Quality of transbronchial biopsy with large forceps compared to cryobiopsy: a randomized controlled, single blinded live animal study
Introduction
The bronchoscopic transbronchial biopsy is the most frequently utilized modalities in obtaining parenchymal lung tissue (1). A standard 2.0 mm forceps is the most utilized tool despite its low diagnostic yield. The need for higher yields in sampling led to the emergence of the transbronchial cryobiopsy. The higher yield comes at the expense of frequent significant complications, with airway bleeding the commonest. Cryobiopsy is often performed with rigid bronchoscopy in order to accommodate empiric deployment of an occlusion balloon in the segment biopsied in order to tamponade significant hemorrhage (2,3). For patients deemed not safe candidates for rigid bronchoscopy (e.g., unfavorable anatomy for neck extension), cryobiopsy can be obtained safely when an occlusion balloon can be simultaneously loaded via double-lumen endotracheal tube (ETT). Our experience related to major bleeding and procedure tolerability has been favorable when utilizing a non-standard 2.8 mm large forceps (LF) as an alternative to cryobiopsy as it does not require rigid bronchoscopy but expectedly sustains a higher diagnostic yield compared to standard forceps biopsy (4). We therefore conducted this prospective single-blinded animal study comparing LF biopsy to cryobiopsy to examine the quality of biopsy specimens and procedural complication rates.
We present the following article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-936/rc).
Methods
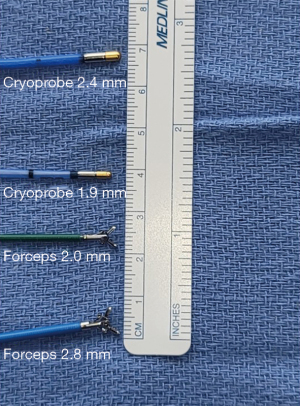
This is a prospective, randomized, controlled singled-blinded study with three arms. Experiments were performed under permission granted by Rowan University IACUC2 017-022 (17-007B), in compliance with Cooper IACUC institutional guidelines for the care and use of animals. The aim of the study is to compare lung biopsy techniques using either a 2.4 mm cryoprobe, a 1.9 mm cryoprobe, or a LF transbronchial lung biopsy (LF-TBLB) (Boston Scientific Radial Jaw M00515200 7.3 mm cup opening size and 2.8 mm required working scope channel). A study protocol, including the research question, key design features analysis plan, was prepared prior to initiation of the study without registration
The primary endpoint is the histopathologic quality of the specimen graded by two blinded pathologists to the technique used to obtain the specimens. The grading system was based on sample size, surface of alveolated area, presence of bronchial tissue, extent of crush artifact, and the presence of hemorrhage. Secondary endpoints included complications such as bleeding or pneumothorax, number of attempts to acquire three samples per lobe, duration of the sampling procedure defined by time for tool in to tool out of the bronchoscope channel, and duration of fluoroscopy activation.
Bleeding after biopsy was graded according to Table 1. Pneumothorax management included intermittent radiographic monitoring using fluoroscopy and chest tube insertion if worsening pneumothorax or if any onset of clinical instability (hypoxemia, hypotension).
Table 1
| Bleeding score | Interventions |
|---|---|
| 0 | No bleeding or only traces of blood not requiring suction |
| 1 | Bleeding requiring suction to clear |
| 2 | Bleeding requiring wedging of the biopsied segment and/or ice cold saline |
| 3 | Bleeding requiring inflation of a bronchial blocker |
| 4 | Bleeding causing cardiopulmonary instability |
Original source from Arya R, Boujaoude Z, Rafferty WJ, Abouzgheib W. Usefulness and safety of transbronchial biopsy with large forceps during flexible bronchoscopy. Proc (Bayl Univ Med Cent) 2020;34:232-6. copyright© 2020 Baylor University Medical Center, reprinted by permission of Taylor & Francis Ltd., http://www.tandfonline.com on behalf of Baylor University Medical Center.
Our hypothesis is that all 3 biopsy tools used during transbronchial biopsy would yield comparable histopathologic quality specimens.
Animals
Three animals (pigs) were used for this study. Pigs were selected given their similar anatomic size and distribution to human lung. Mean weight was 68±2 kg. The animals were housed at Cooper’s Vivarium housing for 5 days prior to study date for adaptation purposes. Because three separate tools were utilized, we opted to use three separate animals, one for each tool. They were provided a standard diet and allowed free access to water. All animals were presumed healthy and no exclusion criteria was set. Each animal was premedicated with a combination of Telazol (0.5 mg/kg), Dexmedetomidine (0.3 mg/kg), and Butorphanol (0.04 mg/kg). The animal was then moved onto the operating table and positioned in sternal recumbency. A mask and breathing circuit were then attached to the animal’s nose. Oxygen was turned on and isoflurane gas anesthesia was initiated. A 20–22-gauge intravenous catheter was placed into animal’s ear and intravenous (IV) fluids initiated. Once anesthesia is achieved, Isoflurane was turned off and the mask was removed. Oral intubation was performed using a laryngoscope and an ETT which was connected to a breathing circuit and oxygen flow turned on to 5 L/min. Manual bagging was performed to obtain an end tidal CO2 between 35–45 mmHg. Then ETT was connected to a ventilator at a respiratory rate of 8–10/min and tidal volume of 10 mL/Kg. The animal was then placed in dorsal recumbency. SpO2, ETCO2, HR, and BP were being monitored. If the animal showed any evidence of pain, a 10 mg of IV bolus butorphanol was given or Isoflurane was up titrated. After completion of the procedure, euthanasia IV solution was administered, and death was confirmed with cessation of heartbeat.
Procedural equipment
A therapeutic flexible bronchoscope (Olympus) was used through the ETT and advanced through the tracheobronchial tree in a standard fashion until the target lobe was reached. A standard C-arm fluoroscope was used for fluoroscopic guidance. A 1.9 mm diameter cryoprobe (Erbe) and another 2.4 mm diameter cryoprobe (Erbe) was used for transbronchial cryobiopsy in animal A and animal B respectively. Both probes were connected to a cryoprobe (Erbe) and activation times for both probes were 3 seconds. A standard 2.8 mm forceps (Boston Scientific Radial Jaw M00515200 7.3 mm cup opening size and required working scope channel) was used to obtain transbronchial forceps biopsies in animal C. A Cohen endobronchial bronchial blocker (Cook Medical G44122) was placed in the targeted lobar airway prior to biopsy maneuvers and inflated as needed depending on the degree of bleeding after reintroducing the bronchoscope and reassessing the bleeding severity. Thrombin (Recothrom, Baxter, IL, USA) 5,000 IU was premixed and drawn in a 60-cc syringe and flushed through the bronchoscope channel when needed for bleeding. Iced saline was also drawn in a separate 60-cc syringe and used as needed through the bronchoscope channel. A standard pneumothorax kit with an 8 French chest tube was available to use in the event of pneumothorax.
Biopsy
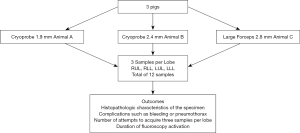
Each animal was randomized to one biopsy tool (Figure 1). Animal A was assigned Cryoprobe 1.9 mm (Cryo 1.9), Animal B assigned Cryoprobe 2.4 mm (Cryo 2.4) and animal C 2.8 mm LF (LF 2.8). All biopsies were done under fluoroscopic guidance. The bronchoscope was inserted through the ETT and airway secretions were cleared. The bronchial blocker was placed into the desired lobe and the occlusion balloon was tested. The sequence and location of the biopsies followed as: right upper lobe, right lower lobe, left upper lobe, and left lower lobe. Three samples per lobe were to be obtained, with a total of 12 specimens per animal. If a biopsy attempt did not yield a sample, the attempt was repeated. The number of attempts to acquire a sample was recorded. Once all samples were obtained, the animal was then euthanized. Any complication occurring before all biopsies were obtained from each animal was treated. The biopsy time, defined as tool in and out channel, was recorded. Duration of fluoroscopy needed for each biopsy was recorded. All biopsies were done by two interventional pulmonologists in random order.
The cryobiopsies, with either probe, were obtained in the standard fashion. The cryoprobe was inserted through the bronchoscope channel into the desired lobe under fluoroscopic guidance until the pleural line was identified. The probe was then retracted approximately 2 cm. The cryoprobe foot pedal was activated for 3 seconds and the specimen, cryoprobe, and bronchoscope was removed en bloc. The specimen was detached from probe which was then pulled out of the bronchoscope channel. The bronchoscope was then advanced back through the ETT to the targeted lobe evaluate any bleeding.
The forceps was used in standard fashion. It was inserted through the bronchoscope channel and advanced in the targeted lobe under fluoroscopic guidance until it reached the pleural line. It was pulled back 2 cm, opened and advanced 1 cm then the biopsy was obtained and pulled out through the channel. The bronchoscope remained in place to control any potential bleeding.
There was a predetermined sequence for bleeding control with each subsequent step utilized if bleeding persisted. In order of escalating severity, the following interventions were performed: Instillation of iced saline, injection of lidocaine mixed with epinephrine, then injection of thrombin, and deployment of a bronchial blocker.
Specimen
Specimens were acquired from each animal. All biopsy samples were placed and fixed in formalin which were processed in paraffin blocks and stained with hematoxylin and eosin in an outside facility in order to eliminate bias and maintain blinding of both pathologists. Each sample was labeled with A, B, or C letter depending on which animal, targeted lobe, followed by number of the sample. The slides were then provided to two separate pathologists who were blinded to the corresponding biopsy tool. If the sample contained multiple fragments, the number of fragments was recorded and then combined in a cell block.
The histologic quality of each sample was assessed under low power field. This assessment used a score which accounted for, by order of importance, the percentage of alveolated tissue, percentage of bronchial tissue, specimen size, presence of crush artifact, and presence of hemorrhage.
For example, large samples with a high alveolated tissue/bronchial tissue ratio, minimal crush artifact, and minimal hemorrhage were scored highly. Small samples with a low alveolated/bronchial tissue ratio, with significant crush artifact, and evidence of hemorrhage were given lower scores (Table 2).
Table 2
| Overall quality | Description | Lung alveolar tissue area |
|---|---|---|
| 4 | Good | ≥4 mm2 |
| 3 | Acceptable | ≥2 mm2 |
| 2 | Scant lung alveolar tissue | <2 mm2 |
| 1 | No lung alveolar tissue | 0 |
Statistical analysis
One-Way ANOVA test with Tukey post hoc tests were used for normally distributed continuous variables. Kruskall Wallis test was used for non-parametric continuous variables. All other secondary analysis was completed with the use of a Pearson’s chi-squared test. The significance level was P≤0.05. SPSS 22 was used for analysis.
Results
Three animals with a mean weight of 68±2 kg were used. We planned to obtain 3 samples from each lobe with total of 12 samples per animal (4 lobes per animal). A biopsy attempt was deemed successful when it yielded an appreciable sample. Each sample may contain one or more fragments of tissue. If a biopsy did not yield a sample, repeat attempts were performed until a sample was obtained. In animal A, a 1.9 mm cryoprobe was utilized. Twelve attempts were required to obtain samples from animal A resulting in 21 biopsy fragments. Average size of all fragments was 2.7 mm. Twelve attempts were required in animal B which used a 2.4 mm cryobiopsy probe with 24 biopsy fragments obtained. The average size of all fragments was 3.98 mm. Eighteen attempts were required in animal C using a 2.8 mm biopsy forceps with 31 biopsy fragments obtained. Average size of all fragments was 1.44 mm (Tables 3,4).
Table 3
| Animal | Number of biopsy pieces | Average size mm | Std deviation | P value |
|---|---|---|---|---|
| A | 21 | 2.7 | 1.511 | <0.001 |
| B | 24 | 3.975 | 2.155 | |
| C | 31 | 1.44 | 0.859 |
Table 4
| Pairwise comparison | P value |
|---|---|
| Animal A vs. Animal B | 0.033 |
| Animal A vs. Animal C | 0.007 |
| Animal B vs. Animal C | <0.001 |
When analyzing the biopsy techniques, we recorded biopsy time, the duration of fluoroscopy, and the excision rate of obtaining samples during biopsy attempts. The mean biopsy time for animal A was 60±38.8, animal B was 63±35.8 and animal C was 51±28.5 (P=0.598) seconds. The median fluoroscopy time was 15 (IQR: 9.25–20) seconds for Animal A, 15 (IQR: 11.25–24.25) seconds for Animal B and 25 (IQR: 16–36.5) seconds for Animal C (P=0.071). The only significant relationship was between the rate of sample excision (number of biopsy attempts to obtain 12 samples per animal) by animal. Animal #C had a 66.7% sample excision rate while animal #A and #B had 100% sample excision rate (P=0.009).
When analyzing the total average size of samples between animal A, B and C, there was a statistically significant difference between all animals (P<0.001). There was a significant difference when comparing animal A vs. animal B (P=0.033), when comparing animal A vs. animal C (P=0.007) and when comparing animal B vs. animal C (P<0.001). Samples obtained in animal B were bigger than animal A and both samples from animal A and B were bigger than those obtained from animal C (Tables 3,4).
The alveolar tissue surface area was recorded when samples were reviewed and scored by pathologists. Medians and interquartile ranges were used for data distribution (Table 5). There was no statistical difference when comparing the alveolar tissue surface area of specimens obtained from all 3 animals using all 3 different biopsy tools (P=0.054).
Table 5
| Statistic | Value |
|---|---|
| Animal A | |
| N | 12 |
| 25th percentile | 0.8125 |
| Median | 1.75 |
| 75th percentile | 2.3 |
| Animal B | |
| N | 12 |
| 25th percentile | 0.25 |
| Median | 2.125 |
| 75th percentile | 4.375 |
| Animal C | |
| N | 12 |
| 25th percentile | 0 |
| Median | 0.125 |
| 75th percentile | 1.8 |
| P value | 0.054 |
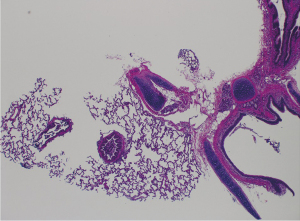
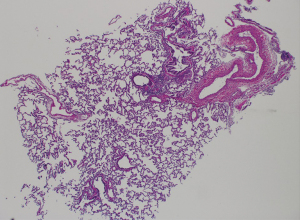
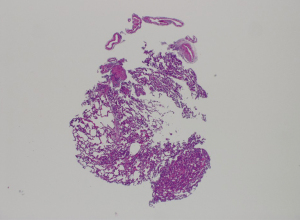
The primary outcome of the study was the quality of the samples obtained from each animal, scored by two blinded pathologists. There was no significant difference in the quality of samples between the three animals (Tables 6,7). Animal A had a mean quality of 2.33±1.15, animal B had a mean quality of 2.67±1.23 and animal Chad a mean quality score of 1.75±0.87 (P=0.132). In addition, pairwise comparisons were performed between each two animals and showed no significant difference (Figures 2-4 shows examples of pathology slides from all three animals).
Table 6
| Quality | N | Mean | Std Dev | P value |
|---|---|---|---|---|
| Animal A | 12 | 2.33 | 1.15 | 0.132 |
| Animal B | 12 | 2.67 | 1.23 | |
| Animal B | 12 | 1.75 | 0.87 |
Table 7
| Pairwise comparisons—lung alveolar tissue area | P value |
|---|---|
| Animal A vs. Animal B | 0.738 |
| Animal A vs. Animal C | 0.403 |
| Animal B vs. Animal C | 0.116 |
Bleeding scores were also recorded and analyzed (Table 8). We also recorded interventions used when bleeding occurred depending on severity. Combination of incremental interventions were available to manage worsening severities of bleeding (Table 1). There was no significant difference in the incidence of bleeding (P=1.000) or interventions (P=0.854) when comparing animal A and B. There was a significant difference in incidences of bleeding and interventions when comparing animal A and B to animal C. Animal A and B had 100% bleeding incidences while animal C had no incidence of bleeding (P<0.001). Out of 32 possible interventions, animal A and B had 18 (56.3%) while animal C had no interventions (P<0.001).
Table 8
| Animal A & B | Animal C | P value | Animal A | Animal B | P value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | Percent | N | n | Percent | N | n | Percent | N | n | Percent | |||||
| Bleeding | 8 | 8 | 100% | 5 | 0 | 0% | <0.001 | 3 | 3 | 100% | 5 | 5 | 0% | <0.001 | ||
| Interventions | 32 | 18 | 56.30% | 20 | 0 | 0%% | <0.001 | 12 | 7 | 58.30% | 20 | 0 | 0%% | <0.001 | ||
N, available interventions; n, used interventions.
One pneumothorax occurred in animal C. Considering it was the sole occurrence, no statistical analysis was made.
Conclusions
TBLB using flexible forceps is the most common utilized method to obtain a parenchymal lung biopsy (1,2). No guidelines have standardized the forceps size for tissue acquisition by TBLB. The most common forceps used is a standard oval 5 mm cup opening that requires a minimum 2.0 mm working channel (Figure 5). These forceps generally yield size samples that are considered small and are associated with a low diagnostic yield. The role of larger forceps for TBLB has not been well defined. Some studies have aimed at addressing forceps size have favored a larger forceps due to larger specimen sizes with the potential benefit of increased diagnostic yield (4,5).
The need for larger tissue samples and better diagnostic yield has led to the emergence of transbronchial lung cryobiopsy (cTBLB). This has gained popularity as an alternative to forceps transbronchial biopsy and surgical lung biopsy, particularly for the diagnosis of idiopathic interstitial pneumonias. cTBLB provides a larger specimen size and retains architectural integrity at the cost of an elevated risk of major bleeding and pneumothorax. Additionally, cTBLB requires experienced operator technique and in most circumstances done via rigid bronchoscopy or with flexible bronchoscopy with bronchial balloon blocker (2,3). Mortality from cTBLB can be up to 0.3% while the rate of pneumothorax can be as high as 30% (2). The more concerning complication is major bleeding after specimen retrieval. Since the specimen is retracted en bloc with the bronchoscope, there is an interval of unobserved time where controlling bleeding is impaired. Bleeding rate can be as high as 14.7% (2). Bleeding during cTBLB is commonly moderate and preemptive inflation of a bronchial blocker after each biopsy is recommended (2,3). It is often our experience that successful inflation of the blocker is complicated by dislodgement, either from friction on retraction of the bronchoscope or from overinflation and subsequent herniation of the blocker.
Our experience with using LF was reported in a retrospective study demonstrating the safety and feasibility of large-forceps TBLB for the diagnosis of diffuse parenchymal lung disease (4). We have favored the use of LF for TBLB for two main reasons. First, the LF acquires larger specimens compared to a standard forceps and thus portends a higher diagnostic yield. Second, the use of LF (when compared to cTBLB) allows for specimen retrieval through the bronchoscope channel, obviating the need to completely remove the bronchoscope with cTBLB. This advantage is instrumental for response time when faced with major bleeding and answers the therapeutic delay experienced with cTBLB.
In contrast, the en bloc retrieval of the specimen with the cryoprobe under rigid bronchoscopy requires the removal of the flexible bronchoscope, which frequently leads to dislodgment of the occlusion balloon, decanting of blood into proximal airways, and poor visualization when re-introducing the bronchoscope after the biopsied specimen is removed ex vivo. This potential safety issue was addressed by Yarmus et al. (6) who described the use of a smaller cryoprobe with an over-sheath that can be rracted through the working channel of the bronchoscope. This removes the need to completely retract the bronchoscope out the rigid barrel. Through this technique, the risk of late recognition of major bleeding and bronchial blocker dislodgement would be mitigated. The authors compared the sheath cryoprobe (1.1 mm diameter) to both the standard cryoprobe (1.9 mm diameter) and forceps (2.0 mm diameter) each in three different animal models. They concluded the sheath cryoprobe and standard cryoprobe biopsies provided larger biopsy specimens when compared to a standard 2.0 mm forceps biopsy. All approaches had similar safety profiles. No significant bleeding occurred, and the pneumothorax rate was 2.5–7.5% for all three tools (6).
Our study was conducted in a similar fashion but focused on comparing transbronchial cryobiopsy as currently practiced with either 2.4 or 1.9 mm cryoprobe without a sheath to transbronchial forceps biopsy with LF (2.8 mm).
The time required to perform the biopsy and fluoroscopy time with all three tools was not statistically significant which mirrored data in previous studies (6). We argue the study design does not mirror standard clinical practice. We speculate cryobiopsy in humans would require longer total procedural and subsequently anesthesia time to obtain samples in humans if compared to transbronchial forceps biopsy. In this study, all tools were utilized in a similar fashion with regards to using the bronchial blocker after each biopsy. The bronchial blocker balloon was not inflated until after retrieval of the specimen and reintroduction of the bronchoscope in order to assess and grade any bleeding. This approach is different than standard clinical practice of cryobiopsy where the balloon is preemptively inflated immediately after biopsy. This preemptive balloon inflation prolongs procedure duration more than transbronchial forceps biopsy, where bronchial blocker is not traditionally utilized.
When analyzing sample sizes in our study, mean sample sizes were larger with Cryo 2.4 when compared to Cryo 1.9 and LF 2.8. Mean sample sizes were larger with Cryo 1.9 compared to LF 2.8. Although it is intuitive to expect a larger specimen from a large cryoprobe given its larger contact area, there was no statistical difference in mean alveolar surface area between the three tools. This emphasizes a distinguishing difference between the quality of a specimen and its size. A larger specimen obtained with a cryoprobe is explained likely from excess bronchial tissue, rather than lung parenchyma.
Our primary outcome, sample quality, was evaluated by two blinded pathologists and yielded no statistically significant difference. Note that this study was not powered at attaining pathologic diagnosis, but rather to characterize specimen quality. The animal models were assumed healthy. We believe the larger sample sizes (Cryo 2.4 > Cryo 1.9 > LF 2.8) yet similar alveolar surface area and sample quality are explained by larger amount of bronchial tissue collected with larger samples. This finding shows the LF to be equivalent to 2.4 and 1.9 mm cryoprobes in obtaining similar specimen quality.
One pneumothorax occurred with LF 2.8. No statistical tests could be run considering the occurrence of one event. The pneumothorax required tube thoracostomy insertion due to hemodynamic instability.
When analyzing bleeding complications after biopsy, we accounted for bleeding score/severity and number of interventions needed to control any given bleeding event. The bleeding rate was significantly higher in both Cryo 2.4 and 1.9 when compared to LF 2.8, but not statistically different when comparing Cryo 2.4 vs. Cryo 1.9.
No bleeding requiring interventions occurred with LF 2.8. This finding mirrors our experience in clinical practice (4). We always use LF 2.8 under fluoroscopic guidance. The area to biopsy is chosen after advancing the closed forceps to pleural line, pulling back about 2–3 cm, opening the forceps jaw, and then advancing gently and collecting the specimen. This technique allows to avoid proximal biopsies which could be associated with inadvertent biopsies of bronchial circulation. A similar technique is described for transbronchial cryobiopsy (7), aimed at minimizing bleeding.
Bleeding events were not minor with either cryoprobe. Such events required numerous combined interventions to control bleeding including iced saline, thrombin instillation, and bronchial blocker inflation. As outlined earlier, we believe cryobiopsy in general collects more bronchial tissue with each specimen and this effect is manifested with more bleeding due to the disruption of bronchial circulation.
Our study has two major limitations. The first is the randomization of one animal each to one tool only. This may portend toward bias however we chose this sequence in order to reduce total anesthesia time. Also, this may also be considered a confounder. Secondly, is the single blinding. Operators cannot be reasonably blinded as the technique for forceps biopsy is different than cryobiopsy. Moreover, the tools are visualized in the bronchoscope channel and on fluoroscopy. We randomly rotated the sequence of operators so not one operator is obtaining all the biopsies from one animal. Overall, this also highlights the need for experienced operators. Often these procedures are performed by pulmonologists with additional training either through formal interventional pulmonology fellowship or from informal mentorship.
In summary, this pilot animal study proves the utility of transbronchial LF biopsy with comparable specimen quality when compared to transbronchial cryobiopsy. The safety profile with respect to major bleeding favors LF biopsy. We therefore highlight the diagnostic utility, safety, and specimen comparability to cTBCB.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-936/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-936/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-936/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-936/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under permission granted by Rowan University IACUC2 017-022 (17-007B), in compliance with Cooper IACUC institutional guidelines for the care and use of animals. A study protocol, including the research question, key design features analysis plan, was prepared prior to initiation of the study without registration
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Andersen HA, Harrison EG Jr. Transbronchoscopic lung biopsy in diffuse pulmonary disease. Ann Otol Rhinol Laryngol 1965;74:1113-9. [Crossref] [PubMed]
- Hetzel J, Maldonado F, Ravaglia C, et al. Transbronchial Cryobiopsies for the Diagnosis of Diffuse Parenchymal Lung Diseases: Expert Statement from the Cryobiopsy Working Group on Safety and Utility and a Call for Standardization of the Procedure. Respiration 2018;95:188-200. [Crossref] [PubMed]
- Inomata M, Kuse N, Awano N, et al. Prospective multicentre study on the safety and utility of transbronchial lung cryobiopsy with endobronchial balloon. ERJ Open Res 2020;6:e00008-2020. [Crossref] [PubMed]
- Arya R, Boujaoude Z, Rafferty WJ, et al. Usefulness and safety of transbronchial biopsy with large forceps during flexible bronchoscopy. Proc (Bayl Univ Med Cent) 2020;34:232-6. [Crossref] [PubMed]
- Loube DI, Johnson JE, Wiener D, et al. The effect of forceps size on the adequacy of specimens obtained by transbronchial biopsy. Am Rev Respir Dis 1993;148:1411-3. [Crossref] [PubMed]
- Yarmus LB, Semaan RW, Arias SA, et al. A Randomized Controlled Trial of a Novel Sheath Cryoprobe for Bronchoscopic Lung Biopsy in a Porcine Model. Chest 2016;150:329-36. [Crossref] [PubMed]
- Colella S, Haentschel M, Shah P, et al. Transbronchial Lung Cryobiopsy in Interstitial Lung Diseases: Best Practice. Respiration 2018;95:383-91. [Crossref] [PubMed]