Surgical treatment of primary tracheobronchial tumors: 16-year experience in a single center
Introduction
Primary tracheobronchial tumor (TBT) is a rare disease with an incidence of 0.1–0.4% (1), originating in different parts of the trachea. Due to the low incidence and non-specific clinical manifestations, TBT is often misdiagnosed as bronchial asthma, bronchitis and other diseases in the early stage. Severe respiratory symptoms will appear when the trachea is blocked by more than 75%. Surgical treatment is usually the most important treatment for TBT (2-4). Survival after diagnosis was significantly longer for patients undergoing curative intent resection, with a median overall survival (OS) of 82–198 months, as compared with 3–92 months for patients who did not undergo surgery (2,5-8).
However, due to the low incidence of primary TBT, the reported prognostic factors affecting the survival of TBT are unclear. Previous studies have reported that age (young), lymph node involvement (N0), small tumor size, margin status (negative), histology [adenoid cystic carcinoma (ACC)] and the use of radiotherapy were associated with an improved survival of tracheobronchial patients (8-12). A report used the National Cancer Database (NCDB) demonstrated that insurance status, cancer grade, residual tumors status, histology and tumor extension were significantly associated with OS for patients with resected primary tracheal carcinoma (13). However, recognized risk factors are still lack. More experience should be accumulated to further clarify the prognostic factors associated with surgical treatment effect of primary TBT.
In this study, the clinical data of 69 patients diagnosed with primary TBT and accepted surgical treatment from January 2004 to January 2020 in our hospital was reviewed and analyzed to identify the potential prognostic factors affecting the surgical outcomes.
We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1791/rc).
Methods
Patients
The clinical data of 69 patients diagnosed as primary TBT and accepted surgical treatment at The Second Affiliated Hospital of Air Force Medical University between January 2004 and January 2020 were retrospectively reviewed and analyzed. And the univariate analysis and multivariate analysis were conducted on the malignant cases. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of the Second Affiliated Hospital of Air Force Medical University (No. K202111-04) and individual consent for this retrospective analysis was waived.
Clinical information and follow-up
Patient clinical and treatment data were collected from Medical Record System of The Second Affiliated Hospital of Air Force Medical University. Clinical data included age, tumor size, gender, length of stay, histological type, smoking history, data of surgery, primary tumor location and symptoms. Based on previous studies, 2 cm of tumor size was used as cut-off for analyzing (1). Treatment data included tumor extension (E1, primary tumor confined to the trachea/bronchus; E2, primary tumor spread outside the trachea/bronchus or spread to adjacent organs) (8,14), lymph node status (N0, no lymph node metastasis; N1, lymph node metastasis; Nx, without dissection of lymph nodes), residual tumors status (R0, no residual tumor after surgery; R1, microscopic residual tumor after surgery) and postoperative treatment [adjuvant therapy was performed for patients with R1 or N1 status. And the decision of conducting adjuvant therapy for patients with E2 status was made based on the multi-disciplinary treatment (MDT)]. Patient follow-up data were collected from Medical Record System and contacting with patients by telephone calls. Computed tomography scan examinations were conducted for evaluation after surgery. And the follow-up was generally performed every 3 months for the first two years, and then every 6 months. OS was defined as the time from the date of surgery to the date of death. Deadline for follow-up was December 16, 2020.
Statistical analysis
Continuous variables were presented as the mean ± standard deviation (SD) while categorical variables were presented as number and percentage. Survival curves were estimated via the Kaplan-Meier method and compared using a log-rank test. Potential factors affecting survival were explored using Cox regression model analysis. A 2-sided P<0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics version 25 (IBM Corp, Armonk, NY, USA).
Results
Characteristics of patients
A total of 72 patients with primary TBT were treated by surgery from 2004 to 2020. And the operative and 30-day mortality were 0% and 4.17% respectively. Finally, 69 patients were included in analysis. The demographic and clinical data of the overall population were summarized in Table 1. Of all 69 patients, there were 62 malignant cases [squamous cell carcinoma (SCC): 16; ACC: 26; mucoepidermoid carcinoma: 10; carcinoids: 4; adenocarcinoma: 4; epithelia-myoepithelial carcinoma: 1; sarcoma: 1] and 7 benign cases (inflammatory pseudotumor: 3; vascular tumor: 1; leiomyoma: 1; schwannoma: 1; plasmacytoma: 1). A total of 29 men and 40 women, with a mean age of 45.3±13.9 years, were included. SCC patients tended to be older than ACC patients (P<0.05) and patients with other histology (P<0.05). The mean tumor size was 2.5±1.2 cm. The mean length of stay was 19.4±8.6 days; 22 patients were smokers, and SCC patients were more smokers than ACC patients (P=0.06) and other patients (P=0.02). Tumors in 17 patients (24.6%) were located in the cervical trachea, 28 (40.6%) were located in the intrathoracic trachea, 5 (7.2%) were located in the carina, 12 (17.4%) were located in the right main bronchus, 7 (10.1%) were located in the left main bronchus. The major symptoms included cough, wheeze, bloody sputum, hemoptysis, breathless, dyspnea, fever and hoarseness. The most initial symptom was cough (65.2%). And there was no statistically significance between different symptoms by histology. The incidence of postoperative complications was 44.9%. Pneumonia was the most common postoperative complications in this study, which accounted for 17.4%. And the incidence of tracheal anastomotic fistula was 10.1%, which was secondly to the pneumonia. Other complications including tracheal stenosis, recurrent laryngeal nerve palsy, chylothorax, fat liquefaction and postoperative bleeding, accounted for 7.2%, 5.8%, 1.4%, 1.4% and 1.4% respectively.
Table 1
| Variable | Overall | SCC (n=16) | ACC | Others | |||
|---|---|---|---|---|---|---|---|
| n=26 | P value | n=27 | P value | ||||
| Age, years, mean ± SD | 45.3±13.9 | 56.8±8.2 | 45.7±11.3 | 0.002 | 38.2±14.5 | <0.001 | |
| Tumor size, cm, mean ± SD | 2.5±1.2 | 2.6±1.1 | 3.0±1.4 | 0.43 | 2.0±0.9 | 0.04 | |
| Length of stay, days, mean ± SD | 19.4±8.6 | 18.8±7.3 | 17.8±6.1 | 0.63 | 21.2±10.9 | 0.45 | |
| Gender, n | |||||||
| Male | 29 | 10 | 10 | 0.13 | 9 | 0.06 | |
| Female | 40 | 6 | 16 | 18 | |||
| Smoking, n | |||||||
| Never | 47 | 7 | 19 | 0.06 | 21 | 0.02 | |
| Ever | 22 | 9 | 7 | 6 | |||
| Location, n | |||||||
| Cervical | 17 | 4 | 8 | 0.36 | 5 | 0.79 | |
| Intrathoracic trachea | 28 | 5 | 12 | 11 | |||
| Others | 24 | 7 | 6 | 11 | |||
| Symptoms | |||||||
| Cough | 65.2% | 68.8% | 61.5% | 0.64 | 66.7% | 0.89 | |
| Wheeze | 59.4% | 62.5% | 65.4% | 0.85 | 46.7% | 0.31 | |
| Bloody sputum/hemoptysis | 23.2% | 25.0% | 15.4% | 0.44 | 26.7% | 0.90 | |
| Breathless | 8.7% | 12.5% | 7.7% | 0.61 | 6.7% | 0.50 | |
| Dyspnea | 7.2% | 6.3% | 3.8% | 0.72 | 10.0% | 0.67 | |
| Fever | 1.4% | 0.0% | 0.0% | – | 3.3% | 0.46 | |
| Hoarseness | 1.4% | 6.3% | 0.0% | 0.20 | 0.0% | 0.17 | |
| No obvious symptoms | 2.9% | 0.0% | 3.8% | 0.43 | 3.3% | 0.46 | |
SCC, squamous cell carcinoma; ACC, adenoid cystic carcinoma; SD, standard deviation.
Treatment characteristics
All patients underwent surgical treatment. Tracheal resection with end-to-end anastomosis was main surgical technique in this study, which was performed for 65.2% (45/69) of patients, and remain patients were conducted with bronchus and lung resections (13/69), carinal resections (11/69). And posterolateral thoracotomy was the most surgical approach (50/69), followed by cervical incision (10/69) and median sternotomy (9/69). For patients with carinal tumors, if the tumor was small and involved the orifice of main bronchus, partial carina resection and reconstruction was performed (2/5); otherwise, a total carina resection and reconstruction needed (3/5). And for patients with bronchial tumors, bronchial sleeve resection without lobectomy would be performed (9/19) when the orifice of the upper lobe was not involved, while others received bronchial sleeve resection with lobectomy (10/19). The staging and treatment data of all patients were summarized in Table 2. There were 33 patients with tumors smaller than 2 cm and 36 patients with tumors larger than 2 cm. For tumor extension, 46 patients were E1 and 23 patients were E2. There were 49 patients known lymph node status, and 20 patients didn’t. And lymph node metastases were found in 16.3% of patients with known lymph node status. A complete resection (R0) was achieved in 85.5%. Most patients underwent surgery only (59.4%), and 40.6% accepted radiotherapy or chemotherapy after surgery. Adjuvant therapy was performed for patients with R1 or N1 status. And the decision of conducting adjuvant therapy for patients with E2 status was made based on the multi-disciplinary treatment (MDT). Furtherly, the treatment strategies for patients between ≥50 group and <50 group were not different. Specifically, for both of patients with ≥50 and <50, tracheal resection was the main surgical technique. And Chi-square test showed that there were no differences in types of resection for primary TBTs between ≥50 and <50 patients (P>0.05). Although adjuvant therapy postoperatively was more common in patients ≥50, the difference was not significant between ≥50 and <50 patients (P>0.05). Briefly, patients were treated with adjuvant therapy postoperatively in ≥50 and <50 group was 48.4% (15/31) and 34.2% (13/38) respectively.
Table 2
| Variable | Overall | SCC (n=16) | ACC | Others | |||
|---|---|---|---|---|---|---|---|
| n=26 | P value | n=27 | P value | ||||
| Tumor size | |||||||
| ≤2 cm | 33 | 6 (37.5%) | 8 (30.8%) | 0.65 | 19 (70.4%) | 0.04 | |
| >2 cm | 36 | 10 (62.5%) | 18 (69.2%) | 8 (29.6%) | |||
| Tumor extension | |||||||
| E1 | 46 | 8 (50.0%) | 14 (53.8%) | 0.81 | 24 (88.9%) | 0.005 | |
| E2 | 23 | 8 (50.0%) | 12 (46.2%) | 3 (11.1%) | |||
| Lymph node status | |||||||
| N1 | 8 | 5 (31.3%) | 2 (7.7%) | 0.14 | 1 (3.7%) | 0.04 | |
| N0 | 41 | 8 (50.0%) | 17 (65.4%) | 16 (59.3%) | |||
| Nx | 20 | 3 (18.8%) | 7 (26.9%) | 10 (37.0%) | |||
| Residual tumors status | |||||||
| R0 | 59 | 16 (100%) | 18 (69.2%) | 0.01 | 25 (92.6%) | 0.27 | |
| R1 | 10 | 0 (0.0%) | 8 (30.8%) | 2 (7.4%) | |||
| Treatment | |||||||
| Surgery only | 41 | 8 (50.0%) | 14 (53.8%) | 0.75 | 19 (70.4%) | 0.40 | |
| Surgery + radiotherapy | 6 | 1 (6.3%) | 4 (15.4%) | 1 (3.7%) | |||
| Surgery + chemotherapy | 20 | 6 (37.5%) | 7 (26.9%) | 7 (25.9%) | |||
| Surgery + radiotherapy + chemotherapy | 2 | 1 (6.3%) | 1 (3.8%) | 0 (0.0%) | |||
SCC, squamous cell carcinoma; ACC, adenoid cystic carcinoma.
The average tumor size of patients in SCC group was larger than that of patients in others group (2.6 vs. 2.0 cm) (P<0.05). And the percentage of the tumor spreading adjacent structures in SCC group was higher than that in others group (50.0% vs. 11.1%) (P<0.05). All patients with SCC underwent complete resection (R0), which was greater than that of patients with ACC (P<0.05).
Survival and risk factors associated with prognosis
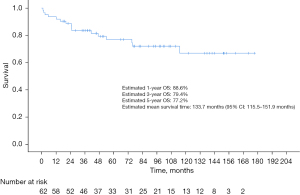
For patients with malignant tumors, the median length of follow-up was 74.7 months for the overall cohort, 48.1 months for patients with ACC, 57.0 months for patients SCC and 89.7 months for patients in the other category. Estimated 1-, 3-, and 5-year OS of the overall population was 88.6%, 79.4%, and 77.2%, respectively, with an estimated mean survival time of 133.7 months (95% CI: 115.5–151.9 months) (Figure 1). And all patients with benign tumors were still alive at the deadline for follow-up (range, 42.67–122.90 months).
The Kaplan-Meier analysis showed that patients with young age, small tumor promised a good prognosis while histological type, postoperative treatment, lymph node status and residual tumors status had no significant effects on OS (P>0.05) (Figure 2A-2F). The univariate Cox regression analysis showed that the age and tumor were associated with prognostic factors for OS (P<0.05). And the multivariate Cox regression analysis showed that the age (≤50 or >50 years) was independent prognostic factors for OS (P<0.05) (Table 3).
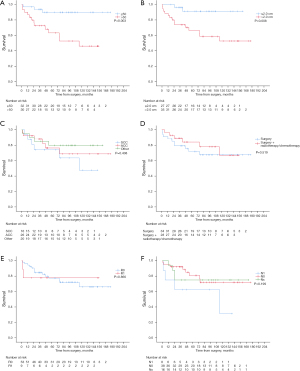
Table 3
| Variable | Univariate Cox regression analysis | Multivariate Cox regression analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender | 0.485 | ||||
| Female | Ref | Ref | |||
| Male | 1.435 (0.521–3.956) | ||||
| Age | 0.008 | 0.037 | |||
| ≤50 years | Ref | Ref | Ref | ||
| >50 years | 5.539 (1.573–19.501) | 4.340 (1.094–17.214) | |||
| Tumor size | 0.019 | 0.085 | |||
| ≤2 cm | Ref | Ref | Ref | ||
| >2 cm | 5.941 (1.347–26.204) | 3.962 (0.827–18.976) | |||
| Tumor extension | 0.784 | ||||
| E1 | Ref | Ref | |||
| E2 | 1.152 (0.418–3.178) | ||||
| Lymph node status | |||||
| N1 | Ref | Ref | Ref | Ref | |
| N0 | 0.358 (0.108–1.192) | 0.094 | 0.489 (0.112–2.128) | 0.340 | |
| Nx | 0.395 (0.098–1.582) | 0.189 | 0.471 (0.075–2.941) | 0.420 | |
| Histological type | |||||
| SCC | Ref | Ref | Ref | Ref | |
| ACC | 0.619 (0.199–1.923) | 0.407 | 1.219 (0.241–6.172) | 0.811 | |
| Others | 0.436 (0.123–1.554) | 0.201 | 1.776 (0.359–8.777) | 0.481 | |
| Smoker | 0.942 | ||||
| Yes | Ref | Ref | |||
| No | 0.962 (0.334–2.772) | ||||
| Postoperative treatment | 0.521 | 0.704 | |||
| Surgery | Ref | Ref | Ref | ||
| Surgery + radiotherapy/chemotherapy | 0.718 (0.261–1.976) | 0.807 (0.266–2.442) | |||
| Residual tumors status | 0.866 | 0.777 | |||
| R0 | Ref | Ref | Ref | ||
| R1 | 1.137 (0.257–5.031) | 1.295 (0.216–7.750) | |||
OS, overall survival; SCC, squamous cell carcinoma; ACC, adenoid cystic carcinoma.
Discussion
In this study, the risk factors associated with 69 surgical treated patients with primary TBT were retrospectively analyzed. For patients with malignant tumors, the univariate Cox regression analysis identified that age and tumor size had significant effects on OS. Further multivariate Cox regression analysis confirmed age was the independent prognostic factor.
For primary TBT, the surgical treatment has been proved a better outcome compared with other treatments. And end-to-end anastomosis is conducted when the tumor size is less than 2 cm. If resection length is between 2–4 cm, it’s necessary to release the tissues around the trachea, and for patients with cervical tumors, a heavy “guardian” suture is placed to prevent excessive extension of the neck in the immediate postoperative period. If the tumor size is more than 4 cm which means length of resection is more than 5 cm usually, the hilum release will be performed for reducing anastomotic tension. The 5-year OS among patients with primary TBT after surgery varies from 47% to 79% based on previous studies (15). A study from Massachusetts General Hospital demonstrated that resection was associated with improved survival, and the rate of survival at 5 years was 87% in patients undergoing resection while 30% in patients without resection (16). A nationwide study in The Netherlands reported that patients treated with surgery had a 5-year survival of 41% (2). In a national analysis in England, the 10-year actuarial palliation-free survival of patients who underwent curative surgery was 60.8%, compared with 19.5% for the condition overall (3). A SEER study on tracheal cancer showed that patients who underwent surgery, with or without adjuvant therapy, had superior OS than those who did not (17). In our study, estimated 5-year OS of the patients with malignant tumor was 77.2%, exhibiting excellent result and consistent with previous studies. Together with previous studies, our study suggested that patients with primary TBT should undergo surgical treatment when possible.
However, the factors affecting the prognosis of surgery were worthy of attention. Previous studies using multivariate Cox regression analysis showed that age, histology, tumor size, lymph node stage, surgical margins, tumor extension, treatment methods are potential prognosis factors for surgical treatment (8,9,13,14,18). A summary of articles related to prognostic factors of patients with tracheal tumor surgery in the past 20 years is shown in Table 4. We incorporated these factors into our analysis, and univariate Cox regression analysis showed age and tumor size had significant effects on the OS. Moreover, in the multivariate analysis, we found age was the only independent risk factor for prognosis of surgery, which was consistent with Bhattacharyya, Mallick, Webb et al. reported (1,5,21). The results suggested that for young patients with tracheal tumors, surgical treatment should be performed as much as possible. The functional status of young people is good in most condition, and it helps to recover the trauma caused by surgery, which is able to maximize the benefits of surgery. Surgery can significantly improve the prognosis of patients. And for older patients, comprehensive factors such as surgery related trauma, surgical complications and patient tolerance should be fully considered. Surgery may reduce the physical and mental state of elderly patients, which can aggravate the aging symptoms and decline the life quality.
Table 4
| Case source† [year of publication] | Era | Tumor | Prognostic factors (univariate analysis) | Prognostic factors (multivariate analysis) |
|---|---|---|---|---|
| NCDB [2020] (6)‡ | 2004–2015 | SCC: 81 | Age, histology, surgery type, residual tumors status | |
| ACC: 137 | ||||
| Others: 67 | ||||
| Chinese Academy of Medical Sciences and Peking Union Medical College [2020] (18) | 1965–2017 | MEC: 101 | Smoking history, tumor size, tumor extension, lymph node status | Lymph node status |
| NCDB [2019] (13) | 2004–2014 | SCC: 234 | Age, insurance status, Charlson/Deyo comorbidity score, chemotherapy, cancer grade, residual tumors status, histology, lymph node status, tumor extension | Insurance status, cancer grade, residual tumors status, histology, tumor extension |
| ACC: 180 | ||||
| Others: 135 | ||||
| Chinese Academy of Medical Sciences and Peking Union Medical College [2019] (8) | 1967–2017 | ACC: 142 | Complaint duration, time of surgery, tumor size, tumor extension, treatment methods | Complaint duration, treatment methods |
| Shanghai Chest Hospital [2016] (7) | 1995–2014 | ACC: 83 | Residual tumors status | |
| Shanghai Chest Hospital [2013] (19) | 2001–2012 | Trachea ACC: 43 | Symptom of dyspnea, resection length | |
| Shanghai Chest Hospital [2013] (19) | 2001–2012 | Bronchial ACC: 26 | Tumor size, relapse | |
| Massachusetts General Hospital [2010] (20) | 1962–2008 | ACC: 108 | Airway margin, extension, lymph node status, perineural growth | |
| Massachusetts General Hospital [2004] (9) | 1962–2002 | ACC: 101 | Histology, age, residual tumors status, lymph node status | Histology, age, residual tumors status, airway margin |
| SCC: 90 | ||||
| This study | 2004–2020 | SCC: 16 | Age, tumor size | Age |
| ACC: 26 | ||||
| Others: 27 |
†, the sources are available from dataset [PubMed]; ‡, this cohort selection criteria are different from the article published in 2019; NCDB, the National Cancer Database; MEC, mucoepidermoid carcinoma; SCC, squamous cell carcinoma; ACC, adenoid cystic carcinoma.
There were still some controversial issues about the treatment of tracheal surgery. Firstly, the adjuvant treatment (radiotherapy and/or chemotherapy) of tracheal tumors after surgery was controversial. There is no consensus on adjuvant therapy for patients with primary tracheal neoplasm after surgery. Although some studies showed that adjuvant therapy postoperatively did not improve the OS for patients with primary tracheal neoplasm (22), most studies demonstrated patients with adjuvant therapy have better OS than those patients without adjuvant therapy (23). However, the characters of patients received adjuvant therapy were not consistent in different centers. Not only patients had either R1 or N1 status received adjuvant therapy, but also patients with the neoplasm spreading to the adjacent tissues or organs received adjuvant therapy (23,24). In our center, other than patients with R1 or N1 status, patients with primary neoplasm that spread to adjacent organs and other structures (E2) will be taken into consideration for adjuvant therapy. And the decision of conducting adjuvant therapy for patients with E2 status was made based on the MDT. Many studies in recent years have also proposed many effective adjuvant therapies. For example, some studies have shown that radiotherapy with additional carbon ion radiotherapy boost has a good effect on local tumor control of ACC (25). And neoadjuvant therapy was effective in tracheal tumors according to some reports (26). Meanwhile, the immunotherapy proposed in recent years may also be one of the tracheal tumor treatments. Therefore, it is necessary to standardize the postoperative adjuvant treatment of tracheal tumors, and it is hoped that effective postoperative treatment can improve the prognosis of patients. Secondly, for tracheal tumor lymph node dissection, there were different opinions. Some reports found that lymph node status had no impact on the prognosis of patients with TBTs (8) while some studies have found that the status of lymph nodes affects the prognosis (9). The possible reason is that the extent and number of lymph node dissection were not standardized, and the tracheal tumor lymph node dissection was only judged by the surgeon. A report from Massachusetts General Hospital found lymph node-positive SCC related to lower survival while no correlation between lymph node status and ACC survival (9), which may indicate that for SCC patients, a more standardized lymph node dissection is very important. Therefore, a standard lymph node dissection method similar to lung cancer or other tumors is necessary for surgery of tracheal tumors. Thirdly, the effect of complete resection on survival remains controversial in primary tracheobronchial neoplasm. Usually, R0 resected is required, and many studies have also proved that R0 resection has a better prognosis than R1 (6). However, several studies indicated that the OS between patients with having complete resection and incomplete resection was not significant (19,27). Zhao et al. (19) pointed that patients with ACC which was a kind of low-grade malignant tumor can receive R1 during surgery, because tension-free anastomosis is more important for patients than R0 resection for ACC. And our result also demonstrated that there was no survival difference between R0 and R1 disease. The reason maybe that patients with R1 disease were mainly ACC (8/10). In our ACC group, positive margin was encountered in 30.8% of patients, which was similar to previous studies (19,28).
An interesting result was noticed in our study. With regards to oncologic outcomes we showed that OS was no statistically significant among ACC, SCC and other pathological types while many previous studies showed strong difference, especially for SCC (5,6,9,13,14). Comparative survival rates after resection varied between previous studies, with 5-year survival rates of 52% to 100% for ACC and 39% to 53% for SCC (2,6,9,29,30). A retrospective analysis from Massachusetts General Hospital reported that 5-year survival of ACC patients with surgery were 78% which was similar to our study (77.6%) (20). But for SCC, a long-term follow-up of 270 patients showed that 39.1% of patients with resected survived at 5 years (9), while 5-year survival for SCC in our study (73.8%) was higher than most studies. A report from Massachusetts General Hospital found that complete resection and age in SCC was associated with improved survival (9). As for mean age of SCC, a national study reported mean age was 65 years and what Massachusetts General Hospital showed was 61 years (6,9), while the mean age of SCC in our study was 56.8 years which was smaller than those of the other studies. On the other hand, R0 resection rate of SCC in our study (100%) was the highest rate of primary TBTs’ studies. The differences in age and R0 resection rate may offer explanation for SCC 5-year survival’s disparity. These findings indicated that other than from the other histological types, for patient with SCC, the R0 resection should be tried the best to achieved, especially for younger patients.
Our study has several limitations. It was a retrospective study in a single center with limited number of patients, which had its intrinsic limitations. However, the present investigation was a pretty large single-center retrospective study of primary TBT compared with other centers. Moreover, the patients in this study included a variety of histological type, providing adequate information which was representative. This study can contribute to the understanding of the clinical characteristics and prognostic factors of primary TBT.
In summary, this study shared the analysis of the prognostic factors and compared with other studies, finding age as independent prognostic factors of primary TBT, and for patient with SCC, the R0 resection should be tried the best to achieved, especially for younger patients, which had important clinical significance for clinical monitoring of primary TBT treated by surgery.
Acknowledgments
We would like to thank Feiyang Yin for her help in polishing our paper. The authors wish to thank all the patients who contributed to this research.
Funding: This work was supported by the National Natural Science Foundation of China (grant No. 82070101); Shaanxi Youth Science and Technology Rising Star Fund (grant No. 2018KJXX-051); The Science and Technology Innovation Fund (grant No. 2019QYTS004); and the Top Talent Fund of Tangdu hospital (grant No. 2019).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1791/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1791/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1791/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1791/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of the Second Affiliated Hospital of Air Force Medical University (No. K202111-04) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bhattacharyya N. Contemporary staging and prognosis for primary tracheal malignancies: a population-based analysis. Otolaryngol Head Neck Surg 2004;131:639-42. [Crossref] [PubMed]
- Honings J, van Dijck JA, Verhagen AF, et al. Incidence and treatment of tracheal cancer: a nationwide study in the Netherlands. Ann Surg Oncol 2007;14:968-76. [Crossref] [PubMed]
- Nouraei SM, Middleton SE, Nouraei SA, et al. Management and prognosis of primary tracheal cancer: a national analysis. Laryngoscope 2014;124:145-50. [Crossref] [PubMed]
- Licht PB, Friis S, Pettersson G. Tracheal cancer in Denmark: a nationwide study. Eur J Cardiothorac Surg 2001;19:339-45. [Crossref] [PubMed]
- Mallick S, Benson R, Giridhar P, et al. Demography, patterns of care and survival outcomes in patients with malignant tumors of trachea: A systematic review and individual patient data analysis of 733 patients. Lung Cancer 2019;132:87-93. [Crossref] [PubMed]
- Benissan-Messan DZ, Merritt RE, Bazan JG, et al. National Utilization of Surgery and Outcomes for Primary Tracheal Cancer in the United States. Ann Thorac Surg 2020;110:1012-22. [Crossref] [PubMed]
- Yang H, Yao F, Tantai J, et al. Resected Tracheal Adenoid Cystic Carcinoma: Improvements in Outcome at a Single Institution. Ann Thorac Surg 2016;101:294-300. [Crossref] [PubMed]
- Wang Y, Cai S, Gao S, et al. Tracheobronchial Adenoid Cystic Carcinoma: 50-Year Experience at the National Cancer Center, China. Ann Thorac Surg 2019;108:873-82. [Crossref] [PubMed]
- Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg 2004;78:1889-96; discussion 1896-7. [Crossref] [PubMed]
- Zhu F, Liu Z, Hou Y, et al. Primary salivary gland-type lung cancer: clinicopathological analysis of 88 cases from China. J Thorac Oncol 2013;8:1578-84. [Crossref] [PubMed]
- Kang DY, Yoon YS, Kim HK, et al. Primary salivary gland-type lung cancer: surgical outcomes. Lung Cancer 2011;72:250-4. [Crossref] [PubMed]
- Yamamoto T, Nakajima T, Suzuki H, et al. Surgical treatment of mucoepidermoid carcinoma of the lung: 20 years' experience. Asian Cardiovasc Thorac Ann 2016;24:257-61. [Crossref] [PubMed]
- Yusuf M, Gaskins J, Trawick E, et al. Effects of adjuvant radiation therapy on survival for patients with resected primary tracheal carcinoma: an analysis of the National Cancer Database. Jpn J Clin Oncol 2019;49:628-38. [Crossref] [PubMed]
- He J, Shen J, Huang J, et al. Prognosis of primary tracheal tumor: A population-based analysis. J Surg Oncol 2017;115:1004-10. [Crossref] [PubMed]
- Wen J, Liu D, Xu X, et al. Nomograms for predicting survival outcomes in patients with primary tracheal tumors: a large population-based analysis. Cancer Manag Res 2018;10:6843-56. [Crossref] [PubMed]
- Gaissert HA, Grillo HC, Shadmehr MB, et al. Uncommon primary tracheal tumors. Ann Thorac Surg 2006;82:268-72; discussion 272-3. [Crossref] [PubMed]
- Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol 2011;34:32-7. [Crossref] [PubMed]
- Wang Y, Cai S, Xue Q, et al. Treatment outcomes of patients with tracheobronchial mucoepidermoid carcinoma compared with those with adenoid cystic carcinoma. Eur J Surg Oncol 2020;46:1888-95. [Crossref] [PubMed]
- Zhao Y, Zhao H, Fan L, et al. Adenoid cystic carcinoma in the bronchus behaves more aggressively than its tracheal counterpart. Ann Thorac Surg 2013;96:1998-2004. [Crossref] [PubMed]
- Honings J, Gaissert HA, Weinberg AC, et al. Prognostic value of pathologic characteristics and resection margins in tracheal adenoid cystic carcinoma. Eur J Cardiothorac Surg 2010;37:1438-44. [Crossref] [PubMed]
- Webb BD, Walsh GL, Roberts DB, et al. Primary tracheal malignant neoplasms: the University of Texas MD Anderson Cancer Center experience. J Am Coll Surg 2006;202:237-46. [Crossref] [PubMed]
- Yang CJ, Shah SA, Ramakrishnan D, et al. Impact of Positive Margins and Radiation After Tracheal Adenoid Cystic Carcinoma Resection on Survival. Ann Thorac Surg 2020;109:1026-32. [Crossref] [PubMed]
- Xie L, Fan M, Sheets NC, et al. The use of radiation therapy appears to improve outcome in patients with malignant primary tracheal tumors: a SEER-based analysis. Int J Radiat Oncol Biol Phys 2012;84:464-70. [Crossref] [PubMed]
- Ning Y, He W, Bian D, et al. Tracheo-bronchial adenoid cystic carcinoma: A retrospective study. Asia Pac J Clin Oncol 2019;15:244-9. [Crossref] [PubMed]
- Lang K, Adeberg S, Harrabi S, et al. Adenoid cystic Carcinoma and Carbon ion Only irradiation (ACCO): Study protocol for a prospective, open, randomized, two-armed, phase II study. BMC Cancer 2021;21:812. [Crossref] [PubMed]
- Jiang BY, Zhang JT, Yan LX, et al. Neoadjuvant immune checkpoint inhibitor plus chemotherapy in rare tracheal tumors. Cancer Commun (Lond) 2021;41:1243-5. [Crossref] [PubMed]
- Maziak DE, Todd TR, Keshavjee SH, et al. Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg 1996;112:1522-31; discussion 1531-2. [Crossref] [PubMed]
- Calzada AP, Miller M, Lai CK, et al. Adenoid cystic carcinoma of the airway: a 30-year review at one institution. Am J Otolaryngol 2012;33:226-31. [Crossref] [PubMed]
- Hazama K, Miyoshi S, Akashi A, et al. Clinicopathological investigation of 20 cases of primary tracheal cancer. Eur J Cardiothorac Surg 2003;23:1-5. [Crossref] [PubMed]
- Regnard JF, Fourquier P, Levasseur P. Results and prognostic factors in resections of primary tracheal tumors: a multicenter retrospective study. The French Society of Cardiovascular Surgery. J Thorac Cardiovasc Surg 1996;111:808-13; discussion 813-4. [Crossref] [PubMed]