
Extracorporeal membrane oxygenation (ECMO) support for acute hypoxemic respiratory failure patients: outcomes and predictive factors
Introduction
Extracorporeal membrane oxygenation (ECMO) is an effective rescue therapy for critically ill patients suffering from ventilatory or circulatory failure (1). In most situations, ECMO is recommended for patients who are refractory to conventional treatment. The goal of ECMO treatment is to maintain oxygenation and organ perfusion until recovery or while awaiting definitive therapy for potential reversible illness. Veno-venous ECMO (VV-ECMO) was reported to be effective in treating acute refractory hypoxemic respiratory failure (2,3), and veno-arterial ECMO (VA-ECMO) was reported to confer benefit in treatment of cardiogenic shock, septic shock, massive pulmonary embolism, and during extracorporeal cardiopulmonary resuscitation (E-CPR) (4-6).
A recent meta-analysis showed improvement in mortality rates among severe acute respiratory distress severe syndrome (ARDS) patients (7); however, ECMO support is expensive and requires a specialized and experienced team. Moreover, serious complications, including bleeding from cannulation site, intracranial hemorrhage, systemic embolization, and acute renal failure (requiring renal replacement therapy) frequently develop, and these complications are associated with high morbidity and mortality (8). Proper case selection for the initiation of ECMO support is therefore essential.
Several mortality prediction scoring systems for patients on ECMO support have been developed, including the Predicting Death for Severe ARDS on VV-ECMO (PRESERVE) score (9), the Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score (10), and the PREdiction of Survival on ECMO Therapy (PRESET) score (11). Despite their proven ability to project mortality in this clinical setting, these scores were developed using ECMO data collected mainly from hospitals in developed countries. It is, therefore, possible that data from the developing world could be comparatively different, which could adversely influence the accuracy of ECMO survival prediction. Accordingly, the aim of this study was to investigate the outcomes and factors associated with mortality in acute hypoxemic respiratory failure patients who received ECMO support, and to externally validate a preexisting ECMO survival prediction scoring system.
We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1460/rc).
Methods
Study design and population
This retrospective cohort study was conducted at the Division of Critical Care of the Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand from September 2010 to February 2021. The study enrolled adult patients aged 18 years old or older who had acute severe hypoxemic respiratory failure, and who underwent either VV-ECMO or VA-ECMO support in the medical or surgical intensive care unit (ICU). Acute severe hypoxemic respiratory failure was defined as including all of the following: (I) onset of severe hypoxemia ≤7 days, (II) PF ratio [partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2)] ≤100, (III) mechanical ventilation with FiO2 ≥0.8, and (IV) positive end-expiratory pressure (PEEP) ≥8 cmH2O or refractory hypoxemia unresponsive to PEEP titration. Patients who had ECMO support for post-cardiac surgery, for postoperative coronary artery bypass, and as bridging therapy for heart or lung transplantation were excluded. Patients with known chronic or end-stage lung disease were also excluded.
Ethical consideration and data collection
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Siriraj Institutional Review Board approved the protocol for this study (approval number Si 203/2018). Due to the study’s retrospective design, written informed consent was not required. Electronic case record forms of all enrolled patients were reviewed. The following data were collection: baseline demographic data, coexisting disease, ICU admission diagnosis, ECMO indications, mechanical ventilator setting, hemodynamic parameters, arterial blood gas analysis, and complications associated with ECMO. Adjunctive therapy, including the use of the prone position, neuromuscular blocking agents, recruitment maneuver, and renal replacement therapy, was also recorded. Critical illness severity scores, SOFA scores and Acute Physiology and Chronic Health Evaluation (APACHE) II scores, together with ECMO survival prediction scores, including PRESERVE scores, RESP scores, and PRESET scores were calculated using the worst parameters within 24 hours before ECMO cannulation (9-11). ECMO duration, the number of ventilator-dependent days, and ICU and hospital length of stay was also recorded. Rate of in-hospital mortality was the primary outcome.
Statistical analysis
All continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test. Variables that deviated from the normal distribution are reported as median and interquartile range (IQR). Variables with normal distribution are shown as mean plus/minus standard deviation (SD). Comparison of non-normal distribution variables was performed by the Mann-Whitney U test, while Student’s t-test was used for variables with a normal distribution. The categorical variables are shown as a number and percentage, and those comparisons were made using either the chi-square test or Fisher’s exact test. Receiving operating characteristic (ROC) curve analysis was performed to identify cut-off values among the continuous variables that showed significant difference between groups. The optimal cut-off point for each variable of interest was determined using the Youden index (12). Continuous variables were then classified into positive or negative groups according to the cutoff point. Univariate analysis was used to identify possible predictors of in-hospital mortality. A univariate analysis was performed on all baseline clinical parameters that showed significant differences between surviving and deceased patients. For the continuous variables, an ROC curve analysis was performed to identify a cut-off value, as mentioned above, before performing a univariate analysis. Variables with a P value less than 0.1 in the univariate analysis were included in a multivariate logistic regression analysis. A P value less than 0.05 was considered statistically significant. Statistical Package for the Social Sciences (SPSS) version 18.0 (IBM Corporation, Armonk, NY, USA) was used for all statistical analyses.
Results
During the study period, 81 patients received ECMO support. Sixteen patients were excluded as follows: eight post-cardiac surgery, four post-cardiac arrest from documented cardiac causes, and four patients who underwent ECMO as bridging therapy for heart and/or lung transplantation. The remaining 65 patients who received ECMO support for acute hypoxemic respiratory failure were enrolled in this study (Figure 1). Patient baseline characteristics are shown in Table 1. The overall median age was 61 years (IQR: 49–70 years), the median BMI was 22.6 kg/m2 (IQR: 20.6–28.0 kg/m2), the median APACHE II score was 27 (IQR: 24–30), and the median SOFA score was 13 (IQR: 11–16). Hypertension (HT) was the leading underlying condition, followed by diabetes mellitus (DM) and chronic kidney disease (CKD). ARDS was the leading diagnosis of acute hypoxemic respiratory failure (49/65, 75.4%), followed by septic shock (10/65, 15.4%). Hospital survivors had lower illness severities as measured by APACHE II, SOFA, PRESERVE, RESP, and PRESET scores. Pulmonary embolism was diagnosed in a significant higher proportion of survivors compared to non-survivors [4/20 (20%) vs. 2/45 (4.4%), P=0.046]. All pulmonary embolism patients presented with refractory hypoxemia, followed by low blood pressure, then received VA- ECMO support for circulatory failure and refractory hypoxemia. Median ventilator-dependent days {1 [1–2] vs. 2 [2–4] days, P=0.07} and hospital admission days {1 (IQR: 1–2) vs. 4 (IQR: 1–11) days, P=0.06} before ECMO initiation were significantly shorter among survivors compared to non-survivors. Patient vital signs, mechanical ventilator setting, blood gas analysis, and respiratory management before ECMO initiation are shown in Table 2. There was no significant difference in baseline body temperature, heart rate, mean arterial pressure, central venous pressure, or ventilator setting between groups. Survivors had a significantly higher median arterial blood pH [7.29 (IQR: 7.22–7.38) vs. 7.20 (IQR: 7.13–7.30), P=0.04], PaO2 [70 (IQR: 60–140) vs. 55 (IQR: 44–71); P<0.001], and PF ratio {73 (IQR: 61–141) vs. 58 [47–75]; P=0.001} than those who died in the hospital. However, significantly lower baseline serum lactate [2.2 (IQR: 1.4–4.5) vs. 4.4 (IQR: 2.1–13.2) mmol/L; P=0.04] was observed in the survivor group.
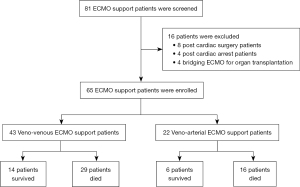
Table 1
| Characteristics | All (N=65) | Survived (n=20) | Died (n=45) | P |
|---|---|---|---|---|
| Age (years) | 61 [49–70] | 61 [48–70] | 61 [50–70] | 0.92 |
| Male gender | 34 (52.0%) | 11 (55.0%) | 23 (51.1%) | 0.77 |
| Body mass index (kg/m2) | 22.6 (20.6–28.0) | 24.4 (20.9–29.3) | 22.5 (19.7–27.5) | 0.37 |
| Severity score | ||||
| APACHE II score | 27 [24–30] | 26 (19–27.8) | 28 [25–32] | 0.03* |
| SOFA score | 13 [11–16] | 12 [10–13] | 14 [11–16] | 0.02* |
| PRESET score | 7 [6–9] | 6 [4–7] | 8 [7–11] | <0.001* |
| PRESERVE score | 5 [4–6] | 4 [3–5] | 5 [4–6] | 0.04* |
| RESP score | 1 [−1 to 2] | 2 [1–4] | 0 [−3 to 2] | 0.001* |
| Underlying conditions | ||||
| Hypertension | 44 (67%) | 15 (75%) | 29 (64.4%) | 0.40 |
| Diabetes mellitus | 25 (38%) | 9 (45%) | 16 (35.6%) | 0.47 |
| Chronic kidney disease | 15 (23%) | 5 (25%) | 10 (22.2%) | 0.81 |
| Coronary arterial disease | 9 (14%) | 4 (20%) | 5 (11.1%) | 0.34 |
| Malignancy | 8 (12%) | 3 (15%) | 5 (11.1%) | 0.66 |
| Diagnosis, n (%) | ||||
| ARDS | 49 (75.3) | 14 (70) | 35 (77.8) | 0.52 |
| Severe pneumonia | 40 (61.5) | 12 (60) | 28 (62.2) | 0.87 |
| Sepsis from other source | 9 (13.8) | 2 (10) | 7 (15.6) | 0.49 |
| Septic shock | 10 (15.4) | 2 (10) | 8 (17.8) | 0.35 |
| Pulmonary embolism | 6 (9.2) | 4 (20) | 2 (4.4) | 0.046* |
| Ventilator days before ECMO | 1 [1–4] | 1 [1–2] | 2 [1–4] | 0.07 |
| Hospital days before ECMO | 2 [1–8] | 1 [1–2] | 4 [1–11] | 0.06 |
Data presented as median and interquartile range or number and percentage. *, a P value <0.05 indicates statistical significance. APACHE II, Acute Physiology and Chronic Health Evaluation score; SOFA, Sequential Organ Failure Assessment score; PRESET, PREdiction of Survival on ECMO Therapy score; PRESERVE, Predicting Death for Severe ARDS on VV-ECMO score; RESP, Respiratory Extracorporeal Membrane Oxygenation Survival Prediction score; ARDS, acute respiratory distress severe syndrome; ECMO, extracorporeal membrane oxygenation.
Table 2
| Parameters | All (N=65) | Survived (n=20) | Died (n=45) | P |
|---|---|---|---|---|
| Body temperature (°C) | 36.5 (35.9–37.4) | 36.9 (36.3–37.8) | 36.5 (35.7–37.3) | 0.12 |
| Heart rate (beats/min) | 112 [99–128] | 114 [91–125] | 111 [100–129] | 0.73 |
| Mean arterial pressure (mmHg) | 74 [65–89] | 84 [64–97] | 74 [65–87] | 0.18 |
| Central venous pressure (mmHg) | 14 [12–21] | 13 [12–20] | 13 [11–16] | 0.69 |
| Lactate (mmol/L) | 3.9 (1.9–10.7) | 2.2 (1.4–4.5) | 4.4 (2.1–13.2) | 0.04* |
| Platelet (×103/mL) | 147 [86–213] | 173 [100–234] | 143 [77–215] | 0.18 |
| Ventilator setting before ECMO | ||||
| PIP (cmH2O) | 30 [28–37] | 30 [23–36] | 32 [28–38] | 0.12 |
| PEEP (cmH2O) | 10 [8–14] | 8 [5–16] | 10 [8–14] | 0.23 |
| Tidal volume (mL/kg) | 6 [5–7] | 6.5 (4.5–7.8) | 6.3 (5.2–7.0) | 0.81 |
| Respiratory rate (time/min) | 30 [24–35] | 32 [24–34] | 30 [24–35] | 0.83 |
| Minute ventilation (L/min) | 11 [8–14] | 11 [9–13] | 10 [7–14] | 0.41 |
| FiO2 | 1 [1–1] | 1 (0.9–1) | 1 [1–1] | 0.27 |
| Arterial blood gas before ECMO | ||||
| pH | 7.22 (7.17–7.34) | 7.29 (7.22–7.38) | 7.20 (7.13–7.30) | 0.04* |
| PaO2 | 60 [49–77] | 70 [60–140] | 55 [44–71] | <0.001* |
| PaCO2 | 50 [36–63] | 40 [31–53] | 52 [41–66] | 0.07 |
| HCO3 | 21 [17–24] | 19 [15–21] | 22 [18–25] | 0.12 |
| PF ratio | 63 [51–84] | 73 [61–141] | 58 [47–75] | 0.001* |
| Rescue therapy, n (%) | ||||
| Prone position | 8 (12.3) | 2 (10.5) | 6 (13.3) | 0.76 |
| Recruitment maneuver | 25 (38.4) | 9 (45) | 16 (35.6) | 0.47 |
| Neuromuscular blocking agents | 37 (56.9) | 16 (80) | 36 (80) | 0.99 |
| Renal replacement therapy | 40 (61) | 8 (40) | 32 (71.1) | 0.02* |
All data presented as median and interquartile range. *, a P value <0.05 indicates statistical significance. ECMO, extracorporeal membrane oxygenation; PIP, peak inspiratory pressure; PEEP, positive end-expiratory pressure; FiO2, fraction of inspired oxygen; pH, acidity or basicity; PaO2, partial pressure of oxygen; PaCO2, partial pressure of carbon dioxide; HCO3, bicarbonate; PF ratio; PaO2/FiO2 ratio.
Both groups showed the same leading indications for ECMO support. These included severe hypoxemia followed by E-CPR and refractory shock. VV-ECMO was used for 43 patients, and 14 of them survived to hospital discharge, while VA-ECMO was used for 22 patients and 6 survived to hospital discharge. There was no significant difference between the proportion of patients who received VV-ECMO or VA-ECMO between the survivor and non-survivor groups (Table 3). Mechanical ventilator-dependent days, ECMO support duration, ICU length of stay, and hospital length of stay were all significantly longer in the survivor group. The most common complication associated with ECMO support was bleeding at the puncture site (23%), followed by limb ischemia, intracranial bleeding, and gastrointestinal bleeding (Table 3).
Table 3
| Data | All (N=65) | Survived (n=20) | Died (n=45) | P |
|---|---|---|---|---|
| Indication for ECMO, n (%) | ||||
| Severe hypoxemia | 47 (72) | 14 (70) | 33 (73.3) | 0.78 |
| E-CPR | 11 (20) | 2 (10) | 9 (20) | 0.31 |
| Refractory shock | 7 (7.7) | 4 (20) | 3 (6.7) | 0.18 |
| ECMO type, n (%) | ||||
| Veno-venous ECMO | 43 (66) | 14 (70) | 29 (64.4) | 0.66 |
| Veno-arterial ECMO | 22 (34) | 6 (30) | 16 (35.6) | 0.66 |
| Duration (days) | ||||
| Ventilator days | 17 [7–25] | 18 [12–26] | 15 [5–24] | 0.077 |
| ECMO duration | 8 [5–15] | 9 [7–12] | 7 [3–15] | 0.23 |
| ICU length of stay | 20 [7–31] | 25 [18–52] | 15 [5–25] | 0.002* |
| Hospital length of stay | 25 [11–41] | 40 [29–78] | 19 [6–32] | <0.001* |
| Complication, n (%) | ||||
| Bleeding at puncture site | 15 (23) | 5 (25) | 10 (22.7) | 0.84 |
| Limb ischemia | 3 (4) | 1 (5) | 2 (4.7) | 0.95 |
| Intracranial bleeding | 3 (4) | 1 (5) | 3 (6.8) | 0.78 |
| GI bleeding | 3 (4) | 0 (0) | 3 (6.8) | 0.3 |
Data presented as number and percentage or median and interquartile range. *, a P value <0.05 indicates statistical significance. ECMO, extracorporeal membrane oxygenation; E-CPR, extracorporeal cardiopulmonary resuscitation; ICU, intensive care unit.
ROC curve analysis was used to identify the cut-off values of the continuous variables that showed significant difference between the survivor and non-survivor groups. All clinically relevant variables, including baseline APACHE II score, SOFA score, quantity of hospital admission days before ECMO initiation, mechanical ventilator-dependent days before ECMO initiation, arterial blood pH, PF ratio, and serum lactate were reclassified into positive or negative groups according to the identified cut-off values. The results of univariate and multivariate analysis (Table 4) revealed four independent predictors of in-hospital mortality, including SOFA score >14 [odds ratio (OR): 8.77, 95% confidence interval (CI): 1.50–51.49; P=0.016], hospitalized >72 hours before ECMO initiation [OR: 13.38, 95% CI: 1.93–92.94; P=0.009], baseline PF ratio <60 [OR: 10.08, 95% CI: 1.45–70.21; P=0.02], and baseline arterial blood pH <7.2 [OR: 14.99, 95% CI: 1.85–121.81; P=0.011]. These factors were then combined to create a new ECMO mortality prediction scoring system (the SOFA EMCO mortality prediction scoring system), SHOP, as follows: S: SOFA >14, H: hospitalize >72 hours, O: PF ratio <60, and P: pH <7.2. The presence of each parameter was given a score of 1 point with the highest possible score of 4. A score of 2 or more predicts in-hospital mortality.
Table 4
| Factors | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Crude OR | P | Adjusted OR | P | ||
| APACHE II >30 | 4.97 (1.02–24.9) | 0.034 | |||
| SOFA >14 | 6 (1.72–20.89) | 0.003 | 8.77 (1.50–51.49) | 0.016* | |
| Hospitalized >72 hour | 5.92 (2.52–23.07) | 0.006 | 13.38 (1.93–92.94) | 0.009* | |
| Ventilator >48 hour | 7.20 (1.49–34.77) | 0.007 | |||
| pH <7.2 | 9.00 (1.85–43.74) | 0.002 | 14.99 (1.85–121.81) | 0.011* | |
| Lactate >2 | 3.50 (1.14–10.76) | 0.025 | |||
| PF ratio <60 | 7.56 (1.92–29.77) | 0.002 | 10.08 (1.45–70.21) | 0.02* | |
| Pulmonary embolism | 0.19 (0.03–1.12) | 0.046 | |||
*, a P value <0.05 indicates statistical significance. ECMO, extracorporeal membrane oxygenation; OR, odds ratio; APACHE II, Acute Physiology and Chronic Health Evaluation score; SOFA, Sequential Organ Failure Assessment score; pH, acidity or basicity; PF ratio; PaO2/FiO2 ratio.
To evaluate the efficacy of preexisting severity scores, ROC curves for each scoring system were generated. Area under the ROC curve (AUC) values are shown in Table 5. Among overall ECMO support patients, the PRESET score had the highest AUC (AUC: 0.798, 95% CI: 0.676–0.919; P<0.001), followed by the RESP score (AUC: 0.766, 95% CI: 0.645–0.887; P=0.001), the SOFA score, and the APACHE II score, whereas the PRESERVE score had the lowest AUC (AUC: 0.654, 95% CI: 0.516–0.793; P=0.048). In the VV-ECMO supported subgroup, the RESP score had the highest AUC, followed by the PRESET, PRESERVE, SOFA, and APACHE II scoring systems. The PRESET score had the highest AUC in the VA-ECMO subgroup, followed by the SOFA, APACHE II, RESP, and PRESERVE scoring systems. The new SHOP scoring system developed and introduced in this study had higher AUC values for predicting mortality in ECMO patients among all patients, and in the VV-ECMO and VA-ECMO subgroups.
Table 5
| System | Overall group (N=65) | VV-ECMO group (n=43) | VA-ECMO group (n=22) | |||||
|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | P* | AUC (95% CI) | P* | AUC (95% CI) | P* | |||
| PRESET | 0.798 (0.676–0.919) | <0.001 | 0.777 (0.614–0.940) | 0.004 | 0.859 (0.691–1.000) | 0.011 | ||
| RESP | 0.766 (0.645–0.887) | 0.001 | 0.817 (0.689–0.944) | 0.001 | 0.771 (0.566–0.976) | 0.056 | ||
| PRESERVE | 0.654 (0.516–0.793) | 0.048 | 0.675 (0.504–0.846) | 0.066 | 0.604 (0.375–0.833) | 0.461 | ||
| APACHE II | 0.669 (0.536–0.803) | 0.030 | 0.608 (0.433–0.783) | 0.254 | 0.781 (0.584–0.978) | 0.047 | ||
| SOFA | 0.688 (0.546–0.824) | 0.018 | 0.628 (0.448–0.808) | 0.178 | 0.797 (0.566–1.000) | 0.036 | ||
| SHOP | 0.873 (0.791–0.956) | <0.001 | 0.866 (0.760–0.971) | <0.001 | 0.891 (0.755–1.000) | 0.006 | ||
*, P value of the AUC. If P<0.05, it indicated that the AUC is significantly different from 0.5, therefore there is evidence that the mortality predicting score has an ability to distinguish between the survivor and non-survivor. If P>0.05, it indicated that the AUC is no different from 0.5, therefore, the mortality predicting score has no ability to distinguish between the survivor and non-survivor. ECMO, extracorporeal membrane oxygenation; VV-ECMO, veno-venous ECMO; VA-ECMO, veno-arterial ECMO; AUC, area under the curve; CI, confidence interval; PRESET, PREdiction of Survival on ECMO Therapy score; RESP, Respiratory Extracorporeal Membrane Oxygenation Survival Prediction score; PRESERVE, Predicting Death for Severe ARDS on VV-ECMO score; APACHE II, Acute Physiology and Chronic Health Evaluation score; SOFA, Sequential Organ Failure Assessment score; SHOP score, SOFA score >14, hospitalized >72 hours before ECMO initiation, PaO2/FiO2 ratio <60, and pH <7.2.
Discussion
Among patients with acute severe hypoxemic respiratory failure who required ECMO support, VV-ECMO was used in two-thirds of patients, and the patient survival rate at hospital discharge was 30.8%. The clinical parameters before ECMO initiation that were found to be independently associated with in-hospital mortality were SOFA score >14, duration of admission >72 hours, PF ratio <60, and pH <7.2. Preexisting ECMO prediction scoring systems, including PRESERVE, RESP, and PRESET, yielded moderately high accuracy in outcome prediction, and the PRESET score was found to be the most accurate. Our developed predictive SHOP score derived from the parameters above was shown to be more accurate for predicting in-hospital mortality among all patients, and in both the VV-EMCO and VA-EMCO subgroups.
The in-hospital mortality rate of 69.2% among our ECMO-supported patients was high compared to the rates reported from other studies. ECMO case volume, patient age, and patient baseline conditions may explain the high mortality rate observed in this study. Two prospective randomized controlled studies from Europe reported hospital mortality rates ranging from 30–50% (2,3). Most centers participating in those studies were experienced in ECMO and had a high annual ECMO support case volume. Higher ECMO support case volume correlated with higher physician and team experience in caring for ECMO patients. This is supported by Barboro et al., who reported that adult patients receiving ECMO at a hospital with over 30 ECMO cases per year had a significantly lower mortality rate (adjusted OR: 0.61, 95% CI: 0.46–0.80) compared to those treated at a low ECMO volume hospital (<6 cases per year) (13). A report from Japan also supports this association. A retrospective observational study that used data from a national administrative database of ECMO for respiratory failure found a hospital mortality rate of 50.4% in high-volume institutions (>16 ECMO per year) compared with 62.5% in low-volume institutions (<8 ECMO per year) (14). Regarding the volume of ECMO support given at the center in the present study, there were 81 ECMO support patients in 11 years, which defines the center as low volume and helps to explain the mortality rate above 60%.
Patient age could influence higher in-hospital mortality. A study with a large patient population reported a lower median age among hospital survivors compared to the median age of those who died [39 years (IQR: 27–51) vs. 45 years (IQR: 30–58)] (10). A similar finding was reported from two other studies (median age 44 and 48 years in the survivor and non-survivor groups, respectively) (9,11). The median age of 61 years (IQR: 49–70) in the present study could be another reason for higher in-hospital mortality.
The presence of hemodynamic instability with or as a consequence of respiratory hypoxemia may also influence mortality. The patient population in the present study included a very high proportion of hemodynamic instability or cardiac arrest. Although these conditions could be considered complications of prolonged hypoxemia, VA-ECMO support was required and the mortality rate among these patients was high. VA-ECMO was employed in 34% of our patients, while in RESP population, VA-ECMO was used in 23%. This study enrolled patients who presented with acute hypoxemic respiratory failure, which is a unique underlying pathophysiologic condition that typically requires VV-ECMO support. However, in severe cases prolonged hypoxemia causes hemodynamic compromise and circulatory collapse, which requires VA-ECMO. The difference in mortality rates of VA-ECMO and VV-ECMO patients from previous reports may be influenced by underlying conditions. In the previously reported study, VA-ECMO was mainly used in ECMO assisted CPR, in which those patients had acute coronary syndrome as an underlying condition (15). In this case, the mortality rates of VA-ECMO were generally much higher than VV-ECMO. In the present study, the mortality rate of VA-ECMO supported patients was 72.7% (n=22), which similar with 67.4% (n=43) among VV-ECMO supported patients. This could be explained by the similarity of acute hypoxemic respiratory failure among the patients due to underlying conditions.
Regarding our evaluation of the efficacy of the preexisting mortality prediction scoring systems, we found the PRESET and RESP systems to be the most accurate in overall patients, and in the VV-ECMO and VA-ECMO subgroups, followed by the PRESERVE score and the two evaluated general severity scores. However, the SHOP scoring system surpassed all other evaluated scoring systems at predicting mortality among ECMO-supported patients. The SHOP score comprises SOFA score >14, duration of admission in the hospital >72 hours, PF ratio <60, and pH <7.2. The SHOP score is a user-friendly and easy to calculate score. During the current COVID-19 pandemic, several new ECMO centers have been established in both developed and developing countries for rescue therapy to treat patients with severe COVID-19 and acute severe respiratory failure (16,17). The newly developed SHOP score could be used to select candidate patients and for prognostic determination at new ECMO centers. This could provide a more accurate score than other scores developed at centers with high ECMO experience.
Limitations
The data included in this study was collected from a single large national tertiary referral center. As such, some findings may not be immediately generalizable to other care settings. Additionally, external validation of the newly developed SHOP score is needed before its application in general practice. We plan to perform both internal validation by using data from new ECMO cases and securing external validation by use of ECMO case data from other hospitals in the future.
Although the proposed SHOP score only has four parameters, it is prudent to remark that the SOFA score itself consists of six components. For centers who do not routinely calculate the SOFA score during clinical care, this may increase complexity prior to SHOP score application. However, the SOFA score evaluates the function of six main organs. In order to calculate the SOFA score, two parameters must be evaluated: mean arterial blood pressure and the Glasgow Coma Scale score. Additionally, four essential laboratory investigations (creatinine, platelet count, bilirubin level and PaO2/FiO2 ratio) must be examined, especially among the critically ill that are candidates for ECMO support. Furthermore, the SOFA score is currently a part of the SEPSIS-3 definition for diagnosis of sepsis and septic shock, which promotes its use worldwide.
Although most of our patients met the diagnostic criteria for ARDS, some patients developed acute hypoxemic respiratory failure from other causes. Acute pulmonary embolism was diagnosed in six patients (9.2%), and four of those survived to hospital discharge. Thus, ECMO support could be an effective treatment for this condition during critical period. Lastly, the long duration of patient enrollment could be considered a study limitation since changes in treatment strategies during this ten-year period could affect patient outcomes.
Conclusions
In-hospital mortality among ECMO-supported patients was high at 69%. SOFA scores >14, hospitalized >72 hours, PaO2/FiO2 ratio <60, and pH <7.2 were found to be independent predictors of in-hospital mortality. A SHOP score of 2 or higher out of a possible 4 significantly predicts in-hospital mortality in ECMO-supported patients. This mortality prediction scoring system requires both internal and external validation before it can be recommended for application in general practice.
Acknowledgments
The authors gratefully acknowledge Mr. Kevin P. Jones and Dr. Adhiratha Boonyasiri for assistance with English editing.
Funding: This study was funded by a grant from the Siriraj Critical Care Research Funding (grant No. SiCCR 005).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1460/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1460/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1460/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1460/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Siriraj Institutional Review Board approved the protocol for this study (approval number Si 203/2018). Due to the study’s retrospective design, written informed consent was not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 2015;7:E166-76. [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Combes A, Hajage D, Capellier G, et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med 2018;378:1965-75. [Crossref] [PubMed]
- Vallabhajosyula S, Prasad A, Bell MR, et al. Extracorporeal Membrane Oxygenation Use in Acute Myocardial Infarction in the United States, 2000 to 2014. Circ Heart Fail 2019;12:e005929. [Crossref] [PubMed]
- Tantibundit P, Mekjarasnapha M, Pulnitiporn A, et al. Extracorporeal cardiopulmonary resuscitation in a woman with twin pregnancy. Perfusion 2021; Epub ahead of print. [Crossref] [PubMed]
- Bréchot N, Hajage D, Kimmoun A, et al. Venoarterial extracorporeal membrane oxygenation to rescue sepsis-induced cardiogenic shock: a retrospective, multicentre, international cohort study. Lancet 2020;396:545-52. [Crossref] [PubMed]
- Combes A, Peek GJ, Hajage D, et al. ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med 2020;46:2048-57. [Crossref] [PubMed]
- Zangrillo A, Landoni G, Biondi-Zoccai G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc 2013;15:172-8. [PubMed]
- Schmidt M, Zogheib E, Rozé H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013;39:1704-13. [Crossref] [PubMed]
- Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 2014;189:1374-82. [Crossref] [PubMed]
- Hilder M, Herbstreit F, Adamzik M, et al. Comparison of mortality prediction models in acute respiratory distress syndrome undergoing extracorporeal membrane oxygenation and development of a novel prediction score: the PREdiction of Survival on ECMO Therapy-Score (PRESET-Score). Crit Care 2017;21:301. [Crossref] [PubMed]
- Perkins NJ, Schisterman EF. The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 2006;163:670-5. [Crossref] [PubMed]
- Barbaro RP, Odetola FO, Kidwell KM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med 2015;191:894-901. [Crossref] [PubMed]
- Muguruma K, Kunisawa S, Fushimi K, et al. Epidemiology and volume-outcome relationship of extracorporeal membrane oxygenation for respiratory failure in Japan: A retrospective observational study using a national administrative database. Acute Med Surg 2020;7:e486. [Crossref] [PubMed]
- Lescouflair T, Figura R, Tran A, et al. Adult veno-arterial extracorporeal life support. J Thorac Dis 2018;10:S1811-8. [Crossref] [PubMed]
- Rabie AA, Azzam MH, Al-Fares AA, et al. Implementation of new ECMO centers during the COVID-19 pandemic: experience and results from the Middle East and India. Intensive Care Med 2021;47:887-95. [Crossref] [PubMed]
- Tongyoo S, Kongsayreepong S. Extracorporeal membrane oxygenation (ECMO) for COVID-19 patients: ECMO for COVID-19. Clin Crit Care 2021;29:e0005. [Crossref]


