
Obstructive sleep apnea and multiple facets of a neuroinflammatory response: a narrative review
Inflammation and sleep-disordered breathing
Obstructive sleep apnea (OSA) is the second most prevalent sleep disorder (1), with higher prevalence in elderly and obese people (2) and incidence of 24% men and 9% of women in 30–60 age group, with further increase in incidence of up to 40–60% for both genders aged sixty five and above (3-5).
Traditionally, the main instigators of OSA-related ramifications have been considered to be the intermittent hypoxia (IH) and sleep fragmentation (SF) (6). However, a more complex picture of OSA-induced injury is emerging (7). The current consensus is that the extent of the associated functional deficits in OSA is likely decided by an intricate interplay of all maladaptive and homeostatic adaptive processes in the brain (7,8). These arguably may include a neuroinflammatory response, along with an individual genetic disposition, a chronic low-grade systemic inflammatory state (2) and any present co-morbidities. In support of the idea that inflammation may play a pivotal role in any such interaction, several studies have highlighted the possible role of a systemic chronic low-grade inflammation in OSA patients (9-11). The presence of inflammatory process is evident by increased levels of the pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) that promote the innate immunological response (10). It should be noted that the very definition of chronic low-grade inflammation remains elusive, and that its use commonly refers to states defined by chronically increased inflammatory markers of the innate immune system [i.e., C-reactive protein, IL-6, TNF-α, white blood count (WBC), neutrophils] (10,12).
In past preclinical studies, the mimicking of the OSA-related mechanical stress in the upper airway has been shown able to induce a localized inflammatory cascade (13). For example, SF has been linked to increased upregulation of TNF-α in mice, and, in pediatric OSA patients, this increase has been reported as directly related to the degree of SF and the body mass index (14,15). Similarly, IH strongly upregulates IL-6 production, which, along with the abundance of TNF-α, may additionally lead to the excessive sleepiness seen in OSA (16). When etanercept, an anti-TNF-α medication for rheumatoid arthritis, is offered to objectively sleepy OSA patients, significant symptomatic and measurable improvement is detected in their sleepiness (17). The magnitude of that improvement is three times higher than the reported of positive airway pressure (PAP), and proportional to the reduced TNF-α and IL-6 levels (17,18).
More recently, another link between OSA and systemic low-grade inflammation has been proposed to occur via an acquired dysbiosis. The OSA-related IH increases the proportion of anaerobic bacteria in the microbiota, while the SF activates directly the innate immune system, and both assist in the increased intestinal permeability, that eventually promotes low-grade endotoxemia and chronic inflammation (19-22). These processes appear to develop even in mild severity cases of OSA (23).
An ever increasing body of literature also supports the notion that the mechanistic links between IH, SF and the pro-inflammatory cytokines could be regulated by the idiosyncratic genetic predisposition and the overall (epigenetic) impact of the environmental factors (10). For example, the -174 G/C IL-6 gene polymorphism for IL-6 has been associated with increased IL-6 levels in OSA patients, but not the -572 G/C (24,25). Also, it has been further advanced that, in cases where CPAP treatment fails to get the pro-inflammatory cytokines under control, other known factors of low-grade inflammation such as obesity and cardiovascular diseases, which often coexist in OSA patients, likely explain that failure in statistical models (24,25).
The prevalence of OSA in patients with obesity/metabolic syndrome (MS) is estimated at 45–60%, and SF is known to promote obesity (26,27). Regrettably, even with the adequate treatment in place (e.g., with PAP), many of the patients report further weight gain (22,28). The MS (i.e., obesity, hypertension, hyperinsulinemia, glucose intolerance and dyslipidaemia) is in turn strongly associated with systemic low-grade inflammation mainly generated from the adipose tissue, and through the secretion of the pro-inflammatory cytokines and leptin resistance (29). Leptin is the satiety hormone, the interactions of which commonly lead to a reduction in calory intake. This effect appears to be decreased in OSA, which may further contribute to development of insulin resistance and diabetes type 2 in such patients (30). Ultimately, it is of note that MS also posits a cocktail of risk factors for cardio-vascular disease (CVD), and presents as the linkage between CVD and OSA, with low-grade inflammation as their shared mechanism (29,31).
Moreover, OSA has been linked to impaired synthesis, secretion, and timing of melatonin (32-34). Melatonin’s beneficial role in suppression of development of insulin resistance, and thus its possible role in correction of ensuing metabolic dysregulation has been argued (35). Melatonin also plays the role in reduction of the formation of free oxygen radicals and prevention of mitochondrial dysfunction, which can lead to oxidative stress (36,37). It also exerts a dual immunomodulatory function, with upregulation of the brain and muscle Arnt-like protein-1 (BMAL1) transcription factor, an established inhibitor of herpes simplex virus and influenza (38). Its role as an anti-inflammatory agent under conditions concerning senescence have similarly been argued, and its main effects appear to lead to reduction of pro-inflammatory cytokines and upregulation of the anti-inflammatory ones (39,40). Thus, even smaller modulation of this effective anti-aging agent might lead to reduction of its role against ‘inflammaging’ and contribute to a low grade systemic chronic inflammation in patients with OSA (41).
In this perspective review, we evaluate and appraise the current body of evidence which supports the notion that neuroinflammation may underly several pivotal functional and neuroanatomical effects of OSA on the brain. Moreover, we propose and argue for the potential role for the transmembrane synaptic protein neuroplastin in this process. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1231/rc).
Neuroinflammation in OSA
One of the important unresolved questions in the sleep medicine is whether its second most prevalent sleep disorder, OSA, may indeed also lead to inflammatory responses in the brain (5,6,42,43). This is of particular note as neuroinflammation has been argued to act as a shared archetypal mechanism in the pathogenesis of Alzheimer’s disorder (AD), depression and several other major neurologic and psychiatric disorders with which OSA appears to share a complex bidirectional link (6,42,44). Our group has long argued that accumulating data does suggest that neuroinflammatory process occurs in OSA, and that it drives specific structural and behavioural changes known to afflict some susceptible OSA patients (42,43,45-49). More recently, we have been able to demonstrate that under OSA-like conditions in rodents, the neuroinflammatory response is indeed instigated in the forebrain and the septal nuclei regions, important cholinergic regions of the brain, with a later widespread and marked chronic component. Furthermore, we have demonstrated that subsequent structural changes develop in distinct neuroanatomical regions with monosynaptic connections to initial frontal and basal forebrain cortical sites of inflammatory response (6). It is widely accepted that acetyl-choline-mediated enhanced processing of sensory information underlies the cognitive process of attention, known to be impaired in patients with OSA (42). It has been argued that a variety of attention-related cognitive operations that together contribute to the detection and discrimination of stimuli are significantly impacted in OSA, and inflammatory processes in these regions may contribute to this (7,45). Moreover, as the integrity of attention processes contributes to the efficacy of higher-order cognitive functions such as learning and memory, it is perhaps unsurprising that these domains have been similarly shown as impaired in some patients with OSA (7,45). In addition, numerous behavioral studies have also implicated basal forebrain cortical cholinergic inputs in sustained attention functioning (50). Most notably, though, the neuroinflammation-driven changes were also shown to underlie several specific observed behaviours, including development of agitated (mal)adaptive behaviour under episodes of stress, and an increased ability to gain weight (6).
Several pivotal preclinical studies over the last several decades have similarly supported this notion. For example, data from rodent studies suggest that SF significantly increases systemic IL-6 serum concentration and hippocampal transcription of IL-6 in mice without further cognitive impairment (51). Further studies have shown that few hours of sleep deprivation can cause increase in astrocytic phagocytosis of presynaptic elements in mouse cerebral cortex, which could be a compensatory response to increased synaptic activity after prolonged wake. Moreover, there is indirect evidence, from both clinical and preclinical studies, that sleep loss may be similarly detrimental to oligodendrocytes, and to the production of myelin (52,53). It has been reported that chronic sleep loss can reduce myelin thickness (52). This effect may have important functional consequences in patients with OSA too, especially given the fundamental role of myelin in optimizing the information flow throughout the brain (53-55). Chronic sleep deprivation, in addition, activates microglia and their phagocytic activity (56). Another study that determined effects of sleep disturbance in mice showed increased IL-6 levels and induced microglial activation in hippocampus, but not in cortex, one and seven days after 24-hour long sleep disturbance (57). Mice (C57BL/6) exposed to chronic IH for 4 weeks showed elevation of Toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MyD88), TIR-domain-containing adapter-inducing interferon-β (TRIF), pro-inflammatory cytokines and oxidative stress which was ameliorated by concomitant atorvastatin administration (58).
On the other hand, in rats, chronic sleep deprivation (21 days) results in anxiogenic behaviour and memory decline and in increased levels of pro-inflammatory cytokines (TNF-α, IL-1β) in hippocampus and piriform cortex, as well as increased expression of glial fibrillary acidic protein, GFAP and Iba1 (59). Another study showed that IH (2-min intervals, 10.5% O2, for one, three, or 14 days) caused increase in gene expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), TNF-α, IL-1β, and IL-6 in rat cortex, medulla, and spinal cord. Additionally, microglial TLR4 mRNA level was upregulated after hypoxia in a region- and time-dependent manner (60-62).
Neuroinflammation has been argued to present a key linking element that interacts with the three neurobiological correlates of major depressive disorder too (63). Unregulated inflammatory response in the brain has been argued to lead to depletion of brain serotonin, dysregulation of the hypothalamus-pituitary-adrenal (HPA) axis, as well as to alteration of the continuous production of adult-generated neurons in the dentate gyrus of the hippocampus (63). In that background, it has been proposed that kynurenine pathway alteration and HPA axis dysregulation may have the common effect of increasing extracellular glutamate levels and glutamate neurotransmission, which can then impact adult hippocampal neurogenesis (63). This pathophysiological cascade appears to be correspondingly triggered during sleep deprivation (64), and it may present the common link between affective disorders, insomnia, OSA and neurodegenerative disorders, including Alzheimer’s disease (AD) (42). Interestingly, an early antidepressogenic effect of the TLR2-dependant neuroinflammatory response in OSA has been demonstrated in the animal model, functionally linked to a distinct fronto-brainstem subcircuitry (6). In past, activation of a similar network in mice has been reported to favor effortful behavioral responses to challenging situations (65). For instance, a selective activation of a subclass of prefrontal cells that project to the brainstem has been shown to induce a rapid and reversible effect on selection of the active behavioral states (65). Based on this, it is tempting to argue that any such initial inflammatory response may play an adaptive role, and that it may initially help the organism to focus on continued trying to find its way out of a complex predicament (6). With time, however, any such prolonged behavioral response would conversely develop into an ‘agitated’ depressive profile, with strong maladaptive anxiety component (6). In keeping, similar mixed anxiety and depression endophenotype has been previously described in some patients with OSA (66), and it has been traditionally linked with higher suicide risks in depressed patients (6,67).
Taken together, and in spite of certain controversies, both preclinical, as well as human studies that investigated inflammatory markers in patients with OSA (68-84), increasingly indicate that there is reliable cumulative evidence supporting OSA as a low-grade chronic inflammatory disease which likely can also induce neuroinflammation and neuronal injury (10).
Shared pathomechanism with neurologic and neurodegenerative disorders
Over the last decade, the links between OSA and earlier onset of neurodegenerative changes and cognitive decline have been emphasised (42,62,85-87). The additive effect of changes in sleep quality and structure, cerebral blood flow and the cellular redox status in OSA patients may contribute to cognitive decline, and may further aggravate AD and other neurodegenerative processes’ (88) progression (42,44). Also, a recent meta-analysis suggests that patients with AD may have a five times higher chance of presenting with OSA than cognitively non-impaired individuals of similar age (44). Moreover, it has been similarly argued that around half of patients with AD will have experienced OSA at some point after their initial diagnosis (44). In addition, OSA prevalence increases with age (89-91), and a recent two-year longitudinal study showed an increase in markers of amyloid burden, a hallmark of AD in the cerebrospinal fluid (CSF) in elderly OSA patients (85,86). Furthermore, CSF levels of total and phosphorylated (P) tau (87), and inflammatory protein YKL-40 (neuroinflammation/astrocyte activation marker) predict poor sleep in cognitively healthy adults, older than 65 years, with increased Aβ42 CSF values (92). Studies have reported that patients with OSA are more likely to develop mild cognitive impairment (MCI) and AD at a younger age (93). In the same vein, a recent meta-analysis of longitudinal studies reported that individuals presenting sleep disturbances, such as insomnia, OSA, sleep-wake rhythm disorders, have a high risk of developing all-cause dementia, AD, and vascular dementia (94). Moreover, insomnia increased the risk of AD but not vascular or all-cause dementia, whilst OSA was associated with an increased risk of all-cause dementia, AD, and vascular dementia (94). Shared pathological findings between OSA and AD include sleep architecture disturbances, neurogenic neuroinflammation, changes in multipartite synapse and impaired clearance of toxic Aβ and tau (42). Several studies, including a recent cross-sectional study, suggest that there is an increase in brain amyloid in OSA patients, in comparison to healthy controls (86,95,96). However, conversely, a number of studies have failed to report changes in CSF tau protein levels in patients with OSA (87,97). Nonetheless, it appears that OSA could act to induce a faster longitudinal increase in CSF tau levels in patients with MCI and AD (85,87). Distinct gender effects and links with a specific limbic phenotype of AD have also been argued (98). However, the exact molecular mechanisms leading from SF and apnoeic events to neurodegeneration remain unclear.
Accumulating body of evidence suggests that various innate cellular adaptive and plasticity mechanisms are triggered in neurodegeneration and occur in an unregulated manner eventually aggravating neuropathologic and clinical findings (99). Moreover, when one considers some of the features of main AD pathological hallmarks, a clear convergence of at least two different types of tissue response to injury emerges: (I) reactivation of fetal phosphorylation pattern of tau protein, which contributes to cytoskeletal disorganization and impaired axonal transport; (II) immune reaction to amyloid formation and accumulation, which leads to chronic neuroinflammation and further structural and functional alterations and neurodegeneration (99,100).
Although typical neurofibrillary degeneration and amyloid deposits are being distributed in a specific spatio-temporal pattern in AD, they likely present common final points of long-lasting cellular changes in different neurodegenerative disorders and are triggered by mostly un-known cause(s). It has been long speculated that in understanding and elucidating the very early molecular alterations, one may learn how to unravel and prevent the formation of the core of several shared pathomechanisms of neurologic and neurodegenerative disorders (7,42). Having in mind that synaptic remodeling and plasticity is of pivotal importance for functions of brain tissue, and that dynamic cross-talk between neurons and different types of glial cells is involved in maintenance of synaptic homeostasis, searching for specific molecular mediators of neuron-glial interactions in (patho)physiological conditions becomes particularly promising line of enquiry. Indeed, several recent studies indicate new potential partners in such an interplay between microglia and neurons, namely TLR2 and neuroplastin. As reported by Polsek et al. (6), TLR2 is involved in initiating and modulating inflammatory response in specific brain areas in the murine OSA model. Moreover, immune response and structural changes triggered by microglial activation and TRL2 actions have been shown to influence on expression of several neuroplasticity markers such as neuroplastin. Transmembrane synaptic protein neuroplastin, which belongs to a family of cell-adhesion molecules (101,102), has been source of much attention since its discovery (103). Its role in long-term potentiation, synaptic plasticity and cognition has been widely acknowledged (104-107). Nonetheless, to date majority of neuroplastin actions have been predominantly reported in the preclinical studies, whilst just a few studies report on its expression and distribution in the human brain (108,109). More recently, however, neuroplastin has been argued as potential biomarker of AD progression, and its involvement in human hippocampal tissue reorganization has been demonstrated, i.e., plasticity response in the early AD neurodegeneration process (103,109). Arguably, a functionally relevant interplay between TLR2 and neuroplastin may form also through their intertwined intracellular signaling pathways. In keeping, it has been suggested that this interplay may occur through the same adaptor protein TNF receptor-associated factor 6, TRAF6 (110,111). Similarly, the early dysfunction of microglia-neuron cross-talk in OSA could also be a consequence of disturbed interactions between TLR2 and neuroplastin (6) (also see Figure 1). Given that TLR2 and neuroplastin share intracellular signaling cascade, and they are both involved in adaptive cellular mechanisms vital for neurons, i.e., regulation of immune response and synaptic plasticity it is tempting to argue that these membrane proteins may indeed play an important role in the bigger archetypal puzzle of brain’s vulnerability to SF and oxidative stress (101,111).
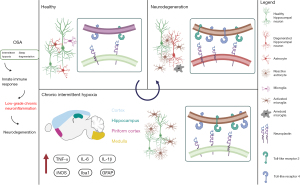
Conclusions and future directions
It is undisputable that cognitive and neurologic dysfunction occur in the majority of patients with OSA (7). The evidence towards its links with the major psychiatric and neurologic disorders is similarly accumulating (7). However, the exact nature of the mechanisms that cause these effects remain to be defined, as does the extent of the relationship and directionality between these factors and any potential inflammatory process in the brain. It is hoped that well defined future multimodal imaging studies, ideally performed in patients with OSA but without any overt co-morbidities, will enable us to finally resolve whether the neuroinflammatory process may indeed present in OSA.
Acknowledgments
Funding: Prof. Steier’s contribution was partially supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. KI was supported by Croatian Science Foundation grant NeuroReact, IP-2016-06-8636 (to SKB), and European Union through the European Regional Development Fund, Operational Programme Competitiveness and Cohesion, grant agreement No. KK.01.1.1.01.0007 (CoRE–Neuro).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease for the series “Sleep Section”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1231/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1231/coif). The series Sleep Section was commissioned by the editorial office without any funding or sponsorship. KI was supported by Croatian Science Foundation grant NeuroReact, IP-2016-06-8636 (to SK-B), and European Union through the European Regional Development Fund, Operational Programme Competitiveness and Cohesion, grant agreement No. KK.01.1.1.01.0007 (CoRE–Neuro). PD received consulting fees from Jazz pharmaceutical on OSA and excessive daytime sleepiness. Jazz pharmaceuticals also funded an OSA preceptorship at the sleep center at GSTT. JS serves as an unpaid editorial board member of Journal of Thoracic Disease. SKB declares there has been no support from any non-academic organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Malhotra A, Heinzer R, Morrell MJ, et al. Late Breaking Abstract - European prevalence of OSA in adults: Estimation using currently available data. Eur Respir J 2018;52:OA4961.
- Lévy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers 2015;1:15015. [Crossref] [PubMed]
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. [Crossref] [PubMed]
- Kerner NA, Roose SP. Obstructive Sleep Apnea is Linked to Depression and Cognitive Impairment: Evidence and Potential Mechanisms. Am J Geriatr Psychiatry 2016;24:496-508. [Crossref] [PubMed]
- Rosenzweig I, Williams SC, Morrell MJ. The impact of sleep and hypoxia on the brain: potential mechanisms for the effects of obstructive sleep apnea. Curr Opin Pulm Med 2014;20:565-71. [Crossref] [PubMed]
- Polsek D, Cash D, Veronese M, et al. The innate immune toll-like-receptor-2 modulates the depressogenic and anorexiolytic neuroinflammatory response in obstructive sleep apnoea. Sci Rep 2020;10:11475. [Crossref] [PubMed]
- Rosenzweig I, Gosselin N, Bucks RS. Cognitive and Neurologic Aspects of Obstructive Sleep Apnea. Encyclopedia of Respiratory Medicine. 2nd ed: Elsevier; 2021.
- Rosenzweig I, Williams SC, Morrell MJ. CrossTalk opposing view: the intermittent hypoxia attending severe obstructive sleep apnoea does not lead to alterations in brain structure and function. J Physiol 2013;591:383-5; discussion 387,389.
- Unnikrishnan D, Jun J, Polotsky V. Inflammation in sleep apnea: an update. Rev Endocr Metab Disord 2015;16:25-34. [Crossref] [PubMed]
- Kheirandish-Gozal L, Gozal D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int J Mol Sci 2019;20:459. [Crossref] [PubMed]
- Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax 2009;64:631-6. [PubMed]
- Besedovsky L, Lange T, Haack M. The Sleep-Immune Crosstalk in Health and Disease. Physiol Rev 2019;99:1325-80. [Crossref] [PubMed]
- Almendros I, Carreras A, Ramírez J, et al. Upper airway collapse and reopening induce inflammation in a sleep apnoea model. Eur Respir J 2008;32:399-404. [Crossref] [PubMed]
- Gozal D, Serpero LD, Kheirandish-Gozal L, et al. Sleep measures and morning plasma TNF-alpha levels in children with sleep-disordered breathing. Sleep 2010;33:319-25. [Crossref] [PubMed]
- Kaushal N, Ramesh V, Gozal D. TNF-α and temporal changes in sleep architecture in mice exposed to sleep fragmentation. PLoS One 2012;7:e45610. [Crossref] [PubMed]
- Li Y, Vgontzas AN, Fernandez-Mendoza J, et al. Objective, but Not Subjective, Sleepiness is Associated With Inflammation in Sleep Apnea. Sleep 2017;40:zsw033. [Crossref] [PubMed]
- Vgontzas AN, Zoumakis E, Lin HM, et al. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab 2004;89:4409-13. [Crossref] [PubMed]
- Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003;163:565-71. [Crossref] [PubMed]
- Moreno-Indias I, Torres M, Montserrat JM, et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur Respir J 2015;45:1055-65. [Crossref] [PubMed]
- Moreno-Indias I, Torres M, Sanchez-Alcoholado L, et al. Normoxic Recovery Mimicking Treatment of Sleep Apnea Does Not Reverse Intermittent Hypoxia-Induced Bacterial Dysbiosis and Low-Grade Endotoxemia in Mice. Sleep 2016;39:1891-7. [Crossref] [PubMed]
- Poroyko VA, Carreras A, Khalyfa A, et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci Rep 2016;6:35405. [Crossref] [PubMed]
- Kheirandish-Gozal L, Peris E, Wang Y, et al. Lipopolysaccharide-binding protein plasma levels in children: effects of obstructive sleep apnea and obesity. J Clin Endocrinol Metab 2014;99:656-63. [Crossref] [PubMed]
- Ko CY, Liu QQ, Su HZ, et al. Gut microbiota in obstructive sleep apnea-hypopnea syndrome: disease-related dysbiosis and metabolic comorbidities. Clin Sci (Lond) 2019;133:905-17. [Crossref] [PubMed]
- Thunström E, Glantz H, Yucel-Lindberg T, et al. CPAP Does Not Reduce Inflammatory Biomarkers in Patients With Coronary Artery Disease and Nonsleepy Obstructive Sleep Apnea: A Randomized Controlled Trial. Sleep 2017; [Crossref] [PubMed]
- Kong D, Qin Z, Wang W, et al. Effect of obstructive sleep apnea on carotid artery intima media thickness related to inflammation. Clin Invest Med 2017;40:E25-33. [Crossref] [PubMed]
- Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med 2007;3:467-72. [Crossref] [PubMed]
- Romero-Corral A, Caples SM, Lopez-Jimenez F, et al. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest 2010;137:711-9. [Crossref] [PubMed]
- Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax 2015;70:258-64. [Crossref] [PubMed]
- Jean-Louis G, Zizi F, Clark LT, et al. Obstructive sleep apnea and cardiovascular disease: role of the metabolic syndrome and its components. J Clin Sleep Med 2008;4:261-72. [Crossref] [PubMed]
- Kelesidis T, Kelesidis I, Chou S, et al. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med 2010;152:93-100. [Crossref] [PubMed]
- Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ 2000;321:199-204. [Crossref] [PubMed]
- Reutrakul S, Siwasaranond N, Nimitphong H, et al. Associations between nocturnal urinary 6-sulfatoxymelatonin, obstructive sleep apnea severity and glycemic control in type 2 diabetes. Chronobiol Int 2017;34:382-92. [Crossref] [PubMed]
- Hernández C, Abreu J, Abreu P, et al. Nocturnal melatonin plasma levels in patients with OSAS: the effect of CPAP. Eur Respir J 2007;30:496-500. [Crossref] [PubMed]
- Ulfberg J, Micic S, Strøm J. Afternoon serum-melatonin in sleep disordered breathing. J Intern Med 1998;244:163-8. [Crossref] [PubMed]
- Cardinali DP, Hardeland R. Inflammaging, Metabolic Syndrome and Melatonin: A Call for Treatment Studies. Neuroendocrinology 2017;104:382-97. [Crossref] [PubMed]
- Caruso C, Candore G, Colonna Romano G, et al. HLA, aging, and longevity: a critical reappraisal. Hum Immunol 2000;61:942-9. [Crossref] [PubMed]
- Hardeland R. Neuroprotection by radical avoidance: search for suitable agents. Molecules 2009;14:5054-102. [Crossref] [PubMed]
- Lim RK, Wambier CG, Goren A. Are night shift workers at an increased risk for COVID-19? Med Hypotheses 2020;144:110147. [Crossref] [PubMed]
- Zhang R, Wang X, Ni L, et al. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci 2020;250:117583. [Crossref] [PubMed]
- Kireev RA, Tresguerres AC, Garcia C, et al. Melatonin is able to prevent the liver of old castrated female rats from oxidative and pro-inflammatory damage. J Pineal Res 2008;45:394-402. [Crossref] [PubMed]
- Hardeland R. Melatonin and the theories of aging: a critical appraisal of melatonin's role in antiaging mechanisms. J Pineal Res 2013;55:325-56. [Crossref] [PubMed]
- Polsek D, Gildeh N, Cash D, et al. Obstructive sleep apnoea and Alzheimer's disease: In search of shared pathomechanisms. Neurosci Biobehav Rev 2018;86:142-9. [Crossref] [PubMed]
- Gnoni V, Drakatos P, Higgins S, et al. Cyclic alternating pattern in obstructive sleep apnea: A preliminary study. J Sleep Res 2021;30:e13350. [Crossref] [PubMed]
- Emamian F, Khazaie H, Tahmasian M, et al. The Association Between Obstructive Sleep Apnea and Alzheimer's Disease: A Meta-Analysis Perspective. Front Aging Neurosci 2016;8:78. [Crossref] [PubMed]
- Bucks RS, Olaithe M, Rosenzweig I, et al. Reviewing the relationship between OSA and cognition: Where do we go from here? Respirology 2017;22:1253-61. [Crossref] [PubMed]
- Rosenzweig I, Glasser M, Crum WR, et al. Changes in Neurocognitive Architecture in Patients with Obstructive Sleep Apnea Treated with Continuous Positive Airway Pressure. EBioMedicine 2016;7:221-9. [Crossref] [PubMed]
- Khazaie H, Veronese M, Noori K, et al. Functional reorganization in obstructive sleep apnoea and insomnia: A systematic review of the resting-state fMRI. Neurosci Biobehav Rev 2017;77:219-31. [Crossref] [PubMed]
- Rosenzweig I, Kempton MJ, Crum WR, et al. Hippocampal hypertrophy and sleep apnea: a role for the ischemic preconditioning? PLoS One 2013;8:e83173. [Crossref] [PubMed]
- Rosenzweig I, Morrell MJ. Hypotrophy versus Hypertrophy: It's Not Black or White with Gray Matter. Am J Respir Crit Care Med 2017;195:1416-8. [Crossref] [PubMed]
- Squire LR. Encyclopedia of neuroscience. Amsterdam; London: Academic; 2009.
- Vacas S, Degos V, Maze M. Fragmented Sleep Enhances Postoperative Neuroinflammation but Not Cognitive Dysfunction. Anesth Analg 2017;124:270-6. [Crossref] [PubMed]
- Bellesi M, Haswell JD, de Vivo L, et al. Myelin modifications after chronic sleep loss in adolescent mice. Sleep 2018;41:zsy034. [Crossref] [PubMed]
- de Vivo L, Bellesi M. The role of sleep and wakefulness in myelin plasticity. Glia 2019;67:2142-52. [Crossref] [PubMed]
- Rosenzweig I, Vukadinovic Z, Turner AJ, et al. Neuroconnectivity and valproic acid: the myelin hypothesis. Neurosci Biobehav Rev 2012;36:1848-56. [Crossref] [PubMed]
- Rosenzweig I, Glasser M, Polsek D, et al. Sleep apnoea and the brain: a complex relationship. Lancet Respir Med 2015;3:404-14. [Crossref] [PubMed]
- Bellesi M, de Vivo L, Chini M, et al. Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex. J Neurosci 2017;37:5263-73. [Crossref] [PubMed]
- Zhu B, Dong Y, Xu Z, et al. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis 2012;48:348-55. [Crossref] [PubMed]
- Deng Y, Yuan X, Guo XL, et al. Efficacy of atorvastatin on hippocampal neuronal damage caused by chronic intermittent hypoxia: Involving TLR4 and its downstream signaling pathway. Respir Physiol Neurobiol 2015;218:57-63. [Crossref] [PubMed]
- Manchanda S, Singh H, Kaur T, et al. Low-grade neuroinflammation due to chronic sleep deprivation results in anxiety and learning and memory impairments. Mol Cell Biochem 2018;449:63-72. [Crossref] [PubMed]
- Smith SM, Friedle SA, Watters JJ. Chronic intermittent hypoxia exerts CNS region-specific effects on rat microglial inflammatory and TLR4 gene expression. PLoS One 2013;8:e81584. [Crossref] [PubMed]
- Yao L, Kan EM, Lu J, et al. Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J Neuroinflammation 2013;10:23. [Crossref] [PubMed]
- Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: Relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res 2015;93:1778-94. [Crossref] [PubMed]
- Troubat R, Barone P, Leman S, et al. Neuroinflammation and depression: A review. Eur J Neurosci 2021;53:151-71. [Crossref] [PubMed]
- Bhat A, Pires AS, Tan V, et al. Effects of Sleep Deprivation on the Tryptophan Metabolism. Int J Tryptophan Res 2020;13:1178646920970902. [Crossref] [PubMed]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 2012;492:428-32. [Crossref] [PubMed]
- Rezaeitalab F, Moharrari F, Saberi S, et al. The correlation of anxiety and depression with obstructive sleep apnea syndrome. J Res Med Sci 2014;19:205-10. [PubMed]
- Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry 2005;62:1249-57. [Crossref] [PubMed]
- Ciftci TU, Kokturk O, Bukan N, et al. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine 2004;28:87-91. [Crossref] [PubMed]
- Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab 1997;82:1313-6. [Crossref] [PubMed]
- Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2006;174:824-30. [Crossref] [PubMed]
- Carpagnano GE, Kharitonov SA, Resta O, et al. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest 2002;122:1162-7. [Crossref] [PubMed]
- Liu H, Liu J, Xiong S, et al. The change of interleukin-6 and tumor necrosis factor in patients with obstructive sleep apnea syndrome. J Tongji Med Univ 2000;20:200-2. [Crossref] [PubMed]
- Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 2002;105:2462-4. [Crossref] [PubMed]
- Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 2003;107:1129-34. [Crossref] [PubMed]
- Minoguchi K, Tazaki T, Yokoe T, et al. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest 2004;126:1473-9. [Crossref] [PubMed]
- Bravo Mde L, Serpero LD, Barceló A, et al. Inflammatory proteins in patients with obstructive sleep apnea with and without daytime sleepiness. Sleep Breath 2007;11:177-85. [Crossref] [PubMed]
- Barceló A, Barbé F, Llompart E, et al. Effects of obesity on C-reactive protein level and metabolic disturbances in male patients with obstructive sleep apnea. Am J Med 2004;117:118-21. [Crossref] [PubMed]
- Imagawa S, Yamaguchi Y, Ogawa K, et al. Interleukin-6 and tumor necrosis factor-alpha in patients with obstructive sleep apnea-hypopnea syndrome. Respiration 2004;71:24-9. [Crossref] [PubMed]
- Guilleminault C, Kirisoglu C, Ohayon MM. C-reactive protein and sleep-disordered breathing. Sleep 2004;27:1507-11. [Crossref] [PubMed]
- Sharma SK, Mishra HK, Sharma H, et al. Obesity, and not obstructive sleep apnea, is responsible for increased serum hs-CRP levels in patients with sleep-disordered breathing in Delhi. Sleep Med 2008;9:149-56. [Crossref] [PubMed]
- Roytblat L, Rachinsky M, Fisher A, et al. Raised interleukin-6 levels in obese patients. Obes Res 2000;8:673-5. [Crossref] [PubMed]
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: A novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006;166:1725-31. [Crossref] [PubMed]
- Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab 2000;85:1151-8. [Crossref] [PubMed]
- Arnardottir ES, Mackiewicz M, Gislason T, et al. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep 2009;32:447-70. [Crossref] [PubMed]
- Bubu OM, Pirraglia E, Andrade AG, et al. Obstructive sleep apnea and longitudinal Alzheimer's disease biomarker changes. Sleep 2019;42:zsz048. [Crossref] [PubMed]
- Sharma RA, Varga AW, Bubu OM, et al. Obstructive Sleep Apnea Severity Affects Amyloid Burden in Cognitively Normal Elderly. A Longitudinal Study. Am J Respir Crit Care Med 2018;197:933-43. [Crossref] [PubMed]
- Liguori C, Maestri M, Spanetta M, et al. Sleep-disordered breathing and the risk of Alzheimer's disease. Sleep Med Rev 2021;55:101375. [Crossref] [PubMed]
- Wasserman D, Bindman D, Nesbitt AD, et al. Striatal Dopaminergic Deficit and Sleep in Idiopathic Rapid Eye Movement Behaviour Disorder: An Explorative Study. Nat Sci Sleep 2021;13:1-9. [Crossref] [PubMed]
- Mehra R, Stone KL, Blackwell T, et al. Prevalence and correlates of sleep-disordered breathing in older men: osteoporotic fractures in men sleep study. J Am Geriatr Soc 2007;55:1356-64. [Crossref] [PubMed]
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006-14. [Crossref] [PubMed]
- Daulatzai MA. Death by a thousand cuts in Alzheimer's disease: hypoxia--the prodrome. Neurotox Res 2013;24:216-43. [Crossref] [PubMed]
- Fjell AM, Idland AV, Sala-Llonch R, et al. Neuroinflammation and Tau Interact with Amyloid in Predicting Sleep Problems in Aging Independently of Atrophy. Cereb Cortex 2018;28:2775-85. [Crossref] [PubMed]
- Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015;84:1964-71. [Crossref] [PubMed]
- Shi L, Chen SJ, Ma MY, et al. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev 2018;40:4-16. [Crossref] [PubMed]
- Jackson ML, Cavuoto M, Schembri R, et al. Severe Obstructive Sleep Apnea Is Associated with Higher Brain Amyloid Burden: A Preliminary PET Imaging Study. J Alzheimers Dis 2020;78:611-7. [Crossref] [PubMed]
- Owen JE, Benediktsdottir B, Cook E, et al. Alzheimer's disease neuropathology in the hippocampus and brainstem of people with obstructive sleep apnea. Sleep 2021;44:zsaa195.
- Liguori C, Mercuri NB, Nuccetelli M, et al. Obstructive sleep apnea may induce orexinergic system and cerebral β-amyloid metabolism dysregulation: is it a further proof for Alzheimer's disease risk? Sleep Med 2019;56:171-6. [Crossref] [PubMed]
- Ferini-Strambi L, Hensley M, Salsone M. Decoding Causal Links Between Sleep Apnea and Alzheimer's Disease. J Alzheimers Dis 2021;80:29-40. [Crossref] [PubMed]
- Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol 2015;14:388-405. [Crossref] [PubMed]
- Saha P, Sen N. Tauopathy: A common mechanism for neurodegeneration and brain aging. Mech Ageing Dev 2019;178:72-9. [Crossref] [PubMed]
- Beesley P, Kraus M, Parolaro N. The neuroplastins: multifunctional neuronal adhesion molecules--involvement in behaviour and disease. Adv Neurobiol 2014;8:61-89. [Crossref] [PubMed]
- Beesley PW, Herrera-Molina R, Smalla KH, et al. The Neuroplastin adhesion molecules: key regulators of neuronal plasticity and synaptic function. J Neurochem 2014;131:268-83. [Crossref] [PubMed]
- Ilic K, Mlinac-Jerkovic K, Sedmak G, et al. Neuroplastin in human cognition: review of literature and future perspectives. Transl Psychiatry 2021;11:394. [Crossref] [PubMed]
- Smalla KH, Matthies H, Langnäse K, et al. The synaptic glycoprotein neuroplastin is involved in long-term potentiation at hippocampal CA1 synapses. Proc Natl Acad Sci U S A 2000;97:4327-32. [Crossref] [PubMed]
- Bhattacharya S, Herrera-Molina R, Sabanov V, et al. Genetically Induced Retrograde Amnesia of Associative Memories After Neuroplastin Ablation. Biol Psychiatry 2017;81:124-35. [Crossref] [PubMed]
- Desrivières S, Lourdusamy A, Tao C, et al. Single nucleotide polymorphism in the neuroplastin locus associates with cortical thickness and intellectual ability in adolescents. Mol Psychiatry 2015;20:263-74. [Crossref] [PubMed]
- Saito A, Fujikura-Ouchi Y, Kuramasu A, et al. Association study of putative promoter polymorphisms in the neuroplastin gene and schizophrenia. Neurosci Lett 2007;411:168-73. [Crossref] [PubMed]
- Bernstein HG, Smalla KH, Bogerts B, et al. The immunolocalization of the synaptic glycoprotein neuroplastin differs substantially between the human and the rodent brain. Brain Res 2007;1134:107-12. [Crossref] [PubMed]
- Ilic K, Mlinac-Jerkovic K, Jovanov-Milosevic N, et al. Hippocampal expression of cell-adhesion glycoprotein neuroplastin is altered in Alzheimer's disease. J Cell Mol Med 2019;23:1602-7. [Crossref] [PubMed]
- Vemula SK, Malci A, Junge L, et al. The Interaction of TRAF6 With Neuroplastin Promotes Spinogenesis During Early Neuronal Development. Front Cell Dev Biol 2020;8:579513. [Crossref] [PubMed]
- Caplan IF, Maguire-Zeiss KA. Toll-Like Receptor 2 Signaling and Current Approaches for Therapeutic Modulation in Synucleinopathies. Front Pharmacol 2018;9:417. [Crossref] [PubMed]


