Spectrum of FDG uptake in large (>10 cm) esophageal leiomyomas
Introduction
Esophageal leiomyomas while rare, represent the most prevalent benign esophageal tumor (60–70%) (1). Surgical resection is generally recommended to alleviate obstructive symptoms. The prognosis of surgically treated leiomyomas is excellent, and the vast majority of patients fully recover after limited resection.
It is occasionally important to distinguish leiomyomas from more aggressive submucosal esophageal tumors, most notably gastrointestinal stromal tumors (GISTs). GISTs are less common in the esophagus than leiomyomas, but have a worse prognosis, particularly when they are large (>10 cm) and have high mitotic counts (2). As a result, surgeons may elect to manage larger GISTs (>10 cm) differently than leiomyomas, potentially offering more extensive surgery, or attempting to cytoreduce the tumor with induction imatinib (3). Therefore, establishing the diagnosis of leiomyoma or GIST may be helpful in some cases.
Unfortunately, distinguishing esophageal leiomyoma from GIST prior to resection can be challenging. The two types of lesions appear similar on computed tomography (CT) imaging and by endoscopic ultrasound (EUS). The demonstration of c-Kit by immunohistochemistry can be diagnostic for GIST, however fine needle aspirations (FNA) of these submucosal lesions are often inadequate or inconclusive (4). Positron emission tomography (PET) scanning has been used to stratify the aggressiveness of various other tumors in the chest. Furthermore GISTs are commonly FDG avid by PET scanning (3). Therefore PET scanning has great appeal in distinguishing leiomyoma from GIST. We examined the maximum standardized uptake value (SUVmax) in patients with large (>10 cm) leiomyomas of the esophagus to assess the spectrum of FDG uptake in these lesions.
Clinical summary
Three patients with large (>10 cm) esophageal leiomyomas were reviewed. All three patients underwent CT scan, PET scan, EUS and FNA prior to surgery. The clinical and pathologic information is presented in Table 1.

Full table
The PET scans demonstrated a range of FDG avidity (Case 1 = SUVmax of 1.3, Case 2 =5.1 and Case 3 =10.1) (Figure 1). All three patients underwent surgical resection of their primary tumors including video-assisted thoracic surgery (VATS) in two cases, and Ivor Lewis esophagectomy in a patient with a bleeding leiomyoma that had destroyed a large portion of the esophageal mucosa. The post-operative immunohistochemical staining for these tumors were all positive for smooth muscle actin (SMA) and were all negative for c-Kit and S100. All three patients recovered to baseline after surgery with resolution of preoperative symptoms.
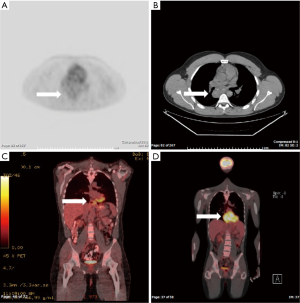
Discussion
Although fusion PET/CT may at times be used to estimate the aggressiveness of a tumor, the wide range of FDG uptake in the current series limits the role of PET in the differential diagnosis of submucosal lesions of the esophagus. In our review of the literature, we identified eight esophageal leiomyomas imaged by PET (only five reports with SUVmax given) with an average SUVmax of 6.6 and a range of 3.8 to 13.4 (5,6).
To the best of our knowledge, this series represents the first evaluation of PET FDG avidity in leiomyomas larger than 10 cm in size (which corresponds to a size in which GISTs are more aggressive and therefore the distinction more important). The wide spectrum of FDG uptake unfortunately precludes PET scanning from reliably distinguishing leiomyomas from more aggressive lesions. Case 3 in particular illustrates this point, as the tumor board that reviewed this patient felt the particularly high FDG uptake was more concerning for GIST and he was actually offered neoadjuvant imatinib at another institution (they were planning repeat FNA to evaluate c-Kit).
Differentiating large mesenchymal esophageal tumors preoperatively could facilitate the most efficacious operative treatment, yet remains a challenge. There are no specific findings in a patient’s clinical presentation, endoscopic results, or CT scan to differentiate esophageal GISTs from leiomyomas. Fine-needle aspiration can result in a conclusive diagnosis but is often avoided with submucosal lesions over concerns that scarring could make enucleation more difficult. Some have suggested that tumors that are larger than 2 cm, showing continued growth, or that are PET avid should undergo EUS with FNA (7). In theory the surgeon could use intraoperative sampling to guide the resection, but unfortunately frozen section may not be able to generate a definitive diagnosis because of the histologic similarities between GISTs and other spindle cell tumors. Therefore the surgeon must often determine the extent of the resection (enucleation or wider excision) based on clinical impression alone. The current case series demonstrates that PET scans are not able to facilitate this challenging decision (because leiomyomas may be quite avid).
Current research conducted on the usefulness of diffusion-weighted imaging (DWI) provides a potential option for pre-surgical diagnosis. A number of studies have shown the utility of DWI along with the apparent diffusion coefficient (ADC) in differentiating between uterine leiomyomas and leiomyosarcomas (8,9). Studies have shown that uterine leiomyomas tend to exhibit low signal intensity on DWI while leiomyosarcomas show an intermediate high signal intensity (9). The ADC values for sarcomas tend to be significantly lower than leiomyomas, further making a specific preoperative diagnosis more likely. It is possible that with further study, the use of DWI and ADC could provide an imaging modality for preoperative diagnosis of esophageal smooth muscle tumors.
Conclusions
These findings indicate that large (>10 cm) esophageal leiomyomas may demonstrate a wide spectrum of FDG uptake and therefore PET scans do not have a role in differentiating leiomyomas from more aggressive malignancies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kernstine KH, Weiss LM. Benign: Leiomyoma. In: Hunter JG, Jobe BA, Thomas CR, editors. Esophageal Cancer: Principles and Practice. New York: Demos Medical, 2009:315-9. Available online: http://www.demosmedical.com/esophageal-cancer.html
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol 2002;10:81-9. [PubMed]
- McAuliffe JC, Hunt KK, Lazar AJ, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol 2009;16:910-9. [PubMed]
- Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc 2010;71:913-9. [PubMed]
- An YS, Kim DY. 18F-fluorodeoxyglucose PET/CT in a patient with esophageal and genital leiomyomatosis. Korean J Radiol 2009;10:632-4. [PubMed]
- Lee HJ, Park SI, Kim DK, et al. Surgical resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg 2009;87:1569-71. [PubMed]
- Blum MG, Bilimoria KY, Wayne JD, et al. Surgical considerations for the management and resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg 2007;84:1717-23. [PubMed]
- Tasaki A, Asatani MO, Umezu H, et al. Differential diagnosis of uterine smooth muscle tumors using diffusion-weighted imaging: correlations with the apparent diffusion coefficient and cell density. Abdom Imaging 2015;40:1742-52. [PubMed]
- Sato K, Yuasa N, Fujita M, et al. Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcoma. Am J Obstet Gynecol 2014;210:368.e1-8.


