Dysfunction of mechanical heart valve prosthesis: experience with surgical management in 48 patients
Although the long-term outcome of mechanical value replacement have improved significantly with the progress of materials, design, manufacturing and surgical techniques, prosthetic valve dysfunction remains a very serious complication after mechanical valve replacement with high mortality and morbidity (1). Numerous studies have been carried out to identify the risk factors for prosthetic valve dysfunction (2-13). An algorithm for management of obstructive thrombosed prosthetic heart valve has been developed recently (4).
Between October 1996 and June 2011, a total of 16,893 cases of mechanical heart valve replacement were performed in our institute. Among these there were 48 (0.28%) cases of reoperations for mechanical heart valve dysfunction. In this retrospective study, we seek to report our experience with surgical management of this group of patients with emphasis on etiology, surgical strategy and early outcomes.
Patients and methods
The Ethics Committee of Fu Wai Hospital and Cardiovascular Institute approved this retrospective study and waived the need for individual patient consent for this study.
Patients
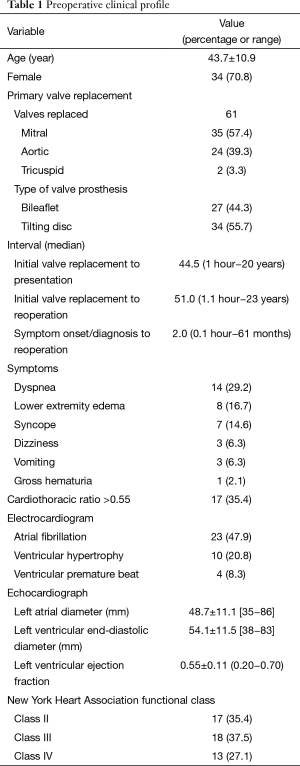
There were 14 males (29.2%) and 34 females (70.8%), with a mean age of 43.7±10.9 years (range, 16–63 years). The median time from the primary valve replacement to onset of symptoms or diagnosis and to reoperation was 44.5 months (range, 1 hour to 20 years) and 51 months (range, 1.1 hours to 23 years), respectively; while the median interval from symptomatic onset or diagnosis to reoperation was 2 months (range, 0.1 hour to 61 months) (Table 1).
Full table
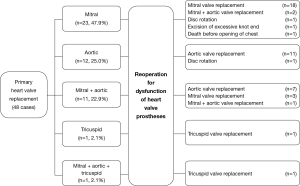
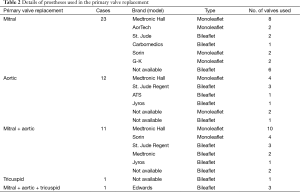
The primary operation was mitral valve replacement in 23 cases (47.9%, including five with a concomitant tricuspid valve repair), aortic valve replacement in 12 (25.0%, including one Bentall operation), aortic and mitral valve replacement in 11 (22.9%), tricuspid valve replacement and aortic + mitral + tricuspid valve replacement, in one each (2.1%) (Figure 1). Table 2 lists the brands (model) and types of 61 valve prostheses used in the primary operation. Among these, 34 were tilting disc (55.7%) and 27 bileaflet (44.3%); 24 were implanted (39.3%) in the aortic (12 tilting disc, 12 bileaflet), 35 (57.4%) in the mitral (22 tilting disc, 13 bileaflet), and 2 bileaflet prostheses (3.3%) in the tricuspid position (Table 2).
Full table
The clinical presentation varied considerably from dyspnea and palpitation with changes in valve clicks on auscultation to sudden onset of ventricular fibrillation immediately following the primary valve replacement. Electrocardiogram was abnormal in 37 cases (77.1%), including atrial fibrillation in 23 (47.9%), ventricular hypertrophy in 10 (20.8%) and ventricular premature beat in 4 (8.3%). Chest roentgenograph showed a cardiothoracic ratio >0.55 in 17 patients (35.4%). Echocardiography showed that the free mobility of the prosthetic disc or leaflet was interfered, which resulted in obstruction or regurgitation of the prosthetic valve. The preoperative New York Heart Association (NYHA) functional class was II in 17 patients (35.4%), III in 18 (37.5%) and IV in 13 (27.1%) (Table 1).
Surgical technique
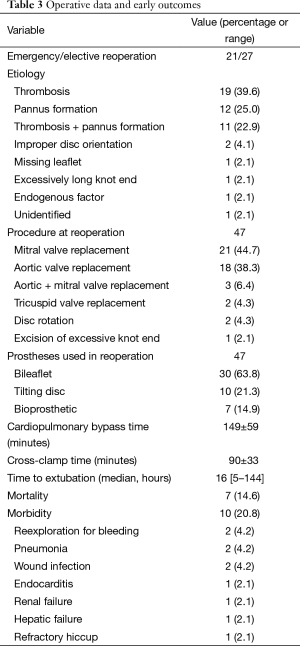
The reoperation was done under moderate hypothermic cardiopulmonary bypass with cold potassium cardioplegia. There were 21 (43.8%) emergency and 27 (65.2%) elective operations. In 46 patients, the operation was performed via a repeat median sternotomy with cannulation of the ascending aorta and vena cava or right atrium. Left thoracotomy and femoral artery cannulation was used in one patient who sustained cardiac arrest after induction of general anesthesia.
Results
The causes for mechanical valve dysfunction were thrombosis in 19 patients (39.6%), pannus formation in 12 (25.0%), pannus formation with thrombosis in 11 (22.9%), improper disc orientation in 2 (4.1%), missing leaflet in 1 (2.1%), excessively long knot end in 1 (2.1%), endogenous factor in 1 (2.1%) and unidentifiable in 1 (2.1%). In one patient who had an aortic valve replacement 10 years ago and suffered from dyspnea for 2 months, one leaflet of the previously implanted bileaflet prosthesis was found to be missing during reoperation.
Reoperations was done in 47 (97.9%) cases, including mitral valve replacement in 21 (43.7%), aortic valve replacement in 18 (37.5%, including one Bentall operation), mitral and aortic valve replacement in 3 (6.3%), tricuspid valve replacement and disc rotation in 2 (4.2%) each, and excision of excessive knot end in 1 (2.1%) (Figure 1). There were 37 mechanical and 7 bioprosthetic valve replacements, using 40 (85.1%) mechanical (10 tilting disc and 30 bileaflet; 20 in mitral, 20 in aortic) and 7 (14.9%) bioprosthetic valves (4 in mitral, 2 in tricuspid and 1 in aortic position) (Table 3). Concomitant procedures included tricuspid valve repair in 13 (27.1%) patients, repair of aneurysm of the sinus of Valsalva in 1 (2.1%).
Full table
The times of cardiopulmonary bypass and aortic cross-clamp were 149±59 (range, 51–391 minutes) and 90±33 minutes (range, 0–142 minutes), respectively. The median time to extubation was 16 hours (range, 5–144 hours).
Seven patients expired (14.6%, 7/48), six of whom (85.7%) died after an emergency reoperation. The mortality rate was significantly higher in emergency (28.6%, 6/21) than in elective patients (3.7%, 1/27; P=0.03). The cause of death was low cardiac output in three cases (6.3%), multi-organ failure in two (4.2%) and intractable ventricular fibrillation in two (4.2%). One female patient expired intraoperatively due to refractory ventricular fibrillation before opening of the chest and the etiology was not identified. Extracorporeal membrane oxygenation was used in one patient who could not wean from cardiopulmonary bypass but failed to save his life.
Morbidity occurred in 10 patients (20.8%), including reexploration for bleeding in two (4.2%), wound infection in two (4.2%) and pneumonia in two (4.2%), endocarditis in one (2.1%), renal failure in one (2.1%), hepatic failure in one (2.1%) and refractory hiccup in one (2.1%) (Table 3). All patients with complications were cured and discharged uneventfully.
Discussion
Prosthetic valve dysfunction is one of the most serious complications after mechanical valve replacement and the optimal management remains controversial. In literature, the incidence was 0.1–6.0% per patient year (1,5-10) and the mean interval between the initial valve replacement and reoperation was 10–16 years (9,12,14,15). In this series, mechanical valve prosthetic dysfunction accounted for 0.28% of the mechanical valve replacements in our institution and the median duration from the initial valve implantation to reoperation was 51 months.
The clinical presentation varied from no symptoms to acute heart failure even sudden cardiac arrest. On auscultation, valve clicks may be diminished or absent with additional systolic or diastolic murmurs. Detection of the “double click” may be useful for early diagnosis of dysfunction of bileaflet prostheses. Although transthoracic echocardiography (TTE) is the most commonly utilized imaging modality (4), it is less sensitive in detecting the reduced prosthetic disc/leaflet motion than cinefluoroscopy. Transesophageal echocardiography (TEE) (14,16) especially 3-dimensional TEE (17) may be the best diagnostic tool, which can define the causes better and helps in guiding therapy, risk stratification and monitoring follow-up outcomes (18). In cases when it is difficult to differentiate pannus from thrombosis (14,19), Doppler study can help in the diagnosis by measuring hemodynamic parameters (8). Besides, multidetector-row computed tomography can identify causes of prosthetic valve dysfunction that constitute surgical indications but are missed at echocardiography or fluoroscopy (20). In this series, the diagnosis was confirmed by Doppler echocardiography in all patients.
Mechanical valve dysfunction can be classified as endogenous and exogenous according to the etiology. Endogenous dysfunctions are caused by valve damage or defect, which has become extremely rare with the improvement of design, materials, manufacture and detection methods in vitro (21). Exogenous causes include inappropriate selection of prosthesis, technical issues or other complications, such as thrombosis, excessive pannus overgrowth into the prosthetic rim, excessively long knot end, residual chordae tendineae stuck in the prosthetic sewing ring, extremely long residual papillary muscles in left ventricle or calcified tissues under the prosthesis hampering leaflet mobility. The most common causes of mechanical valve dysfunction are thrombosis and pannus formation, which was present in 87.5% of patients in this series.
Thrombosis is usually due to inadequate anticoagulation (22) and associated with atrial fibrillation (9) and low cardiac function. However, Maribas et al. reported that valve thrombosis occurs with similar frequency in patients with bioprosthetic valves and in those with mechanical valves who are receiving adequate anticoagulant therapy (23). In this series, thrombosis was seen in 62.5% (30/48) of patients; among them 16 (33.3%) developed atrial fibrillation and 21 (43.7%) were in NYHA functional class III or IV preoperatively. This high incidence of thrombosis highlights the need for adequate anticoagulation and regular follow-up after initial prosthetic valve replacement. Owing to the low pressure of the right heart, the incidence of tricuspid valve dysfunction (almost all caused by thrombosis) is as high as 20% (11). In this series, there were two cases (4.2%) of tricuspid valve dysfunction caused by thrombosis; at reoperation tissue valves were chosen to reduce the risk of recurrent thrombosis.
Pannus formation was found in 47.9% of patients in this series, including 11 (22.9%) with concomitant thrombosis. A study of 87 cases with mitral valve obstruction has found that pannus formation is more frequent than thrombus formation, but thrombosis is of earlier onset than pannus formation (13). Pannus is an overgrowth of fibrous tissue invading the valve orifice and may result from an non-immune inflammatory reaction (13), which is evidenced by elevated levels of plasma transforming growth factor beta 1 (24), protein C and protein S (25). Histologically the pannus tissue incorporates collagen and elastic tissues containing endothelial cells, chronic inflammatory cells and proliferation of myofibroblasts (26). Several studies reported that pannus formation causing prosthetic aortic valve stenosis occurred mainly in female patients with a small body surface area (12,14,24,27). This is corroborated by the 70.8% of female patients in our series. Pannus formation has been identified as the second cause of reoperation for mechanical valve (9). Although mitral valve replacement with partial or complete chordal sparing offers the advantage of preserving left ventricular geometry and function, the unresected mitral valve leaflets may exacerbate the host tissue response to the prosthesis and increase the risk of thrombus and/or pannus formation (28). In our practice, we routinely excise the mitral valve about 2.5 mm from the annulus and trim the residual edge neatly.
For patients with mechanical valve dysfunction, surgical decision-making should be based on the cause of valve dysfunction. For endogenous causes, the only effective option is a redo valve replacement. One patient in this series developed acute valve dysfunction after aortic valve replacement, and TEE showed moderate regurgitation. Immediate inspection ruled out thrombosis, excessive knot end or residual papillary muscles and other exogenous factors. Cardiac activity was not restored after the valve orientation was adjusted twice. Decision was made to use another mechanical valve, which restored cardiac activity and achieved stable hemodynamics. This case suggested that the dysfunction might result from endogenous factors, such as intrinsic defect of the valve. Exogenous factors, such as excessively long knot end or residual chordae tendineae, should be avoided in the initial procedure. To prevent small chordae tendineae from interfering with the free mobility of the prosthetic disc or leaflet, normal saline can be injected to fill the ventricular cavity repeatedly to float the slender chordae and to guarantee complete resection. After the knots are tied, the mobility of discs and length of knot ends should be checked routinely. In three patients of this series with improper disc orientation and excessive knot end, prosthetic valve dysfunction was successfully treated with disc rotation and excision of the excessive knot end. Our experience shows that redo valve replacement may not be necessary in cases with certain exogenous causes, such as improper disc orientation or excessive knot end.
For patients with thrombosis, controversy exists regarding the optimal management. Some believe it is enough to debride the thrombosis instead of prosthetic valve replacement owing to the relatively short surgical duration (5,29), and debridement may have lower risk and mortality than valve replacement. Albeit there are reports that valve function could be normal after debridement, it should be noted that the thrombus usually adheres firmly to the prosthetic valve disc, which makes complete removal difficult (3,9). Any slight damage of the disc surface is likely to cause the recurrence of thrombosis. Although the rate of recurrent thrombosis following clot removal and debridement has been shown to be higher than after valve replacement, the difference in recurrent thrombosis between redo valve replacement and clot debridement was not statistically significant (7,30). Therefore, thrombus removal might be considered in patients with high surgical risks. Deviri and associates argue that the decision to replace or debride the valve should be left to the surgeon, based on anatomic and technical factors at the time of surgery (2). In addition, pannus formation is the result of an exuberant healing process in response to a foreign body and takes longer time to become clinically manifest, often in combination with thrombosis. Because the fibrotic overgrowth generally occurs on both the prosthetic and natural valve surfaces, prosthetic valve replacement is preferred to debridement in patients with pannus (13). Nevertheless, some believe that redo valve replacement might be advisable only in patients with extensive, circumferential pannus (12,27). On the other hand, more recent studies have shown that thrombolysis can restore adequate function of the thrombosed prosthetic valve with high success rate and lower mortality and morbidity rates (22,31,32). In literature, thrombolysis has achieved a success rate of 64–89%; the incidence rate was 5–19% for systemic embolism and 5–8% for major bleeding. But the incidence rate of recurrence was as high as 15–31% with a mortality of 6–12.5% (4,33-37). Based on this, thrombolysis is recommended as the first-line treatment for all patients with left-sided prosthetic valve thrombosis in the Society for Heart Valve Disease guidelines and for patients with low thrombus burden (<0.8 cm2) regardless of NYHA functional class in the American College of Chest Physicians guidelines (35). It is believed that thrombolysis has a higher chance of success if the thrombus is younger than 14 days (22,33).
No matter what treatment would be taken, the mortality rate of prosthetic dysfunction was significantly higher than the primary valvular disease. It was reported that the overall operative mortality was 10.8–15% and 20–40% in emergency operations (2,5,23,38) and as high as 62.5% in early series (39). In this series the overall mortality was 15.2% and 6 of 7 (85.7%) deaths occurred after emergency reoperation. The high mortality of 28.6% in emergency patients may be due to the poor general conditions, worsened cardiac function and lack of sufficient preoperative preparation. While emergency reoperation may be the only life-saving therapy for emergency cases, prompt and sufficient preoperative preparations should be made for patients with chronic prosthetic dysfunction to improve their general conditions and cardiac function, so as to achieve better surgical outcomes.
Limitations
The retrospective study has several limitations, such as the relatively small number of patients and the lack of late follow-up after the reoperation. Specifically, information was not available about the surgical techniques and valve prostheses used in initial valve replacement. Details of anticoagulation management after the initial procedure were also missing, such as the target international normalized ratio (INR), frequency of INR measurements and especially the INR values prior to the occurrence of valve dysfunction.
Conclusions
The results of this study show that thrombus and pannus are two major causes for dysfunction of mechanical valve prosthesis and surgical management is associated with significant mortality and morbidity. Earlier identification and prompt reoperation are vital to achieving better clinical outcomes. The high incidence of thrombosis in this series highlights the need for adequate anticoagulation and regular follow-up after initial mechanical valve replacement. Further study is warranted to determine the long-term outcome of reoperation in patients with mechanical valve dysfunction.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Edmunds LH Jr. Thromboembolic complications of current cardiac valvular prostheses. Ann Thorac Surg 1982;34:96-106. [PubMed]
- Deviri E, Sareli P, Wisenbaugh T, et al. Obstruction of mechanical heart valve prostheses: clinical aspects and surgical management. J Am Coll Cardiol 1991;17:646-50. [PubMed]
- Kontos GJ Jr, Schaff HV, Orszulak TA, et al. Thrombotic obstruction of disc valves: clinical recognition and surgical management. Ann Thorac Surg 1989;48:60-5. [PubMed]
- Huang G, Schaff HV, Sundt TM, et al. Treatment of obstructive thrombosed prosthetic heart valve. J Am Coll Cardiol 2013;62:1731-6. [PubMed]
- Montero CG, Mula N, Brugos R, et al. Thrombectomy of the Björk-Shiley prosthetic valve revisited: long-term results. Ann Thorac Surg 1989;48:824-8. [PubMed]
- Butchart EG, Lewis PA, Grunkemeier GL, et al. Low risk of thrombosis and serious embolic events despite low-intensity anticoagulation. Experience with 1,004 Medtronic Hall valves. Circulation 1988;78:I66-77. [PubMed]
- Martinell J, Fraile J, Artiz V, et al. Reoperations for left-sided low-profile mechanical prosthetic obstructions. Ann Thorac Surg 1987;43:172-5. [PubMed]
- Esmaeilzadeh M, Mirdamadi A, Parsaee M, et al. Is the Peak-to-Mean Pressure Gradient Ratio Useful for Assessment of Aortic Valve Prosthesis Obstruction? J Tehran Heart Cent 2010;5:69-73. [PubMed]
- Rizzoli G, Guglielmi C, Toscano G, et al. Reoperations for acute prosthetic thrombosis and pannus: an assessment of rates, relationship and risk. Eur J Cardiothorac Surg 1999;16:74-80. [PubMed]
- Habets J, Budde RP, Symersky P, et al. Diagnostic evaluation of left-sided prosthetic heart valve dysfunction. Nat Rev Cardiol 2011;8:466-78. [PubMed]
- Lengyel M, Horstkotte D, Völler H, et al. Recommendations for the management of prosthetic valve thrombosis. J Heart Valve Dis 2005;14:567-75. [PubMed]
- Sakamoto Y, Hashimoto K, Okuyama H, et al. Prevalence of pannus formation after aortic valve replacement: clinical aspects and surgical management. J Artif Organs 2006;9:199-202. [PubMed]
- Vitale N, Renzulli A, Agozzino L, et al. Obstruction of mechanical mitral prostheses: analysis of pathologic findings. Ann Thorac Surg 1997;63:1101-6. [PubMed]
- Barbetseas J, Nagueh SF, Pitsavos C, et al. Differentiating thrombus from pannus formation in obstructed mechanical prosthetic valves: an evaluation of clinical, transthoracic and transesophageal echocardiographic parameters. J Am Coll Cardiol 1998;32:1410-7. [PubMed]
- Oh SJ, Park S, Kim JS, et al. Reoperation for non-structural valvular dysfunction caused by pannus ingrowth in aortic valve prosthesis. J Heart Valve Dis 2013;22:591-8. [PubMed]
- Nakatani S, Andoh M, Okita Y, et al. Prosthetic valve obstruction with normal disk motion: usefulness of transesophageal echocardiography to define cause. J Am Soc Echocardiogr 1999;12:537-9. [PubMed]
- Pattabiraman V, Nanda NC, Iqbal F, et al. Incremental value of three-dimensional over two-dimensional transesophageal echocardiography in the assessment of acute dysfunction of mechanical mitral valve prosthesis. Echocardiography 2010;27:885-7. [PubMed]
- Tong AT, Roudaut R, Ozkan M, et al. Transesophageal echocardiography improves risk assessment of thrombolysis of prosthetic valve thrombosis: results of the international PRO-TEE registry. J Am Coll Cardiol 2004;43:77-84. [PubMed]
- Tanis W, Habets J, van den Brink RB, et al. Differentiation of thrombus from pannus as the cause of acquired mechanical prosthetic heart valve obstruction by non-invasive imaging: a review of the literature. Eur Heart J Cardiovasc Imaging 2014;15:119-29. [PubMed]
- Aoyagi S, Fukunaga S, Arinaga K, et al. Prosthetic valve obstruction: diagnostic usefulness of cineradiography and multidetector-row computed tomography. Thorac Cardiovasc Surg 2007;55:517-9. [PubMed]
- Russhard P, Weerasinghe A. Intermittent jamming of a bileaflet mechanical heart valve in the absence of any extrinsic cause of obstruction. Eur J Echocardiogr 2011;12:E19. [PubMed]
- Roudaut R, Lafitte S, Roudaut MF, et al. Management of prosthetic heart valve obstruction: fibrinolysis versus surgery. Early results and long-term follow-up in a single-centre study of 263 cases. Arch Cardiovasc Dis 2009;102:269-77. [PubMed]
- Maribas P. Management of prosthetic heart valve obstruction: Speech for the surgery? Arch Cardiovasc Dis 2009;102:255-7. [PubMed]
- Teshima H, Fukunaga S, Takaseya T, et al. Obstruction of St. Jude medical valves in the aortic position: plasma transforming growth factor type beta 1 in patients with pannus overgrowth. Artif Organs 2010;34:210-5. [PubMed]
- Ohata T, Sakakibara T, Takano H, et al. Acute thrombotic obstruction of mitral valve prosthesis: low protein C level. Asian Cardiovasc Thorac Ann 2002;10:165-6. [PubMed]
- Teshima H, Hayashida N, Yano H, et al. Obstruction of St Jude Medical valves in the aortic position: histology and immunohistochemistry of pannus. J Thorac Cardiovasc Surg 2003;126:401-7. [PubMed]
- Darwazah AK. Recurrent pannus formation causing prosthetic aortic valve dysfunction: is excision without valve re-replacement applicable? J Cardiothorac Surg 2012;7:62. [PubMed]
- Khan NA, Butany J, Leong SW, et al. Mitral valve-sparing procedures and prosthetic heart valve failure: a case report. Can J Cardiol 2009;25:e86-8. [PubMed]
- Ogutu P, Szalay Z, Roth M, et al. Thrombectomy for prosthetic heart valve obstruction. J Card Surg 2007;22:218. [PubMed]
- Antunes MJ. Fate of thrombectomized Björk-Shiley valves. J Thorac Cardiovasc Surg 1986;92:965-6. [PubMed]
- Vitale N, Renzulli A, Cerasuolo F, et al. Prosthetic valve obstruction: thrombolysis versus operation. Ann Thorac Surg 1994;57:365-70. [PubMed]
- Vitale N, Renzulli A, de Luca Tupputi Schinosa L, et al. As originally published in 1994: Prosthetic valve obstruction: thrombolysis versus operation. Updated in 2000. Ann Thorac Surg 2000;70:2182-3. [PubMed]
- Renzulli A, Vitale N, Caruso A, et al. Thrombolysis for prosthetic valve thrombosis: indications and results. J Heart Valve Dis 1997;6:212-8. [PubMed]
- Cáceres-Lóriga FM, Pérez-López H, Morlans-Hernández K, et al. Thrombolysis as first choice therapy in prosthetic heart valve thrombosis. A study of 68 patients. J Thromb Thrombolysis 2006;21:185-90. [PubMed]
- Bonou M, Lampropoulos K, Barbetseas J. Prosthetic heart valve obstruction: thrombolysis or surgical treatment? Eur Heart J Acute Cardiovasc Care 2012;1:122-7. [PubMed]
- Nagy A, Dénes M, Lengyel M. Predictors of the outcome of thrombolytic therapy in prosthetic mitral valve thrombosis: a study of 62 events. J Heart Valve Dis 2009;18:268-75. [PubMed]
- Keuleers S, Herijgers P, Herregods MC, et al. Comparison of thrombolysis versus surgery as a first line therapy for prosthetic heart valve thrombosis. Am J Cardiol 2011;107:275-9. [PubMed]
- Renzulli A, Onorati F, De Feo M, et al. Mechanical valve thrombosis: a tailored approach for a multiplex disease. J Heart Valve Dis 2004;13 Suppl 1:S37-42. [PubMed]
- Moreno-Cabral RJ, McNamara JJ, Mamiya RT, et al. Acute thrombotic obstruction with Björk-Shiley valves: diagnostic and surgical considerations. J Thorac Cardiovasc Surg 1978;75:321-30. [PubMed]