided data for long-term MACE. The pooled analysis using a random effect model shows that compared with non-anemic patients, anemic patients are at high risk for long-term MACE (OR: 2.06, 95% CI, 1.48-2.86, P<0.01) (Figure 5). There is a high degree of heterogeneity among the included studies (I2=91%, P<0.01) (Figure 5).
Publication bias
We cannot evaluate publication bias of the only 2 studies reporting the data of ischemic events, so we evaluate publication bias of the impact of anemia on reinfarction instead. There was no evidence of significant publication bias by formal statistical tests (Egger’s test, P=0.91; Begg’s test P=1.00)
Discussion
This meta-analysis identified 17 case-control studies investigating the impact of anemia on long-term ischemic events in patients undergoing PCI. To our known, this is the first meta-analysis evaluated the impact of anemia on the long-term adverse events in patients undergoing PCI. Our analysis showed that anemia patients were at higher risk for long-term ischemic events and bleeding events. In addition, anemia patients are at higher risk for long-term mortality and MACE.
Previous studies have shown the association between anemia and ischemic events (3,10) in patients in different settings, but studies reporting the association between anemia and composite ischemic events in patients undergoing PCI are still limited. Although there’re only two studies in our meta-analysis provided the data for composite ischemic events, the two studies deduced a same conclusion that anemic patients are at risk for ischemic events. What’s more, the pooled analysis of the impacts of anemia on reinfarction also relate anemia to ischemic events. For long-term TVR, our analysis showed that anemic patients are also at higher risk for TVR than non-anemic patients (OR: 1.77), but the difference was not statistically significant (P=0.29). This result may be explained by the fact that some patients with ischemic events choose drug therapy instead of revascularization, which can weaken the association between anemia and revascularization. In addition, due to some included studies didn’t provide the incidence of total revascularization (they only provided the incidence of TVR, TLR or CABG, but the incidence of total revascularization can’t be simply summarized by the above incidence of single events), we only use the incidence of TVR in these studies. Thus, whether anemia is associated with the incidence of total revascularization need more studies to identify. Overall, Our results agree with previous studies relating anemia to ischemic events, and further identified this association of anemia and long-term ischemic events in patients undergoing PCI.
It has also been shown that anemia is associated with bleeding events (11,51). In our study, four studies provided data for long-term bleeding events, but Tsujita et al. (49) and Voeltz et al. (18) only provided data for TIMI major bleeding or minor bleeding events not the total bleeding events. Considering the incidence of total bleeding events can’t be simply summarized by major and minor bleeding events, we use the data of TIMI major bleeding events in the two studies. Our results agree with previous studies relating anemia to bleeding events. Previous studies show that anemia is associated with in-hospital or long-term mortality, MACE in patients undergoing PCI, our analysis further confirms it.
We have discussed the reason for ischemic events in our previous study (42). In brief, the presence of anemia can decrease oxygen delivery to the myocardium and induce myocardial ischemia through mismatches in oxygen supply and demand, especially in patients with coronary artery stenosis. This effect may induce an increased heart rate and blood volume that is mainly mediated through the activation of the sympathetic nervous system and the renin-angiotensin-aldosterone system (52,53). The latter can sharpen the mismatch between supply and demand of oxygen through ventricular remodeling and cardiac dysfunction, and thus increase the incidence of ischemic events.
The potential mechanisms of higher bleeding events are list as follows. First, anemia may be associated with the reduction of thrombogenesis, the abnormal function of platelet, higher degrees of inflammation, which can increase the risk of bleeding. Second, anemic patients are more often elder patients and with chronic kidney disease. Advanced age has been found to be associated with an increased risk of vascular complications following PCI (54). The presence of local vascular changes, or of more advanced vascular disease, has been postulated as a potential explanation for the increased incidence of bleeding complications in elderly patients. Similarly to advanced age, renal dysfunction has been identified as an important correlate of adverse outcomes following percutaneous cardiac procedures (55,56). Platelet dysfunction (57), elevated levels of anti-Xa (58), impaired clearance of low-molecular-weight heparin (59), and additional abnormalities in the coagulation cascade are all plausible explanations for the increased bleeding risk observed in renally impaired patients.
There are some limitations in our study. First, there are only two studies provided data of composite ischemic events; we cannot get enough data to make the results powerful enough. Second, as we discussed earlier, due to some included studies didn’t provide the incidence of total revascularization or total bleeding events, so we analyzed the impact of anemia on TVR and TIMI major bleeding events instead. This may have some influence on our results. Third, the time for adverse events in our study ranged from 12-46.6 months, we haven’t analyzed the association of anemia and adverse events in different years after PCI. Whether the association between anemia and adverse events in the first year after PCI is the same with the 5 years remains unknown.
Further studies should focus on the further points. First, there’s a need for further clarification and consistency regarding dosage, timing and duration of antithrombotic therapy for the prevention of ischemic events and bleeding events. Second, anemic patients were more likely to be female, elder patients, and often have more comorbidities, which can increase the risk for adverse events. Further studies need to explore whether there’re differences among those different subgroups. Finally, whether the impact of anemia on the outcomes differs at different stage after PCI need more studies to confirm.
Conclusions
Anemic patients undergoing PCI are at higher risk for both long-term ischemic events and bleeding events, and also at higher risk for long-term mortality and MACE. There’s a need for further clarification and consistency regarding dosage, timing and duration of antithrombotic therapy for the prevention of ischemic events and bleeding events in anemic patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Karkouti K, Wijeysundera DN, Beattie WS, et al. Reducing Bleeding in Cardiac Surgery I. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation 2008;117:478-84. [PubMed]
- Peterson PN, Magid DJ, Lyons EE, et al. Association of longitudinal measures of hemoglobin and outcomes after hospitalization for heart failure. Am Heart J 2010;159:81-9. [PubMed]
- Sabatine MS, Morrow DA, Giugliano RP, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation 2005;111:2042-9. [PubMed]
- Horwich TB, Fonarow GC, Hamilton MA, et al. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 2002;39:1780-6. [PubMed]
- von Haehling S, Jankowska EA, van Veldhuisen DJ, et al. Iron deficiency and cardiovascular disease. Nat Rev Cardiol 2015;12:659-69. [PubMed]
- Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med 2006;119:327-34. [PubMed]
- Elwood PC WW, Benjamin IT, Sweetnam PM. Mortality and anaemia in women. Lancet 1974;1:891-4. [PubMed]
- Patel KV, Guralnik JM. Prognostic implications of anemia in older adults. Haematologica 2009;94:1-2. [PubMed]
- Sarnak MJ TH, Manjunath G, MacLeod B, et al. Anemia as a risk factor for cardiovascular disease in The Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol 2002;40:27-33. [PubMed]
- Arant CB, Wessel TR, Olson MB, et al. Hemoglobin level is an independent predictor for adverse cardiovascular outcomes in women undergoing evaluation for chest pain: results from the National Heart, Lung, and Blood Institute Women's Ischemia Syndrome Evaluation Study. J Am Coll Cardiol 2004;43:2009-14. [PubMed]
- Ali ZA, Poludasu S, Qureshi YH, et al. Impact of major bleeding on long-term mortality in anemic versus nonanemic patients undergoing percutaneous coronary intervention using bivalirudin. Am J Cardiol 2014;113:1481-6. [PubMed]
- Kurek T, Lenarczyk R, Kowalczyk J, et al. Effect of anemia in high-risk groups of patients with acute myocardial infarction treated with percutaneous coronary intervention. Am J Cardiol 2010;105:611-8. [PubMed]
- Nikolsky E, Aymong ED, Halkin A, et al. Impact of anemia in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: analysis from the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) Trial. J Am Coll Cardiol 2004;44:547-53. [PubMed]
- Lee PC, Kini AS, Ahsan C, et al. Anemia is an independent predictor of mortality after percutaneous coronary intervention. J Am Coll Cardiol 2004;44:541-6. [PubMed]
- Halkin A, Singh M, Nikolsky E, et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol 2005;45:1397-405. [PubMed]
- Kitai Y, Ozasa N, Morimoto T, et al. Prognostic implications of anemia with or without chronic kidney disease in patients undergoing elective percutaneous coronary intervention. Int J Cardiol 2013;168:5221-8. [PubMed]
- Kunadian V, Mehran R, Lincoff AM, et al. Effect of anemia on frequency of short- and long-term clinical events in acute coronary syndromes (from the Acute Catheterization and Urgent Intervention Triage Strategy Trial). Am J Cardiol 2014;114:1823-9. [PubMed]
- Voeltz MD, Patel AD, Feit F, et al. Effect of anemia on hemorrhagic complications and mortality following percutaneous coronary intervention. Am J Cardiol 2007;99:1513-7. [PubMed]
- Kim TH, Koh YS, Chang K, et al. Improved anemia is associated with favorable long-term clinical outcomes in patients undergoing PCI. Coron Artery Dis 2012;23:391-9. [PubMed]
- Salisbury AC, Kosiborod M, Amin AP, et al. Recovery from hospital-acquired anemia after acute myocardial infarction and effect on outcomes. Am J Cardiol 2011;108:949-54. [PubMed]
- Leshem-Rubinow E, Steinvil A, Rogowski O, et al. Hemoglobin nonrecovery following acute myocardial infarction is a biomarker of poor outcome: a retrospective database study. Int J Cardiol 2013;169:349-53. [PubMed]
- Greenberg G, Assali A, Vaknin-Assa H, et al. Hematocrit level as a marker of outcome in ST-segment elevation myocardial infarction. Am J Cardiol 2010;105:435-40. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Catakoglu AB, Aytekin S, Sener M, et al. Impact of anemia on nonfatal coronary events after percutaneous coronary interventions. Heart Vessels 2007;22:383-8. [PubMed]
- Dündar C, Oduncu V, Erkol A, et al. In-hospital prognostic value of hemoglobin levels on admission in patients with acute ST segment elevation myocardial infarction undergoing primary angioplasty. Clin Res Cardiol 2012;101:37-44. [PubMed]
- Husemann W, Fobker M, Pohlen M, et al. Impact of haemoglobin concentration and chronic kidney disease in patients with coronary heart disease undergoing percutaneous coronary interventions. Nephrol Dial Transplant 2007;22:2563-70. [PubMed]
- Jani SM, Smith DE, Share D, et al. Blood Transfusion and In-hospital Outcomes in Anemic Patients with Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Clin Cardiol 2007;30:II49-56. [PubMed]
- Kim P, Dixon S, Eisenbrey AB, et al. Impact of Acute Blood Loss Anemia and Red Blood Cell Transfusion on Mortality after Percutaneous Coronary Intervention. Clin Cardiol 2007;30:II35-43. [PubMed]
- Maluenda G, Lemesle G, Collins SD, et al. The clinical significance of hematocrit values before and after percutaneous coronary intervention. Am Heart J 2009;158:1024-30. [PubMed]
- Manzano-Fernández S, Marin F, Martinez JA, et al. Anaemia as predictor of gastrointestinal bleeding in atrial fibrillation patients undergoing percutaneous coronary artery stenting. QJM 2008;101:749-51. [PubMed]
- McKechnie RS, Smith D, Montoye C, et al. Prognostic implication of anemia on in-hospital outcomes after percutaneous coronary intervention. Circulation 2004;110:271-7. [PubMed]
- Pilgrim T, Rothenbühler M, Kalesan B, et al. Additive effect of anemia and renal impairment on long-term outcome after percutaneous coronary intervention. PLoS One 2014;9:e114846. [PubMed]
- Pilgrim T, Vetterli F, Kalesan B, et al. The impact of anemia on long-term clinical outcome in patients undergoing revascularization with the unrestricted use of drug-eluting stents. Circ Cardiovasc Interv 2012;5:202-10. [PubMed]
- Reinecke H, Trey T, Wellmann J, et al. Haemoglobin-related mortality in patients undergoing percutaneous coronary interventions. Eur Heart J 2003;24:2142-50. [PubMed]
- Sattur S, Harjai KJ, Narula A, et al. The influence of anemia after percutaneous coronary intervention on clinical outcomes. Clin Cardiol 2009;32:373-9. [PubMed]
- Sharma SK. Is mortality raised in patients receiving percutaneous coronary intervention if they suffer from anemia? Nat Clin Pract Cardiovasc Med 2004;1:74-5. [PubMed]
- Shiraishi J, Kohno Y, Nakamura T, et al. Prognostic impact of chronic kidney disease and anemia at admission on in-hospital outcomes after primary percutaneous coronary intervention for acute myocardial infarction. Int Heart J 2014;55:301-6. [PubMed]
- Steinvil A, Rogowski O, Banai S, et al. Anemia and inflammation have an additive value in risk stratification of patients undergoing coronary interventions. J Cardiovasc Med (Hagerstown) 2015;16:106-11. [PubMed]
- Vrsalovic M, Pintaric H, Babic Z, et al. Impact of admission anemia, C-reactive protein and mean platelet volume on short term mortality in patients with acute ST-elevation myocardial infarction treated with primary angioplasty. Clin Biochem 2012;45:1506-9. [PubMed]
- Wang X, Qiu M, Li J, et al. Impacts of anemia on 3-year ischemic events in patients undergoing percutaneous coronary intervention: a propensity-matched study. J Thorac Dis 2015;7:1951-9.
- Ayhan E, Aycicek F, Uyarel H, et al. Patients with anemia on admission who have undergone primary angioplasty for ST elevation myocardial infarction: in-hospital and long-term clinical outcomes. Coron Artery Dis 2011;22:375-9. [PubMed]
- Hosseini SK, Ansari MJ, Lotfi Tokaldany M, et al. Association between preprocedural hemoglobin level and 1-year outcome of elective percutaneous coronary intervention. J Cardiovasc Med (Hagerstown) 2014;15:331-5. [PubMed]
- Matsue Y, Matsumura A, Abe M, et al. Prognostic implications of chronic kidney disease and anemia after percutaneous coronary intervention in acute myocardial infarction patients. Heart Vessels 2013;28:19-26. [PubMed]
- Nikolsky E, Mehran R, Aymong ED, et al. Impact of anemia on outcomes of patients undergoing percutaneous coronary interventions. Am J Cardiol 2004;94:1023-7. [PubMed]
- Rathod KS, Jones DA, Rathod VS, et al. Prognostic impact of anaemia on patients with ST-elevation myocardial infarction treated by primary PCI. Coron Artery Dis 2014;25:52-9. [PubMed]
- Poludasu S, Marmur JD, Weedon J, et al. Effect of hemoglobin level on long-term all-cause mortality after percutaneous coronary intervention in African-Americans. Am J Cardiol 2009;103:1078-82. [PubMed]
- Tsujita K, Nikolsky E, Lansky AJ, et al. Impact of anemia on clinical outcomes of patients with ST-segment elevation myocardial infarction in relation to gender and adjunctive antithrombotic therapy (from the HORIZONS-AMI trial). Am J Cardiol 2010;105:1385-94. [PubMed]
- Uchida Y, Ichimiya S, Ishii H, et al. Impact of Admission Anemia on Coronary Microcirculation and Clinical Outcomes in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Int Heart J 2015;56:381-8. [PubMed]
- Dauerman HL, Lessard D, Yarzebski J, et al. Bleeding complications in patients with anemia and acute myocardial infarction. Am J Cardiol 2005;96:1379-83. [PubMed]
- Hébert PC, Van der Linden P, Biro G, et al. Physiologic aspects of anemia. Crit Care Clin 2004;20:187-212. [PubMed]
- Anand IS, Chandrashekhar Y, Ferrari R, et al. Pathogenesis of oedema in chronic severe anaemia: studies of body water and sodium, renal function, haemodynamic variables, and plasma hormones. Br Heart J 1993;70:357-62. [PubMed]
- Bertrand OF, Larose E, Rodés-Cabau J, et al. Incidence, predictors, and clinical impact of bleeding after transradial coronary stenting and maximal antiplatelet therapy. Am Heart J 2009;157:164-9. [PubMed]
- Kim JH, Lee JH, Jang SY, et al. Prognostic value of early acute kidney injury after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2014;114:1174-8. [PubMed]
- Takagi K, Chieffo A, Naganuma T, et al. Impact of renal dysfunction on long-term mortality in patients with unprotected left main disease: Milan and New-Tokyo (MITO) Registry. Int J Cardiol 2014;177:1131-3. [PubMed]
- Uçar H, Gür M, Koyunsever NY, et al. Mean platelet volume is independently associated with renal dysfunction in stable coronary artery disease. Platelets 2014;25:274-8. [PubMed]
- Lim W, Dentali F, Eikelboom JW, et al. Meta-analysis: low-molecular-weight heparin and bleeding in patients with severe renal insufficiency. Ann Intern Med 2006;144:673-84. [PubMed]
- Lim W. Low-molecular-weight heparin in patients with chronic renal insufficiency. Intern Emerg Med 2008;3:319-23. [PubMed]