A prospective trial of CT-guided percutaneous microwave ablation for lung tumors
Introduction
Today, surgical resection remains the gold standard treatment for localized non-small cell lung cancer (NSCLC) (1). However, surgery is invasive and not without complications (2). Not all patients are suitable candidates for surgery, especially those with limited pulmonary function or who have undergone extensive prior surgery. Patients classified as “inoperable” by a surgeon are typically treated with other targeted therapies such as radiofrequency ablation (RFA), cryoablation (Cryo) and stereotactic body radiation therapy (SBRT), or systemic therapy (3-5). Percutaneous microwave ablation (pMWA) is an alternative option with newer data for targeted treatment of cancer within the lung compared to the abundance of data that exists with other treatment modalities.
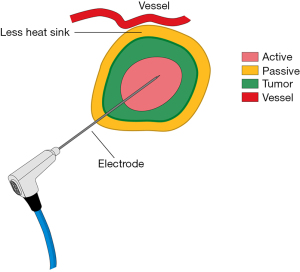
Thermal ablation is an established method for inducing cellular damage. MWA delivers electromagnetic microwaves through biologic tissue and directly to the tumor to produce heat and cause ablation of the target tissue (6) (Figure 1). Heating results in thermal coagulation and localized tissue necrosis. MWA can be performed percutaneously, laparoscopically, through open procedures, or natural orifices.
MWA has several potential advantages over RFA or Cryo (7). In MWA, the heating process is active and delivers continuous heating at optimal temperatures in the tumor allowing for more complete thermal ablation in a shorter period of time agnostic of the surrounding tissue type. In addition, microwaves can penetrate tissues that have a high impedance to electrical current resulting in an ability to create larger tumor ablation fields (7). MWA also has increased affinity for water-based tissue with a minimal heat-sink effect and can utilize multiple applicators to deliver an improved convection profile, with less procedural pain (8,9). Clinical data to support the use of MWA in lung tumors is limited in terms of robust sample sizes with adequate long-term recurrence data. Additionally there are only a few studies that document proof of malignancy prior to ablation. However, preliminary literature suggests that MWA provides good local control with minimal complications for the treatment of lung cancer and may produce a replicable ablation zone in other tissue types (10).
The Emprint Ablation System (Medtronic, Boulder, CO, USA) with Thermosphere Technology (Figure 2) is a unique product for ablating solid organs. The EmprintTM procedure planning application is an interactive tool that predicts the size of an MWA zone based on probe placement, time, power, and target tissue type and represents data visually on pre- or intra-procedure CT images for visual planning. This computerized visual feedback is intended to allow the operator to adjust probe position, energy delivered, or duration of ablation to maximize the efficacy of the ablation procedure (5). The efficacy of this planning technology was evaluated in a recent clinical trial entitled EMPRESS (Clinicaltrials.gov; #NCT02323854).
The system includes three controls to maintain spherical ablation zones:
- Thermal control—advanced antenna cooling prevents the antenna shaft from interfering with the ablation zone.
- Field control—advanced antenna geometry focuses energy at the tip of the device allowing for a precise spherical electromagnetic field.
- Wavelength control—the active antenna buffering maintains the spherical electromagnetic field during the ablation procedure.
Despite the evolution of microwave technology in the lung, there exists a literature gap regarding the use of this particular planning software to determine the appropriate time and power to achieve an adequate ablation margin, and the safety associated with this particular technology.
The aim of this study was to conduct a prospective analysis of safety and feasibility of pMWA using the Emprint system for biopsy proven primary or secondary lung tumors. Additionally, the authors sought to determine the efficacy of the pre-planning system to predict a 5 mm margin and determine the time and power used during ablation. Finally, technical success and patient outcomes were evaluated as a secondary endpoint. We present the following article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1636/rc).
Methods
This was a prospective, non-randomized, single-arm, single center study, designed to demonstrate efficacy and safety of the Emprint™ Ablation System using a percutaneous approach in patients with primary or secondary lung tumors (Clinicaltrials.gov; #NCT02673021). After obtaining approval from the Mayo Clinic Institutional Review Board (IRB #15-001758), patients were consented and enrolled based on the inclusion and exclusion criteria of the protocol, as seen in Table 1. Additionally, patients were excluded if they had prior ablation to the treatment zone, or any clips/staples in this region from a prior resection. Additionally, patients who were candidates for surgical resection were also included in the trial after a multidisciplinary discussion.
Table 1
| Inclusion | Exclusion |
|---|---|
| Subject must be at least 18 years old | Subject is pregnant or breast feeding |
| Subject is able to understand the study procedures and provide informed consent | Subject has a significant clinical disease or condition, e.g., cardiovascular, respiratory, gastrointestinal, renal, hepatic, hematological, psychiatric or neurologic that would preclude enrollment, as determined by the primary investigator |
| Subject is willing and able to complete the entire study as specified in the protocol, including the follow-up visits | Subject has another location of disease that is not controlled, and there are no plans for control |
| Subject has lung lesion that is biopsy-proven cancer (of any type) or suspicious, with confirmation at the time of the procedure | Subject has more than 10 lung nodules |
| Lung lesion(s) are reachable/treatable per clinician opinion | If subject has acute or chronic severe renal (kidney) insufficiency (glomerular filtration rate <30 mL/min/1.73 m2 they will not receive contrast with imaging |
| Subject can have other location of disease if it is controlled, or there are plans for control | Subject has renal dysfunction due to the hepato-renal syndrome or in the perioperative liver transplantation period |
| Subject has 1 or more lung nodules (not more than 10), that have a mean diameter <3 cm on axial CT scan | Prior ablation/metal/staple line within the treatment zone |
| Life expectancy ≥6 months |
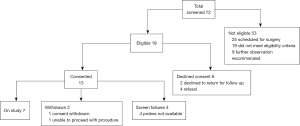
Patients were screened and enrolled by a board-certified thoracic surgeon and subspecialty interventional radiologist as well as reviewed by the multidisciplinary ablation tumor board to determine eligibility criteria, Figure 3. All patients gave informed consent prior to enrollment and the study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Patients were required to have biopsy confirmation of malignancy prior to ablation. Additionally, all patients obtained pulmonary function testing within 3 months prior to ablation and subsequently within 3 months after ablation to assess changes in functional status.
Ablations were carried out using a percutaneous approach via the FDA-approved commercially available Emprint Ablation System (Medtronic USA, Microwave Ablation System, Medtronic). All cases in this study were performed by 4 radiologists trained in lung ablation. Ablations were performed either in the radiology procedural suite or hybrid operating room with both the radiologist and thoracic surgeon present for every case. Patients were intubated with a double lumen endotracheal tube under general endotracheal anesthesia and paralyzed, with single-lung ventilation. Prior to placement of the ablation probe, a planning CT scan was performed and loaded into the Emprint Ablation System software. The software was used to plan the route and depth of microwave probe placement and the power and time of microwave energy to achieve 5 mm minimum margin relative to the index ablation target. After placement of the actual probe, a second CT scan was performed, simulated ablation zones generated using the Emprint Ablation System software. This allowed the initial plan for energy delivery to be altered, if necessary, for small deviations in probe position, differences in lung aeration or other change in physical geometry from the planning CT.
Technical success was defined as successful placement of the microwave probe in the planned location, successful delivery of the planned dose of microwave energy, and complete coverage of the ablation target by the ablation zone. Minimum ablation zone margin was determined on an immediate post-procedure CT by manual measurement of the minimum distance from the edge of the ablation target to the edge of the ablation zone. As a goal of the study was to determine the efficacy of ablation as prescribed by the Emprint Ablation Planning software, actual measured margins were not included in the definition of technical success and repeat ablation was not immediately performed for a minimum margin <5 mm. Safety was defined as less than 20% incidence of a serious adverse event (Grade 3 or above).
Following the procedure, patients were admitted for overnight observation on a thoracic surgical service and monitored for bleeding or pneumothorax. A massive hemoptysis protocol was created and readily available.
Imaging with fluorodeoxyglucose (FDG) PET/CT, and PET/MR was performed one day after the first 3 lesions underwent MWA. Standard of care diagnostic CT was performed within 16–48 hours post-procedure and additionally at 1–2-month and 6-month follow-up. FDG PET/CT was performed within 16–48 hours post procedure and additionally at 1–2-month and 6-month follow-up. FDG PET/MR was performed within 16–48 hours post procedure and at the 1–2-month follow-up visit.
Following discharge from the hospital, patients were seen between three to 10 weeks following the procedure with a CT scan of the chest, PET scan and pulmonary function testing. Imaging was then subsequently repeated at 6 months and 1 year thereafter. The ablation zone was defined as the region of alveolar opacity (ground glass or consolidation) generated by the on the immediate post-ablation CT after needle removal and as the solid or cavitary opacity at the treatment site on later follow-up examinations.
Statistical analysis
Technical success was defined as successful placement of the ablation probe in the planned position in the lung and the delivery of the planned dose of microwave energy.
Efficacy was defined as stability or involution of the ablation zone (i.e., lack of local tumor progression) on subsequent follow up exams to the study endpoint of 1 year.
Local tumor progression was defined as (I) growth of the ablation zone by 20% or more from the baseline post procedure measurement or from any new nadir in size established on subsequent follow-up, (II) new nodularity at the margin of an ablation zone.
The ablation zone was defined as the region of treatment-induced ground glass opacity at the target site on the immediate, post-procedure CT and as the region of solid or cavitary opacity on subsequent follow-up CTs. As the index ablation target is obscured by the ablation zone after treatment, the ablation zone was measured using RECIST criteria as a proxy for the index ablation target. Local tumor progression was defined as an increase in RECIST measurements of 20% from a prior follow-up CT or as new nodularity on the margin of the ablation zone or new FDG avidity in the ablation zone on follow-up exams. CT measurements were performed by a board certified non-blinded radiologist proficient in ablation and interpretation of chest CTs. PET-MRI was independently evaluated by a non-blinded board-certified nuclear medicine radiologist well versed in interpretation of chest imaging. Adverse event data were collected starting from the initial administration of anesthesia until their 12-month post procedure follow-up visit.
Primary endpoints of the study included efficacy and safety. Efficacy was defined as lack of local tumor progression within the 1-year study period. The safety evaluation is based on the major complication rates of performing MWA. Secondary endpoints related to recurrence-free survival and overall radiologic response at 6 months and 1 year.
Results
Between June 2016 and January 2019, a total of 6 patients and 7 lesions were treated under the study protocol. An additional 6 patients were enrolled into the study but ultimately did not receive MWA (one patient declined treatment, one underwent Cryo, and four patients were withdrawn because the ablation probes became unavailable due to a voluntary antennae recall). Additional patients refused to enroll in the study secondary to the need for a staged biopsy prior to treatment. The cohort of treated patients consisted of 3 males and 3 females, respectively with a median age of 67 (IQR, 65–70) years. Three metastatic colorectal tumors and four primary adenocarcinoma lung tumors were treated. Demographic details are included in Table 2.
Table 2
| Patient | Age (years) | Sex | BMI | No. of nodules | Location | Size (mm) | Histology | Distance to pleura (mm) |
|---|---|---|---|---|---|---|---|---|
| 1a | 65 | Female | 21.4 | 10 | LUL | 5 | Colorectal | 31 |
| 1b | 66 | Female | 22.3 | 9 | RUL | 6 | Colorectal | 12 |
| 2 | 55 | Male | 29.8 | 2 | LUL | 7 | Colorectal | 24 |
| 3 | 67 | Female | 38.6 | 1 | RLL | 13 | Lung (AC) | 14 |
| 4 | 72 | Male | 29.9 | 1 | LUL | 22 | Lung (AC) | 13 |
| 5 | 67 | Male | 20.8 | 1 | RML | 8 | Lung (AC) | 39 |
| 6 | 70 | Female | 32.1 | 1 | LUL | 14 | Lung (AC) | 38 |
BMI, body mass index; LUL, left upper lobe; RUL, right upper lobe; RLL, right lower lobe; AC, adenocarcinoma; RML, right middle lobe.
All but 2 patients had one nodule identified on CT that underwent treatment. Patient 1 had 10 nodules identified and underwent 2 separate MWA treatments. She had previously undergone a thoracotomy with right upper lobectomy and right middle lobe wedge resections. At the time of the first treatment, she had one nodule treated with MWA and another nodule on the same side treated with Cryo. At a separate time one nodule was treated in the contralateral lung with MWA and two nodules on the same side were treated with Cryo. Patient 2 had two nodules identified, one was treated by MWA and the other was stable and observed.
Median lesion size was 10.7 (IQR, 6–14) mm. All ablations were completed at 75 W. The median ablation time was 5 (IQR, 2–10) minutes. The mean ablation time was 5.9 (IQR, 3–10) minutes. Technical success rate was achieved in all procedures. Twelve adverse events were reported (1 Grade 3, 3 Grade 2, and 8 Grade 1 events) with no Grade 4 or 5 events.
Of the seven ablations during the 1 year follow-up, there was one local tumor recurrence at 271 days following ablation presenting as new nodularity at the apex of the ablation zone; this was subsequently successfully treated with percutaneous Cryo. One additional local tumor recurrence was identified after the established observation time at 512 days with diffuse enlargement of the ablation zone, which was resected surgically at 839 days from initial treatment. Complete procedural details are located within Table 3.
Table 3
| Patient | Ablation target size (RL × AP × SI) (mm) | Immediate ablation zone size (L × W × H) (mm) | Ablation duration (minutes) | Ablation energy (W) | Plan minimum margin (mm) | Measured minimum margin (mm) | Anesthesia time (hours:minutes) |
|---|---|---|---|---|---|---|---|
| 1a | 5×4×4 | 31×11×11 | 2 | 75 | 6 | 4 | 3:25 |
| 1b | 6×6×6 | 43×24×24 | 5 | 75 | 8 | 8 | 4:48 |
| 2 | 7×4×4 | 35×28×27 | 3 | 75 | 7 | 5 | 1:29 |
| 3 | 13×9×12 | 36×17×15 | 6 | 75 | 7 | 2 | 2:45 |
| 4 | 22×20×16 | 45×33×40 | 10 | 75 | 10 | 5 | 2:24 |
| 5 | 8×7×7 | 35×25×24 | 5 | 75 | 5 | 4 | 2:10 |
| 6 | 9×14×12 | 37×33×40 | 10 | 75 | 5 | 5 | 3:07 |
RL, right left; AP, anterior posterior; SI, superior inferior; L, length; W, width; H, height.
The length of stay following treatment was overnight except for one ablation that required 2 nights for a pneumothorax requiring tube thoracostomy. Twelve adverse events were reported (Table 4). There were no Grade 4 or 5 adverse events. There was one adverse event that was Grade 3, in a patient with upper respiratory tract infection treated with antibiotics. Grade 2 events included pain (n=2), and pneumothorax (n=1).
Table 4
| Patient | Term | Grade | Outcome | Related |
|---|---|---|---|---|
| 1 | Pain | Grade 2 | Resolved spontaneously | Possible |
| 1 | Pneumothorax | Grade 2 | Resolved with treatment | Probable |
| 1 | Pleural effusion | Grade 1 | Resolved spontaneously | Probable |
| 1 | Dyspnea | Grade 1 | Ongoing at study end | Possible |
| 1 | Fatigue | Grade 1 | Ongoing at study end | Unlikely |
| 1 | Pain | Grade 2 | Resolved with treatment | Probable |
| 1 | Atelectasis | Grade 1 | Resolved spontaneously | Probable |
| 1 | Pleural effusion | Grade 1 | Resolved spontaneously | Probable |
| 2 | Pneumothorax | Grade 1 | Resolved spontaneously | Definite |
| 4 | Pain | Grade 1 | Resolved with treatment | Definite |
| 4 | Atelectasis | Grade 1 | Ongoing at study end | Unrelated |
| 6 | Upper respiratory infection | Grade 3 | Resolved with treatment | Unrelated |
Per study protocol, all patients completed 1 year follow-up except one patient. At 1 year, 5 patients were alive. Patient 6 developed bilateral lower lobe aspiration pneumonia unrelated to the procedure and unfortunately expired 4 months after her ablation. Given that she had no symptoms or radiographic evidence of pneumonia immediately following her procedure and the original ablation site was the left upper lobe, this was deemed unrelated to her procedure. One patient expired from extra-thoracic metastatic disease outside the study window, at 20 months post-ablation.
Pre and post procedural pulmonary function testing was captured (Table 5). Notably, the pulmonary function testing was not adversely affected in all but subjects 1 and 5. Subject 1 did not have pulmonary function testing between her surgery and initial MWA; therefore, the 32.3% decrease in her forced expiratory volume in one second (FEV1) could be contributed to multiple lung resections. The mean and median % change in FEV1 for patients 2–6 who had ablation alone was −2% and −2%, respectively. The mean and median % change in diffusion capacity for carbon monoxide (DLCO) for the same patients was −1% and an increase in 4%, respectively (Figure 4A,4B). Additionally in patients 2–6, there was a median change of 0% in vital capacity and forced vital capacity.
Table 5
| Subject | FEV1, L | FEV1, % | DLCO, % | Days post procedure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | % change | Pre | Post | % change | Pre | Post | % change | ||||
| 1a1 | 2.07 | 1.40 | −32.3% | 103% | 68% | −35% | 56% | ND | ND | 163 | ||
| 1b | 1.4 | 1.42 | 1.4% | 68% | 72% | 4% | ND | 39% | ND | 45 | ||
| 2 | 3.2 | 3.17 | −0.1% | 82% | 82% | −0% | 82% | 86% | 4% | 64 | ||
| 4 | 2.52 | 2.28 | −9.5% | 106% | 96% | −10% | 92% | 77% | −15% | 34 | ||
| 5 | 3.04 | 3.64 | 19.7% | 104% | 125% | 21% | 67% | 72% | 5% | 62 | ||
| 6 | 1.65 | 1.59 | −3.6% | 52% | 50% | −2% | 50% | 45% | −5% | 47 | ||
| 7 | 0.9 | 1.25 | 38.9% | 43% | 61% | −18% | 63% | 70% | 7% | 46 | ||
1, pulmonary function tests completed prior to surgery completed before the ablation; FEV1, forced expiratory volume in one second; DLCO, diffusion capacity for carbon monoxide; ND, not done.

Based on CT imaging of each nodule (Figure 5), there was a median decrease of 42.75% based on RECIST criteria at 6 months, and 48.6% at 1 year (Table 6).
Table 6
| Patient | Immediate | ≤6 months | At 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ablation zone size (mm) | RECIST | Ablation zone size (mm) | RECIST | % change | Ablation zone size (mm) | RECIST | % change | |||
| 1a | 5 | 42 | 23 | 34 | −19.1% | 26 | 32 | −23.8% | ||
| 1b | 6 | 66 | NA | NA | NA | 29 | 38 | −42.4% | ||
| 2* | 7 | 63 | 23 | 33 | −47.2% | 18 | 29 | −54.0% | ||
| 3 | 13 | 53 | 12 | 22 | −58.5% | 10 | 18 | −66.0% | ||
| 4 | 22 | 85 | 27 | 43 | −49.4% | 18 | 39 | −54.1% | ||
| 5 | 8 | 60 | 24 | 37 | −38.3% | 20 | 34 | −43.3% | ||
| 6 | 14 | 77 | 29 | 66 | −14.3% | NA | NA | NA | ||
*, patient 2 had continued shrinkage of the lesion and RECIST criteria demonstrated smaller size, but a clearly new distinct nodule at the apex of the ablation zone was characterized as a local tumor recurrence, as seen in Figure 5L. RECIST, Response Evaluation Criteria in Solid Tumors.
PET-MRI data
At 24 hours post-ablation the central necrotic core of the ablation zone showed complete lack of FDG activity, and lack of enhancement (on CT or MRI), increased T1 signal (likely due to blood products and proteinaceous fluid), and increased T2 signal (likely due to edema). Also, at 24 hours post-ablation there was also a thin rim of mild FDG activity and contrast enhancement (CT and MRI) at the edge of the ablation zone and normal lung, likely inflammatory. The rim of enhancement on MRI was best seen at 3–5 minutes post injection of contrast on MRI. The PET activity was best seen when respiratory gating was applied. The rim of FDG activity seen in at 24 hours did not exceed blood pool activity in any case. No focal activity or nodular enhancement was seen to suggest residual cancer was present at the edge of the ablation zone in any case at 24 hours. Even in retrospect, the single tumor ablation site that later showed recurrence (patient 2) did not show findings on CT, MRI or FDG PET suggestive of technical failure/residual disease at 24 hours post ablation (Figures 6,7).
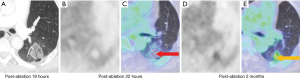
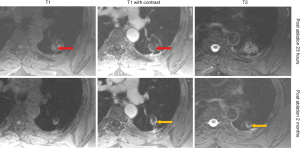
At 1–3 months post-ablation the central ablation zone sometimes cavitated, and other times retained necrotic debris and/or fluid. The rim of the ablation zone thickened and showing mild to moderate FDG activity, enhancement, and T2 signal intensity likely due to the formation of healing granulation tissue. The thickening was sometimes asymmetric and the FDG activity was not always uniform. Activity could be less than or up to twice the SUVmax of liver, without suggesting residual or recurrent disease was present (based on follow-up).
After 2–3 months, the lesions would decrease in size, rim thickness, FDG activity, and T2 signal. FDG activity after 6 months was below blood pool in all cases. The ablation sites stabilized by 6–12 months.
Conclusions
This single institution prospective nonrandomized Phase I trial requiring biopsy of primary or secondary tumors <3 cm in size followed by percutaneous MWA appears to demonstrate safety and efficacy, with possible preservation of pulmonary function.
Multiple other ablative studies, with a variety of energy technologies, have established safety and efficacy of percutaneous ablation for lung cancer (5-7). A large systematic review and pooled analysis comparing SBRT and RFA in stage I non-operable primary lung cancer demonstrated 1 year local control rates of 77% for RFA 97% for SBRT (5) and showed that there was no difference in overall survival. RFA did have a pneumothorax in 31% of patients, and SBRT demonstrated Grade 3 pneumonitis in 2% of patients. It should be noted that Cryo theoretically has the advantage of being resistant to the cold-sink effects of ventilation compared to RFA but there have been no direct studies to prospectively evaluate Cryo vs. RFA vs. MWA (7).
With less than a decade of evaluation and few clinical trials to evaluate efficacy and safety of MWA, this trial aims to address the predictability of the Thermosphere system to achieve predictable margins and establish local control with an appropriate safety profile. One of the largest current international series of patients undergoing MWA (11)established local control rates at 1, 3, 5 years after MWA as 96%, 64%, and 48%, respectively. The median cancer-specific overall survival rate was 47.4 months. The overall survival rates at 1, 2, 3, and 5 years after MWA were 89%, 63%, 43%, and 16%, respectively. Tumors that were ≤3.5 cm were associated with better survival than tumors that were >3.5 cm.
Wolf et al. (12) retrospectively evaluated 50 patients to assess MWA effectiveness. The results of this study showed that 26% (13 of 50) of patients had residual disease at the ablation site and another 22% (11 of 50) of patients had recurrent disease resulting in a 1-year local control rate of 67%, resulting in a mean time to recurrence of 16.2 months.
This is in comparison to the recently published MALT study by Iezzi et al. (13), where 69 MWA treatments were completed in 54 patients with primary and secondary pulmonary tumors. This study reported an overall survival rate of 98.0% and 71.3% at 12 and 24 months, respectively with a local progression rate of 24.7% with an average time to progression of 8.1 months.
A retrospective single-center study by Zheng et al. (14) of 204 MWA sessions of lung tumors was conducted to determine the incidence of major complications. They reported 32 cases (15.7%) of pneumothorax requiring chest tube, 6 cases (2.9%) of pleural effusions requiring chest tube, 6 cases (2.9%) of pneumonia, 1 case (0.5%) of pulmonary abscess and 1 death (0.5%) related to the procedure. After the first procedure of MWA, complete necrosis was observed in 131 of 137 tumors with a maximum diameter of greater than 3 cm and less than 5 cm.
This current study has similar complications and efficacy profiles to the above listed trials and others (Table 7), despite the small sample size. There are few unique studies that required biopsy-proven malignancy prior to intervention and this study additionally evaluated pulmonary testing and PET-MRI evaluation on all patients. Only one recurrence during the study period is documented within the local ablation zone, with a one-year efficacy rate of 86%. The safety profile is also favorable, with a 17% pneumothorax rate requiring a chest tube, 17% pneumonia rate, and no effusions requiring a chest tube, no incidence of abscess, and no deaths. We also demonstrate preservation of forced expiratory volume and diffusion capacity, which is in concordance with Dupuy et al. (25), who studied pulmonary function testing on all patients undergoing RFA at 3 and 24 months post-procedure and found no statistical change from baseline in either metric and an increase in forced vital capacity.
Table 7
| Author | Year | Sample size, nodules | Ablation type | Clinical trial | Study type | Biopsy required | Recurrence-free survival, in years | Histology | Duration of follow-up, in months |
|---|---|---|---|---|---|---|---|---|---|
| Reisenauer et al. | 2021 | 7 | MWA | Yes | Prospective | Yes | 86% (1 year) (L) | Both | 12 months |
| Wolf et al. (12) | 2008 | 50 | MWA | No | Retrospective | No | 67% (1 year) (L) | Both | 10 months (median) |
| Lencioni et al. (15) | 2008 | 183 | RFA | Yes | Prospective | Yes | 88% (1 year) (L) | Both | 24 months |
| Vogl et al. (16) | 2011 | 130 | MWA | Yes | Prospective | Yes | 26.9% (2 years) (L) | Metastatic | 24 months |
| Crabtree et al. (17) | 2013 | 51 | RFA | Yes | Prospective | Yes | 70% (1 year) (L) | Primary NSCLC | 24 months |
| 61% (2 years) | |||||||||
| Yang et al. (11) | 2014 | 47 | MWA | No | Retrospective | Yes | 96% (1 year) (L) | Primary NSCLC | 30 months (median) |
| 64% (3 years) | |||||||||
| 48% (5 years) | |||||||||
| de Baère et al. (18) | 2015 | 1,037 | RFA | Yes | Prospective | No | 40.2% (1 year) (PFS) | Metastatic | 12 months |
| 23.3% (2 years) | |||||||||
| 16.4% (3 years) | |||||||||
| 13.1% (4 years) | |||||||||
| de Baere et al. (19) (ECLIPSE) | 2015 | 60 | Cryo | Yes | Prospective | No | 34% (18 months) (L) | Metastatic | 12 months |
| Macchi et al. (20) (LUMIRA) | 2017 | 26 | MWA vs. RFA | Yes | Randomized | No | – | Metastatic | 12 months |
| Cheng et al. (21) | 2018 | 48 | MWA | No | Retrospective | No | 83% (L) at median time 31 months | Metastatic | 31 months (median survival) |
| Kurilova et al. (22) | 2018 | 90 | MWA | No | Retrospective | No | 93% (1 year) (L) | Metastatic | 25.6 months (median) |
| 86% (2 years) | |||||||||
| 86% (3 years) | |||||||||
| Palussière et al. (23) | 2018 | 42 | RFA | Yes | Prospective | Yes | 81% (3 years) (L) | Primary NSCLC | 36 months |
| Callstrom et al. (24) (SOLSTICE) | 2020 | 224 | Cryo | Yes | Prospective | No | 85.1% (1 year) (L) | Metastatic | 24 months |
| 77.2% (2 years) | |||||||||
| Iezzi et al. (13) (MALT) | 2021 | 69 | MWA | Yes | Prospective | No | 75.3 (2 years) (L) | Both | 24 months |
| Total | 2008–2020 | 2,090 | RFA: 1,339; MWA: 467; Cryo: 284 | No: 235; yes: 1,855 | Retrospective: 235; prospective: 1,855 | No: 1,630; yes: 460 | 67–96% at 1 year (L) | Primary & secondary | 10–36 months |
MWA, microwave ablation; L, local; RFA, radiofrequency ablation; NSCLC, non-small cell lung cancer; PFS, progression free survival; Cryo, cryoablation.
Ideal imaging to predict recurrence following MWA of lung tumors has been extensively studied and reported. The utility of contrast-enhanced MRI alone has been demonstrated in the literature. A recent evaluation of 77 lung metastases post-MWA with MRI 24 hours later and confirmed comparable efficacy for prediction of long term recurrence and complication and potentially reduce radiation risks associated with follow-up CT imaging (26). Evaluation of lesions with combined PET-MRI long-term is unique to this study. Albeit limited size, PET-MRI consistently demonstrated a reduction in T2 signal and FDG avidity after 2–3 months and return to blood pool at 6 months. This correlates with known CT data regarding the variability of radiographic changes at the ablation site in the immediate post-procedural course and can last up to 3 months. Previous studies in up to 900 patients with various malignancies who have undergone PET-MR imaging have not elucidated a clear difference in diagnostic accuracy for up front staging, however, whether MR will emerge as a predictive modality for recurrence remains to be seen (27). Future studies will be warranted to reliably develop prediction patterns for early local treatment failure.
This study has several limitations. Despite being a prospective study, the sample size of this study is low and therefore major statistical conclusions cannot be drawn. There were many patients who underwent ablation during the period of the study who elected not to participate due to need for pre-procedural biopsy. Additionally, this study consisted of primary and secondary pulmonary tumors and further larger sample studies are warranted to analyze distinct recurrence patterns between the two histologies. Finally, this study did not directly compare planned predicted margins to actual margins. The goal was to see if the software prescribed an accurate dosage to achieve adequate margins as measured in native lungs. Because the ablation zone is larger than the target lesion and obscures the target on follow-up imaging as well as shrinkage of the surrounding tissue, the size of the initial ablation zone must be used as a proxy for lesion size for the purposes of RECIST measurements (19,24). Further investigations are needed in this area.
In conclusion, this is the first study to require biopsy and prospectively enroll patients with primary and metastatic pulmonary tumors for MWA ablation to demonstrate safety and efficacy. Percutaneous MWA is a viable option in inoperable patients with primary and secondary pulmonary tumors. MWA preserves lung parenchyma and lung function and preliminary PET-MR data suggests resolution of post-ablation changes at 2–3 months with baseline at 6 months.
Acknowledgments
Funding: This trial was partially funded by Covidien (Medtronic).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1636/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1636/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1636/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1636/coif) and report that financial support in the amount of $200,000 was provided by Covidien for conducting this study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Mayo Clinic Institutional Review Board (IRB #15-001758) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kawachi R, Tsukada H, Nakazato Y, et al. Early recurrence after surgical resection in patients with pathological stage I non-small cell lung cancer. Thorac Cardiovasc Surg 2009;57:472-5. [Crossref] [PubMed]
- Uramoto H, Nakanishi R, Fujino Y, et al. Prediction of pulmonary complications after a lobectomy in patients with non-small cell lung cancer. Thorax 2001;56:59-61. [Crossref] [PubMed]
- Roesch J, Andratschke N, Guckenberger M. SBRT in operable early stage lung cancer patients. Transl Lung Cancer Res 2014;3:212-24. [PubMed]
- Meng MB, Wang HH, Zaorsky NG, et al. Risk-adapted stereotactic body radiation therapy for central and ultra-central early-stage inoperable non-small cell lung cancer. Cancer Sci 2019;110:3553-64. [Crossref] [PubMed]
- Bi N, Shedden K, Zheng X, et al. Comparison of the Effectiveness of Radiofrequency Ablation With Stereotactic Body Radiation Therapy in Inoperable Stage I Non-Small Cell Lung Cancer: A Systemic Review and Pooled Analysis. Int J Radiat Oncol Biol Phys 2016;95:1378-90. [Crossref] [PubMed]
- Carrafiello G, Laganà D, Mangini M, et al. Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg 2008;6:S65-9. [Crossref] [PubMed]
- Hinshaw JL, Lubner MG, Ziemlewicz TJ, et al. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation—what should you use and why? Radiographics 2014;34:1344-62. [Crossref] [PubMed]
- Wright AS, Sampson LA, Warner TF, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005;236:132-9. [Crossref] [PubMed]
- Shock SA, Meredith K, Warner TF, et al. Microwave ablation with loop antenna: in vivo porcine liver model. Radiology 2004;231:143-9. [Crossref] [PubMed]
- Vogl TJ, Basten LM, Nour-Eldin NA, et al. Evaluation of microwave ablation of liver malignancy with enabled constant spatial energy control to achieve a predictable spherical ablation zone. Int J Hyperthermia 2018;34:492-500. [Crossref] [PubMed]
- Yang X, Ye X, Zheng A, et al. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol 2014;110:758-63. [Crossref] [PubMed]
- Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 2008;247:871-9. [Crossref] [PubMed]
- Iezzi R, Cioni R, Basile D, et al. Standardizing percutaneous Microwave Ablation in the treatment of Lung Tumors: a prospective multicenter trial (MALT study). Eur Radiol 2021;31:2173-82. [Crossref] [PubMed]
- Zheng A, Wang X, Yang X, et al. Major complications after lung microwave ablation: a single-center experience on 204 sessions. Ann Thorac Surg 2014;98:243-8. [Crossref] [PubMed]
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 2008;9:621-8. [Crossref] [PubMed]
- Vogl TJ, Naguib NN, Gruber-Rouh T, et al. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology 2011;261:643-51. [Crossref] [PubMed]
- Crabtree T, Puri V, Timmerman R, et al. Treatment of stage I lung cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033). J Thorac Cardiovasc Surg 2013;145:692-9. [Crossref] [PubMed]
- de Baère T, Aupérin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 2015;26:987-91. [Crossref] [PubMed]
- de Baere T, Tselikas L, Woodrum D, et al. Evaluating Cryoablation of Metastatic Lung Tumors in Patients—Safety and Efficacy: The ECLIPSE Trial—Interim Analysis at 1 Year. J Thorac Oncol 2015;10:1468-74. [Crossref] [PubMed]
- Macchi M, Belfiore MP, Floridi C, et al. Radiofrequency versus microwave ablation for treatment of the lung tumours: LUMIRA (lung microwave radiofrequency) randomized trial. Med Oncol 2017;34:96. [Crossref] [PubMed]
- Cheng G, Shi L, Qiang W, et al. The safety and efficacy of microwave ablation for the treatment of CRC pulmonary metastases. Int J Hyperthermia 2018;34:486-91. [Crossref] [PubMed]
- Kurilova I, Gonzalez-Aguirre A, Beets-Tan RG, et al. Microwave Ablation in the Management of Colorectal Cancer Pulmonary Metastases. Cardiovasc Intervent Radiol 2018;41:1530-44. [Crossref] [PubMed]
- Palussière J, Chomy F, Savina M, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in patients ineligible for surgery: results of a prospective multicenter phase II trial. J Cardiothorac Surg 2018;13:91. [Crossref] [PubMed]
- Callstrom MR, Woodrum DA, Nichols FC, et al. Multicenter Study of Metastatic Lung Tumors Targeted by Interventional Cryoablation Evaluation (SOLSTICE). J Thorac Oncol 2020;15:1200-9. [Crossref] [PubMed]
- Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer 2015;121:3491-8. [Crossref] [PubMed]
- Roman A, Kaltenbach B, Gruber-Rouh T, et al. The role of MRI in the early evaluation of lung microwave ablation. Int J Hyperthermia 2018;34:883-90. [Crossref] [PubMed]
- Czernin J, Ta L, Herrmann K. Does PET/MR Imaging Improve Cancer Assessments? Literature Evidence from More Than 900 Patients. J Nucl Med 2014;55:59S-62S. [Crossref] [PubMed]