A comparison of short-term outcomes between Ivor-Lewis and McKeown minimally invasive esophagectomy
Introduction
Surgery is still the main treatment for esophageal cancer, however, the complication and mortality rate of open esophagectomy is high. As a result, the thoracoscopic-laparoscopic minimally invasive esophagectomy (MIE) was developed. The previous study showed significant advantages of MIE over traditional open surgery (1,2). Now, the MIE comprised three surgical approaches: laparoscopic transhiatal, MIE McKeown approach (cervical anastomosis) and MIE Ivor-Lewis approach (intrathoracic anastomosis). Currently, the thoracoscopic and laparoscopic esophagectomy with cervical anastomosis is the mainstream technique. However, cervical anastomosis is still an invasive approach with high incidence of recurrent laryngeal nerve (RLN) injury and anastomotic leak (3).
The MIE with intrathoracic anastomosis (Ivor-Lewis) is increasingly used for the treatment of mid and lower esophageal cancers. Nguyen et al. preferred the operative approach is the laparoscopic/thoracoscopic Ivor Lewis resection (4). Other studies have also shown that the MIE Ivor-Lewis is safe and effective (5-8), particularly in reducing perioperative complications such as RLN injury, lung infection, and anastomosis fistula (3,9). However, Walther et al. (10) found that the additional esophageal resection of 5 cm in the neck group did not increase tumor removal and survival; on the other hand, it did not adversely influence morbidity, anastomotic diameter or eating. However, Bruno Walther’s conclusions are based on the thoracotomy and esophagogastric manual anastomoses in the neck. Whether these conclusions are suitable for new technologies?
This paper tried to compare the safety, feasibility, and short-term outcomes between MIE Ivor-Lewis approach and MIE McKeown approach for the treatment of middle and lower thoracic esophageal cancer.
Materials and methods
From January 2013 to October 2014, 72 patients have received the MIE Ivor-Lewis and MIE with cervical anastomosis at Weifang People’s Hospital, Weifang Medical College. Demographic characteristics, pathological data, operative procedures, and perioperative complications and 90-day mortality in patients were retrospectively analyzed.
This was a retrospective study. The ethics committee of our institution approved the study protocol. All the patients collected after the research begun had clear indications and clinical purpose for MIE and provided written informed consents.
Patients
The data of 72 consecutive patients with esophageal cancer who underwent MIE McKeown or MIE Ivor-Lewis was retrospectively analyzed. All patients were diagnosed as esophageal cancer by pathological criteria using upper endoscopy and biopsy specimen analysis. Simultaneously, every patient had a comprehensive pre-operative evaluation consisting of clinical presentation, physical examination, pulmonary function tests, electrocardiography, cardiac echocardiography, contrast-enhanced computed tomography (CT) scans of the chest and abdomen, and barium meal assessment. Based on clinical assessments, the inclusion criteria of this study were as follows:
- Patients with clinically staged T1-3N0M0 tumors;
- Patients with mid esophageal tumors and located below the carina or lower esophageal tumors;
- Patients without a previous history of cancer;
- Patients without a previous history of neck or chest surgery;
- Patients without neoadjuvant chemoradiotherapy;
- The application of mechanical circular stapler for anastomosis.
The exclusion criteria of this study were as follows:
- Patients with pre-existing COPD/asthma/interstitial lung disease;
- Patients with heart/liver/renal/diabetes dysfunction;
- Patients with esophagogastric manual anastomoses in the neck;
- Patients with hybrid MIE.
Procedure
MIE McKeown approach
The thoracoscopic portion of the MIE McKeown was first performed to evaluate the resection of the tumor. The thoracoscopic procedure for esophageal mobilization and mediastinal lymphadenectomy has been previously described (11,12).
After general anesthesia and endotracheal intubation, the patient was placed in the left lateral decubitus position. The surgeon and thoracoscopic technician standed on ventrally part of the patient. Three ports were made: a 1 cm optical port was placed in the 7th intercostal space at mid-axillary line; the utility port, a 2 cm incision expanded with a protection sleeve, was placed in the 5th intercostal space at the anterior axillary line; the other port was 1.5 cm incisions placed in scapular line at the 8th intercostal space. First, the right RLN lymph nodes were dissected and the mediastinal pleura were exposed at the level of the inferior pulmonary vein to commence esophageal mobilization. The azygos venous arcade was ligated using silk to expose the esophagus. After mobilizing the thoracic esophagus from the hiatus to the thoracic inlet, an aggressive mediastinal regional lymphadenectomy was carried out. If necessary, the thoracic duct was mobilized and ligated above the diaphragm.
After completing the thoracoscopic procedure, the patient was rotated to a dorsal decubitus, with the neck extended and turned toward the right. The surgeon stood at right side of the patient, with surgical assistance positioned to the right (camera) and the left side of the patient. Pneumoperitoneum was established with 12−15 mmHg with CO2, following which, five abdominal trocars were inserted, a forcep is placed through the 5 mm trocar below the xiphoid process to grasp the gastrohepatic ligament for liver retraction. The procedure of laparoscopic abdominal exploration was described in detail by Dr. Tan (13).
An approximate 5 cm oblique incision was made over the anterior border of the left sternocleidomastoid muscle. The mobilization of neck esophagus, left neck lymphadenectomy and construction of gastric conduit (diameter 3−5 cm) was described in detail by Dr. Zhu (11) and Dr. Tan (13). With the control and skill of the technique, a lot of surgical refinements were added to the knowledge on technology. Ultimately, the gastric conduit was pulled up to the left neck through the posterior mediastinum, assisted by the rubber tube to enable esophagogastric mechanical anastomosis. Nasogastric feeding was performed in MIE McKeown Group.
MIE Ivor-Lewis approach
The laparoscopic portion of the MIE Ivor-Lewis approach was first performed and the thoracoscopic procedure was similar to the MIE McKeown approach. The only difference was that the adipose tissue of lesser curvature need to be cleared in abdominal cavity.
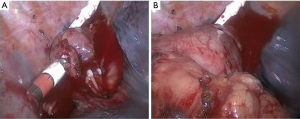
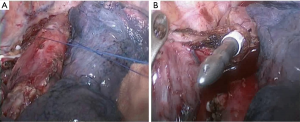
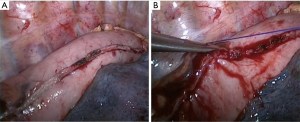
After completing the laparoscopic procedure, the patient was rotated to the left lateral decubitus position. The surgeon and thoracoscopic technician stood on ventrally part of the patient. Three ports were made: a 1 cm optical port was placed in the 7th intercostal space at mid-axillary line; the utility port, a 2 cm incision expanded with a protection sleeve, was placed in the 5th intercostal space at the anterior axillary line; the other incision was 3 cm incisions placed in scapular line at the 8th intercostal space. The mobilization of the thoracic esophagus and mediastinal regional lymphadenectomy was similar to the MIE McKeown. The hand-sewn purse string suture for esophagus was performed close to superior aperture of thorax (Figure 1). An incision was made 2−3 cm distal to the purse string. An anvil was placed into the esophagus and the purse string was tied and tightened. The esophagus was transected 5−10 mm distal to the purse sting. The gastric fundus was firstly resected using 1-2firings of Echelon 60 stapler (Ethicon Endo-Surgery, Cincinnati, Ohio, USA). A 3 cm incision was made in the lesser curvature and the body of the circular stapler (CDH stapler, Ethicon Endo-Surgery, USA) was connected to the anvil through the incision. An end-to-side gastroesophageal anastomosis was completed in the right thoracic cavity (Figure 2). The esophagus and lesser curvature were resected and the incision was blocked using 1-2firings of Echelon 60 stapler. The staple line of the gastric conduit was embedded with gastric muscular and serosa layers using prolene suture (Ethicon, Somerville, NJ, USA) (Figure 3) running suture. Nasogastric feeding was performed by interventional radiologic procedure if the post-operative potential or definite anastomotic leak was discovered.
Statistical analysis
Clinical data, including patient demographics, tumor characteristics, operative features and the complications of perioperative period of the two groups, were collected. Medical charts were reviewed to identify complications as per the Society of Thoracic Surgeons National Database. Clinical information was recorded in Microsoft EXCEL for further processing. Statistical analyses were performed using the SPSS software (version 17.0) and using the Student’s t-test, and χ2 test. A two-sided P value of less than 0.05 was considered to be statistically significant.
Results
This retrospective study included a total of 72 patients who received either MIE Ivor-Lewis or MIE McKeown. There were no significant differences in demographic and pathologic characteristics of patients (Table 1).

Full table
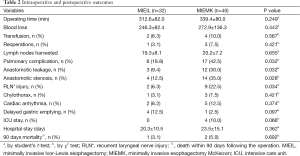
The intraoperative and postoperative outcomes are shown in Table 2. The mean operative time is 312.6±82.0 vs. 339.4±80.0, respectively (P>0.05). The mean number of lymph nodes harvested is 19.3rves vs. 20.2rves, respectively (P>0.05). One (3.1%) patient of MIE Ivor-Lewis group and three (7.5%) patients of MIE McKeown group required reoperations because of chylothorax. Four (10%) patients of MIE McKeown group required sending to ICU as a consequence of complications. There was no statistical significance with the mean hospital stay between two groups.
Full table
There was a significantly lower rate of pulmonary complications, anastomotic leak, anastomotic stenosis and RLN injury between the two groups. There was no statistical significance with the disparity of chylothorax, cardiac arrhythmia, delayed gastric emptying between the two groups. Unfortunately, two patients in MIE Mckeown group died of acute respiratory distress syndrome (ARDS) within 90 days. One patient in MIE Ivor-Lewis group died of severe pulmonary infection within 90 days. There was no statistical significance with the 90-day mortality between the two groups.
Discussion
In the current study, the blood loss, morbidity, surgical outcomes and the 90-day mortality were not significantly different between the MIE Ivor-Lewis and the MIE McKeown groups, and this showed that the MIE Ivor-Lewis approach was safe and feasible. The number and location of lymphadenectomy, the location of tumor and the residual tumor of cutting edge were not significantly different between the two groups, and so the oncologic outcomes were equivalent. These findings are consistent with other reported studies (3,9) except for operative time. In the current study, the operative time was not significantly different between two groups because the intrathoracic anastomosis and the embedding of the staple line in the MIE Ivor-Lewis group were more complex and wasted more time.
In the present study, the MIE Ivor-Lewis approach resulted in lesser respiratory complications and lesser RLN injury. Cervical lymphadenectomy can result in more RLN injury, while, the MIE Ivor-Lewis can reduce the RLN injury. The injury of the RLN lesion can result in aspiration, disability of expectoration, and these will increase the incidence of respiratory complication. Safranek et al. (14) observed that the RLN injury will lead to a series of complications and poor prognosis. Luketich et al. (3) reported that the MIE Ivor-Lewis was associated with reduced RLN injury, and then, they preferred MIE-chest approach.
The lower anastomotic leakage and stenosis in MIE Ivor-Lewis group was found compared with MIE McKeown group, the potential reason including: (I) due to the omission of cervical incision, the remnant esophagus has a good vascular supply; (II) a shorter gastric conduit will permit a more extended gastric resection and will, because of a good vascular supply, lead to less anastomotic leakages; (III) avoiding the compression from inferior aperture of thorax, gastric conduit has a good vascular supply.
However, there are some disadvantages for this kind of MIE Ivor-Lewis approach as such pleural pollution, not standard gastric conduit. During esophagogastric anastomosis, gastric contents would inevitably pollute thoracic cavity, and so the douching of thoracic cavity with massive disinfection fluid and physiologic saline was necessary. In the present study, the incidence of delayed gastric emptying and sour regurgitation in MIE Ivor-Lewis group was higher in compared with MIE McKeown, although there was no significant difference. The non-standard gastric conduit may be the potential reason. Lee et al. (15) described that a narrow gastric tube improved gastric emptying in a flow-visualization model. Shen et al. (16) reported that narrow gastric tubes were longer and less interfered in perfusion, which contributed to lower incidence of anastomotic leakage following minimally invasive esophagectomy.
The open Ivor-Lewis esophagectomy has been the classical operation for patients with mid and lower esophageal cancer. Owing to the technically demanding nature of this procedure, access to MIE Ivor-Lewis has been limited to select specialized centers (17,18). The MIE McKeown procedure is more convenient and easy to grasp for the beginners. However, perioperative complications such as RLN injury, lung infection, and anastomotic fistula with the MIE McKeown is more and more reconstructed. With the development of operative technic, the MIE Ivor-Lewis has become increasingly prevalent and showed preliminarily good outcomes (19-23).
However, Ivor-Lewis MIE is technically demanding. It requires extensive experience in both open and minimally invasive surgery. We gained some valuable lessons from the operations: (I) the adipose tissue of lesser curvature should be cleared in abdominal cavity to increase the length and decrease the width of gastric conduit; (II) if the gastric fundus was firstly resected using 1-2firings of Echelon 60 stapler before esophagogastric anastomosis, the gastric conduit will be easy to shape; (III) it is necessary to obtain a tight seal of the esophageal tissue around the anvil to avoid potential anastomotic leakage that an extra silk was tied and tightened around the anvil after the purse-string suture; (IV) the anastomotic site should be close to the vascular arcus, meanwhile, the blood vessels should be avoid to embedding the anastomotic stoma to prevent bleeding of anastomotic stoma.
In conclusion, the incidence of respiratory complications, anastomotic leakage and RLN lesion were found to lower in the MIE Ivor-Lewis group, meanwhile, the operative time, blood loss, perioperative mortality, the 90-day mortality and oncological outcomes were not significantly different. This suggests that the kind of MIE Ivor-Lewis approach is safe and feasible and seems to superior outcomes. The limitations of our study were its retrospective design and lack of exploration of the long-term effects, especially on quality of life. Therefore, a randomized controlled trial has been designed for expansion on our initial findings of following MIE Ivor-Lewis.
Acknowledgements
The authors would like to express their thanks to Dr. Lijie Tan for his comment on this work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [PubMed]
- Meng F, Li Y, Ma H, et al. Comparison of outcomes of open and minimally invasive esophagectomy in 183 patients with cancer. J Thorac Dis 2014;6:1218-24. [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [PubMed]
- Nguyen NT, Hinojosa MW, Smith BR, et al. Minimally invasive esophagectomy: lessons learned from 104 operations. Ann Surg 2008;248:1081-91. [PubMed]
- Ai B, Zhang Z, Liao Y. Laparoscopic and thoracoscopic esophagectomy with intrathoracic anastomosis for middle or lower esophageal carcinoma. J Thorac Dis 2014;6:1354-7. [PubMed]
- Li H, Hu B, You B, et al. Combined laparoscopic and thoracoscopic Ivor Lewis esophagectomy for esophageal cancer: initial experience from China. Chin Med J (Engl) 2012;125:1376-80. [PubMed]
- Zhang R, Kang N, Xia W, et al. Thoracoscopic purse string technique for minimally invasive Ivor Lewis esophagectomy. J Thorac Dis 2014;6:148-51. [PubMed]
- Shen G, Pan SB, Wu M, et al. Use of efficient purse-string stapling technique for esophagogastric anastomosis in minimally invasive Ivor Lewis esophagectomy. J Thorac Dis 2013;5:898-901. [PubMed]
- Lin J, Kang M, Lin J, et al. Short-term efficacy comparison between Ivor-Lewis approach and McKeown approach in minimally invasive esophagectomy. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:888-91. [PubMed]
- Walther B, Johansson J, Johnsson F, et al. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg 2003;238:803-12; discussion 812-4. [PubMed]
- Chen B, Zhang B, Zhu C, et al. Modified McKeown minimally invasive esophagectomy for esophageal cancer: a 5-year retrospective study of 142 patients in a single institution. PLoS One 2013;8:e82428. [PubMed]
- Shen Y, Zhang Y, Tan L, et al. Extensive mediastinal lymphadenectomy during minimally invasive esophagectomy: optimal results from a single center. J Gastrointest Surg 2012;16:715-21. [PubMed]
- Feng M, Shen Y, Wang H, et al. Thoracolaparoscopic esophagectomy: is the prone position a safe alternative to the decubitus position? J Am Coll Surg 2012;214:838-44. [PubMed]
- Safranek PM, Cubitt J, Booth MI, et al. Review of open and minimal access approaches to oesophagectomy for cancer. Br J Surg 2010;97:1845-53. [PubMed]
- Lee JI, Choi S, Sung J. A flow visualization model of gastric emptying in the intrathoracic stomach after esophagectomy. Ann Thorac Surg 2011;91:1039-45. [PubMed]
- Shen Y, Wang H, Feng M, et al. The effect of narrowed gastric conduits on anastomotic leakage following minimally invasive oesophagectomy. Interact Cardiovasc Thorac Surg 2014;19:263-8. [PubMed]
- Pennathur A, Awais O, Luketich JD. Technique of minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg 2010;89:S2159-62. [PubMed]
- Tapias LF, Morse CR. Minimally invasive Ivor Lewis esophagectomy: description of a learning curve. J Am Coll Surg 2014;218:1130-40. [PubMed]
- Xie MR, Liu CQ, Guo MF, et al. Short-term outcomes of minimally invasive Ivor-Lewis esophagectomy for esophageal cancer. Ann Thorac Surg 2014;97:1721-7. [PubMed]
- Runkel N, Walz M, Ketelhut M. Abdominothoracic esophageal resection according to Ivor Lewis with intrathoracic anastomosis: standardized totally minimally invasive technique. Chirurg 2015;86:468-75. [PubMed]
- Price TN, Nichols FC, Harmsen WS, et al. A comprehensive review of anastomotic technique in 432 esophagectomies. Ann Thorac Surg 2013;95:1154-60; discussion 1160-1. [PubMed]
- Okabe H, Tanaka E, Tsunoda S, et al. Intrathoracic esophagogastric anastomosis using a linear stapler following minimally invasive esophagectomy in the prone position. J Gastrointest Surg 2013;17:397-402. [PubMed]
- Kim K, Park JS, Seo H. Early outcomes of video-assisted thoracic surgery (VATS) Ivor Lewis operation for esophageal squamous cell carcinoma: the extracorporeal anastomosis technique. Surg Laparosc Endosc Percutan Tech 2013;23:303-8. [PubMed]