Left ventricular thrombus associated with arteriovenous extra corporeal membrane oxygenation
Introduction
Extra corporeal membrane oxygenation (ECMO) indications, usage, and outcomes have strikingly progressed over the past 20 years; it became tool essential in the care of adults and children with severe cardiac and pulmonary dysfunction refractory to conventional management (1,2). Nowadays ECMO has become more reliable with improvement of equipment, and increased experience, which is reflected in improving results. Her we will present a case of a rare complication of ECMO due to left ventricular distension and stasis.
Case presentation
Nineteen years old male with no significant past medical history, other than current history of worsening fatigue and dyspnea for 2 weeks prior to his presentation to referring hospital. He was initially diagnosed with flu and treated with Tamiflu. He was admitted to the referring hospital in florid pulmonary edema and was immediately intubated. Echocardiogram (ECHO) revealed an ejection fraction (EF) of 10-15%. Intra-aortic balloon pump was placed and supported with a combination of inotropes and pressor. The patient was transferred to our hospital for further management. He was taken emergently to the operating room for institution of central venoarterial (VA) ECMO. The patient had cardiac arrest on the induction of anesthesia and cardiopulmonary resuscitation was initiated. Emergent sternotomy was performed and central VA ECMO was initiated (right atrium—aorta). The chest was temporarily packed and closed due to coagulopathic bleeding. Anticoagulation was started on POD 2. He returned to the operating room on POD 3 fr washout, placement of LV vent, and delayed sternal closure. Follow up TEE on POD 4 showed a new large thrombus (Figures 1,2), measuring 35×50 millimeter. The patient was separated from ECMO on POD 14 following recovery of adequate cardiac function. Figure 3 shows well-formed LV clot obtained on POD 23. Unfortunately the patient suffered of severe, irreversible anoxic encephalopathy during the cardiac arrest. CT scan of the head did not demonstrate any thromboembolic events. The families choose to withdrawal care.
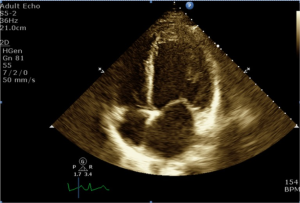
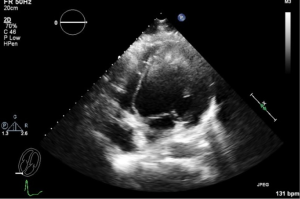

Discussion
VA ECMO provides excellent hemodynamic support in for adult patients in cardiogenic shock or following cardiac arrest. ECMO is provided until the patient’s cardiac function recovers or receives other types of circulatory support including ventricular assist device or cardiac transplantation. Historic survival rate reported in literature ranges between 30-40% among patients who received the VA ECMO for cardiac arrest, severe cardiogenic shock, or failure to wean from cardiopulmonary bypass following cardiac surgery (1,2). Risks of left ventricle dysfunction and distension have been described in both central and peripheral VA cannulation (3-6). Because of poorly contracting, non-ejecting left ventricle or atrium, intracardiac clots may develop as the result of decreased blood flow to the left ventricle, increased afterload from the arterial cannula, and LV distention with resultant stasis (3-5). Ideally, patient should be maintained on inotropic support to maintain LV contracting and LV vent should be inserted. If peripheral VA ECMO is used; percutaneous atrial septostomy, transapical LV vent through left thoracotomy, adding Impella represent alternative options (5-7). Anticoagulation should be resumed as early as possible to avoid the risk of intracardiac thrombus, recognizing that the high ECMO circuit flow might protect developing clots inside the ECMO circuit but not the left side of the heart.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Combes A, Leprince P, Luyt CE, et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med 2008;36:1404-11. [PubMed]
- Smedira NG, Blackstone EH. Postcardiotomy mechanical support: risk factors and outcomes. Ann Thorac Surg 2001;71:S60-6; discussion S82-5.
- Weis F, Beiras-Fernandez A, Bruegger D. Huge intracardiac thrombosis in a patient on veno-arterial extracorporeal membrane oxygenation support. Interact Cardiovasc Thorac Surg 2009;8:247-9. [PubMed]
- Gaide-Chevronnay L, Durand M, Rossi-Blancher M, et al. Cardiac thrombosis in a patient during extracorporeal life support. J Cardiothorac Vasc Anesth 2012;26:664-5. [PubMed]
- Avalli L, Maggioni E, Sangalli F, et al. Percutaneous left-heart decompression during extracorporeal membrane oxygenation: an alternative to surgical and transeptal venting in adult patients. ASAIO J 2011;57:38-40. [PubMed]
- Aiyagari RM, Rocchini AP, Remenapp RT, et al. Decompression of the left atrium during extracorporeal membrane oxygenation using a transseptal cannula incorporated into the circuit. Crit Care Med 2006;34:2603-6. [PubMed]
- Kawashima D, Gojo S, Nishimura T, et al. Left ventricular mechanical support with Impella provides more ventricular unloading in heart failure than extracorporeal membrane oxygenation. ASAIO J 2011;57:169-76. [PubMed]


