A diagnostic test: combined detection of heparin-binding protein, procalcitonin, and C-reactive protein to improve the diagnostic accuracy of bacterial respiratory tract infections
Introduction
Respiratory tract infection (RTI) is one of the most common diseases in clinical settings, and its pathogenic microorganisms include bacteria, viruses, fungi, and mycoplasma. The incidence of RTI increases year by year due to environmental pollution; thus, RTIs pose a serious threat to human health (1). Bacterial RTIs are common in hospitalized patients. Sputum culture remains the gold standard for the diagnosis of RTIs, but its diagnostic efficiency is limited by the low sampling quality of the sputum specimens and by the low positivity rate, poor reliability, and long testing period of the culture (2). Inflammatory markers, including heparin-binding protein (HBP), procalcitonin (PCT) and C-reaction protein (CRP), as they can help diagnose bacterial infection quickly and early, have been increasingly used in the diagnosis of bacterial RTIs (3-5); however, a single marker can be released in the blood due to the occurrence of one or more illness, often fails to support an accurate diagnosis of the disease. The analysis of inflammatory markers in combination or along with other tests could be helpful. There have been relatively few studies on the combination of these markers in the diagnosis of bacterial RTIs. So we retrospectively investigated the performance of each inflammatory marker and combinations of these markers in the patients with bacterial RTIs, aims to improve their sensitivity and specificity in the diagnosis of bacterial RTIs. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-260/rc).
Methods
General data
A total of 87 inpatients with RTIs at the Department of Respiratory Medicine of the East Hospital of Sichuan Provincial People’s Hospital from January 2019 to December 2019 were enrolled in this study and divided into the bacterial infection group (n=43) and the non-bacterial infection group (n=44) based on bacterial culture results. Additionally, 44 age- and sex-matched healthy volunteers from the Health Check-Up Center, during the same period, were enrolled as the healthy controls.
Inclusion criteria
To be eligible for inclusion in the study, patients had to meet the following inclusion criteria: (I) be an inpatient with complete clinical data at the Department of Respiratory Medicine of our hospital; (II) be aged ≥18 years; (III) for the case group, meet the diagnostic criteria for respiratory tract bacterial infection set out in the Guidelines for the Diagnosis and Treatment of Respiratory Diseases (6), for the bacterial infection group, have a positive culture result, and for the healthy control group, be a healthy individual who had received a health check-up at our hospital; and (IV) have provided written informed consent (this could have been provided by either the subject and/or a family member).
Exclusion criteria
Patients were excluded from the study if they met any of the following exclusion criteria: (I) had incomplete test results or missing information; (II) were aged <18 years; (III) showed poor compliance; (IV) had received immunosuppressive therapy; (V) had an active autoimmune disease; and/or (VI) had received antibiotic treatment for >3 d before enrollment.
Judgment criteria
The diagnosis of RTI was based on the definition and diagnostic criteria for RTI set out in the Guidelines for the Diagnosis and Treatment of Respiratory Diseases (6). Specifically, the patients had to (I) present with a cough, mucous sputum, and wet rales in the lungs, and have 1 of the following signs: (i) a fever; (ii) an increased white blood cell (WBC) count and/or proportion of neutrophils; or (iii) inflammatory infiltration in the lungs on X-ray; and (II) have a chronic airway disease during the stable phase (chronic bronchitis with or without obstructive emphysema, asthma, or bronchiectasis) accompanied by a secondary acute infection, and microbiological analysis or X-ray chest results that revealed significant changes or new lesions compared to those at admission.
Collection of clinical data
Clinical data, including the name, gender, age, admission number, clinical diagnosis, HBP level, PCT level, and CRP level, were collected from 43 patients with bacterial RTIs and 44 patients with respiratory non-bacterial infections. In addition, clinical data, including the name, gender, age, HBP level, PCT level, and CRP level, were collected from the 44 healthy subjects who had received health check-ups at our hospital. A retrospective analysis was performed using these data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was approved by the Medical Clinical Research Ethics Committee of Sichuan Provincial People’s Hospital, China (No. 2019–309-1). All the participants voluntarily participated in this study and signed the informed consent form.
Measurement of plasma HBP, PCT, and CRP levels
After the clinical data were collected and recorded, 3 mL of peripheral blood was collected from the patients with RTIs at hospital admission, and then anticoagulated with sodium citrate (at a ratio of 1:9). After the blood was centrifuged at 3,000 rpm for 15 min, the plasma was pipetted and gently dispensed into Eppendorf tubes. The assay was completed within 1 h.
The plasma HBP level was measured by an enzyme-linked immunosorbent assay (ELISA) with a novel dry immunofluorescence analyzer (Jet-istar 3000) and its reagents (Joinstar, Zhejiang, China) with a 95% confidence interval (CI) of 0.00–10.00 ng/mL. The PCT level was determined by the electrochemiluminescence method using a Roche E602 instrument and its reagents (Roche Diagnostics GmbH, Mannheim, Germany) with a 95% CI of 0.00–100.00 ng/mL. The plasma CRP level was determined by immunoturbidimetric assay using a Beckman Specific Protein Analyzer and its reagents (Beckman Coulter Inc., CA, USA) with a 95% CI of 0.00–10.00 mg/L. All the measurements were completed in the Clinical Laboratory of our hospital.
Bacterial culture and identification of sputum specimens
- Specimen collection: sputum specimens were collected early in the morning after admission. Before each sputum expectoration, all patients rinsed their mouths with normal saline or received oral nursing. The first sample of sputum was discarded, and the remaining sputum was collected (7).
- Bacterial isolation and identification: the specimens were first screened under a microscope, and a squamous epithelial cell count of <10 and a WBC count of >25 under a low-power field of view were regarded as qualified. The specimens were then inoculated onto sheep-blood agar and MacConkey agar for bacterial identification according to the method described in Bergey’s Manual of Systematic Bacteriology.
Statistical methods
Statistical analyses and graphs were completed using GraphPad Prism ver.7.0 (GraphPad Software, San Diego, CA, USA). All the data were tested for normality using the Kolmogorov-Smirnov (K-S) test. The normally distributed data are presented as the mean ± standard deviation, and the non-normally distributed data are presented as the 50th percentile (P50; median) or P25–P75 (interquartile range). The t-test was used for pairwise comparisons, and the chi square test was used for counting data comparisons. A binary logistic regression analysis was used to calculate the probability of bacterial RTIs by the use of combined biomarker testing. First, the predicted probabilities of different combinations of markers were calculated in parallel by a binary logistic regression analysis. Second, the receiver operating characteristic (ROC) curves were constructed and the areas under the ROC curve (AUCs) were calculated to assess the diagnostic performance of each marker and their combinations. The chi-square test was used to compare the percentages. All tests were two-tailed and P<0.05 was considered significantly different.
Results
Patient clinical features
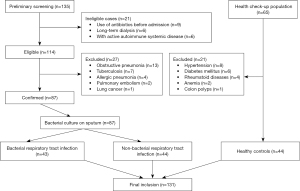
After the preliminary screening, 135 inpatients at the Department of Respiratory Medicine of our hospital were identified. After the history taking and physical examination, 21 ineligible patients and 27 patients with other pulmonary diseases were excluded from the study. Ultimately, 87 patients with bacterial RTIs were included in the study. Of the 65 individuals who received health check-ups, 21 were excluded from the study due to the presence of chronic diseases and 44 were included in the study. Thus, 131 subjects were included in the final analysis (see Figure 1). There were 43 patients (19 male and 24 female) aged 67.0–85.0 years (median: 74.0 years) in the bacterial infection group, 44 patients (18 male and 26 female) aged 66.0–80.0 years (median: 75.0 years) in the non-bacterial infection group, and 44 subjects (20 male and 24 female) aged 56.0–67.0 years (median: 63.0 years) in the healthy control group.
Comparison of plasma HBP, PCT, and CRP levels
The levels of inflammatory markers in each group were subjected to a K-S test, which showed that the P values in all groups were below 0.05, indicating that the data were non-normally distributed. Thus, P50 (P25, P75) was used to describe the levels of inflammatory markers in each group (see Table 1). The differences were statistically significant between the bacterial infection and non-bacterial infection groups, and between the bacterial infection group and the healthy control group.
Table 1
| Group | HBP (ng/mL) | PCT (ng/L) | CRP (mg/L) |
|---|---|---|---|
| Bacterial infection group | 30.01 (22.39, 44.75)* | 3.75 (2.04, 8.42)* | 23.09 (12.16, 40.27)* |
| Non-bacterial infection group | 16.66 (9.46, 21.95)# | 0.28 (0.04, 1.58)# | 13.08 (6.48, 18.95)# |
| Healthy control group | 8.54 (6.13, 12.16) | 0.05 (0.03, 0.80) | 7.22 (5.33, 9.19) |
*, Z=22.70, 25.08, 21.86; P<0.05, compared to the healthy control group; #, Z=22.09, 26.34, 6.39; P<0.05, compared to the healthy control group. HBP, heparin-binding protein; PCT, procalcitonin; CRP, C-reaction protein.
Results of ROC curve analysis for each marker
A ROC curve analysis was performed to assess the ability of the 3 markers (i.e., plasma HBP, PCT, and CRP) to diagnose bacterial RTIs (see Figure 2). The sensitivity, specificity, positive predictive value, negative predictive value, and AUC of each marker are set out in Table 2. The AUCs of all 3 markers were >0.85, indicating that these markers had good diagnostic ability to distinguish between patients with bacterial RTIs and healthy individuals.
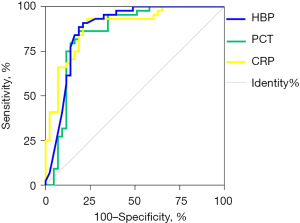
Table 2
| Marker | SEN | SPE | PPV | NPV | +LR | –LR | Odds ratio | Yuden | Cut-off | AUC |
|---|---|---|---|---|---|---|---|---|---|---|
| HBP (ng/mL) | 0.895 | 0.816 | 0.791 | 0.909 | 4.871 | 0.129 | 37.778 | 0.700 | 18.965 | 0.886 |
| PCT (ng/L) | 0.875 | 0.829 | 0.814 | 0.886 | 5.141 | 0.151 | 34.125 | 0.700 | 1.148 | 0.879 |
| CRP (mg/L) | 0.854 | 0.826 | 0.814 | 0.864 | 4.909 | 0.177 | 27.708 | 0.678 | 11.855 | 0.854 |
HBP, heparin-binding protein; PCT, procalcitonin; CRP, C-reaction protein; RTI, respiratory tract infection; SEN, sensitivity; SPE, specificity; PPV, positive predictive value; NPV, negative predictive value; +LR, positive likelihood ratio; –LR, negative likelihood ratio; AUC, areas under the receiver operating characteristic curve.
A ROC curve analysis was performed to assess the value of plasma HBP, PCT, and CRP in distinguishing between bacterial RTIs and non-bacterial RTIs, and the AUCs were calculated to assess their diagnostic performance (see Figure 3). The sensitivity, specificity, positive predictive value, negative predictive value, and AUC of each marker are set out in Table 3. The AUCs of all 3 markers were <0.8, indicating that these markers had limited value in distinguishing between patients with bacterial RTIs and those with non-bacterial RTIs.
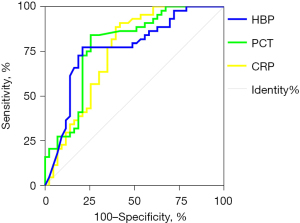
Table 3
| Marker | SEN | SPE | PPV | NPV | +LR | –LR | Odds ratio | Yuden | Cut-off | AUC |
|---|---|---|---|---|---|---|---|---|---|---|
| HBP (ng/mL) | 0.821 | 0.771 | 0.744 | 0.841 | 3.580 | 0.233 | 15.377 | 0.585 | 24.170 | 0.785 |
| PCT (ng/L) | 0.773 | 0.791 | 0.791 | 0.773 | 3.691 | 0.287 | 12.844 | 0.563 | 1.846 | 0.767 |
| CRP (mg/L) | 0.839 | 0.696 | 0.605 | 0.886 | 2.763 | 0.232 | 11.929 | 0.491 | 21.045 | 0.748 |
HBP, heparin-binding protein; PCT, procalcitonin; CRP, C-reaction protein; RTI, respiratory tract infection; SEN, sensitivity; SPE, specificity; PPV, positive predictive value; NPV, negative predictive value; +LR, positive likelihood ratio; –LR, negative likelihood ratio; AUC, areas under the receiver operating characteristic curve.
Correlation analysis
A binary logistic regression analysis was performed to investigate the relationships among these 3 inflammatory markers. Positive correlations were found among HBP, PCT, and CRP (see Table 4).
Table 4
| Marker | Item | HBP | PCT | CRP |
|---|---|---|---|---|
| HBP | Pearson correlation | 0.802** | 0.814** | |
| Significance (2-sided) | 0.000 | 0.000 | ||
| PCT | Pearson correlation | 0.802** | 0.646** | |
| Significance (2-sided) | 0.000 | 0.000 | ||
| CRP | Pearson correlation | 0.814** | 0.646** | |
| Significance (2-sided) | 0.000 | 0.000 |
**, significantly correlated at the 0.01 level (2-sided). HBP, heparin-binding protein; PCT, procalcitonin; CRP, C-reaction protein.
Diagnostic value of combined detection of HBP, PCT, and CRP in differentiating between bacterial and non-bacterial RTIs
The ROC curves of multiple combined detection markers were constructed based on the results of the binary logistic regression analysis (see Figure 4). The diagnostic performance of these combinations is set out in Table 5. The results showed that the combined HBP + CRP assay had the largest AUC.
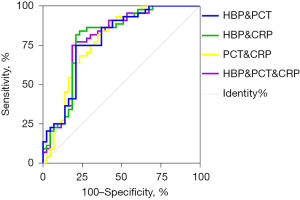
Table 5
| Marker | SEN | SPE | PPV | NPV | +LR | −LR | Odds ratio | Yuden | AUC |
|---|---|---|---|---|---|---|---|---|---|
| HBP + PCT | 0.756 | 0.786 | 0.791 | 0.750 | 3.526 | 0.311 | 11.333 | 0.541 | 0.783 |
| HBP + CRP | 0.809 | 0.800 | 0.791 | 0.818 | 4.048 | 0.238 | 17.000 | 0.609 | 0.797 |
| PCT + CRP | 0.778 | 0.706 | 0.651 | 0.886 | 2.762 | 0.232 | 11.929 | 0.469 | 0.780 |
| HBP + PCT + CRP | 0.761 | 0.804 | 0.814 | 0.750 | 3.899 | 0.297 | 13.125 | 0.564 | 0.794 |
HBP, heparin-binding protein; PCT, procalcitonin; CRP, C-reaction protein; SEN, sensitivity; SPE, specificity; PPV, positive predictive value; NPV, negative predictive value; +LR, positive likelihood ratio; –LR, negative likelihood ratio; AUC, areas under the receiver operating characteristic curve.
Discussion
Bacterial RTI is one of the most common and deadly conditions among hospitalized patients. The abuse of antibiotics and immunosuppressants in recent decades has led to an increase in antibiotic resistance, which has seriously affected the therapy outcomes (8). Thus, it is important to find biomarkers that can be used to diagnose bacterial RTIs early, accurately and effectively and to identify their advantages and limitations. The present study showed that the combined detection of HBP, PCT, and/or CRP was valuable in the early diagnosis of bacterial RTIs, which is helpful in guiding the rational use of antibiotics, reducing the mortality rate and hospitalization costs, and lowering the socioeconomic burden. We found that plasma HBP, PCT, and CRP were significantly increased in patients with bacterial RTIs and were significantly correlated with inflammation severity. Additionally, the ROC curve analysis revealed that the diagnostic performance was significantly improved by combining these inflammatory markers.
PCT and CRP are the commonly used markers in the clinical diagnosis of bacterial RTIs, and HBP is a relatively new marker. PCT, a protein of 116 amino acids, is the peptide precursor of calcitonin. It is present in very small amounts in the sera of normal individuals; however, it can be detected 2 hours after the onset of bacterial infection and reaches its peak in 6–8 hours (9). In our current study, the PCT level gradually increased with inflammation severity, as observed in the bacterial infection group, the non-bacterial infection group, and the healthy control group, and the differences between the groups were statistically significant. With a PCT cut-off value of 1.846 ng/L, the AUC was 0.767, with a sensitivity of 0.773, a specificity of 0.791, a positive predictive value of 0.791, and a negative predictive value of 0.773, for diagnosing bacterial RTIs. Notably, PCT level had a higher diagnostic specificity than the other markers. CRP is an acute-phase reactant produced in the liver. Generally, CRP secretion begins within 4–6 h of inflammation and peaks at 36–50 h. A variety of non-infectious factors (e.g., trauma, surgery, and burns) can cause an increase in CRP, which affects the sensitivity and specificity of CRP in the diagnosis of bacterial infections (4,10).
HBP, also known as azurocidin or CAP37, is a neutrophil-derived mediator of inflammation (11). HBP has anti-microbial activity, and its effect against bacteria is accomplished by binding to bacterial cells, followed by phagocytosis by monocytes. In addition to its antimicrobial properties, HBP also induces the chemotaxis of inflammatory cells and increases the anti-inflammatory response. Additionally, HBP alters vascular permeability (12-14). Blood HBP levels are extremely low in healthy individuals. When an infection occurs, some of the organisms invade the blood and activate neutrophils to release HBP, which leads to an increase in the blood HBP level (15-17). In our current study, the HBP levels differed significantly among the 3 groups and were closely correlated to the severity of the disease. With a HBP cut-off value at 24.170 ng/mL, the AUC was 0.785, with a sensitivity of 0.821, a specificity of 0.771, a positive predictive value of 0.744, and a negative predictive value of 0.841, for diagnosing bacterial RTIs. Notably, HBP had the highest diagnostic performance compared to the other 2 markers.
However, in the new era of precision medicine, any single marker, which is limited by its sensitivity and specificity, can fail to support an accurate diagnosis of a disease. Thus, the combinations of multiple markers are often required to meet the new requirements for the precise diagnosis and treatment of a specific disease. To identify the optimal combination for the diagnosis of bacterial RTIs, we adopted the method suggested by Doseeva et al. (18) in the present study. First, the predicted probabilities of different combinations of markers were calculated by a binary logistic regression analysis. Second, a ROC curve analysis was performed based on the predicted probabilities, and the diagnostic performance of the combined testing was compared by calculating the AUCs. The results showed that the combined HBP + CRP assay had the largest AUC. Thus, we concluded that the combined HBP + CRP test is the best combination for the diagnosis of bacterial RTIs, and may enable the rapid and efficient diagnosis of bacterial RTIs and thus inform clinical decision-making in a timely and effective manner.
Our study had some limitations. First, the small sample size limits the generalizability of our findings. Second, the possibility that some subjects used antibiotics and antipyretic-analgesics outside our hospital could not be completely ruled out. Third, all the subjects were elderly people and most of them had underlying conditions, such as hypertension and diabetes, and we cannot exclude the possibility that these underlying diseases affected the test results. In our future studies, we will increase the sample size, and engage in strict history taking to rule out the above-mentioned interfering factors.
Acknowledgments
Funding: This study was supported by the Sichuan Provincial Health and Family Planning Commission Scientific Research Program (No. 18PJ117).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-260/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-260/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-260/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was approved by the Medical Clinical Research Ethics Committee of Sichuan Provincial People’s Hospital, China (No. 2019–309-1). All the participants voluntarily participated in this study and signed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vitkina TI, Veremchuk LV, Mineeva EE, et al. The influence of weather and climate on patients with respiratory diseases in Vladivostok as a global health implication. J Environ Health Sci Eng 2019;17:907-16. [Crossref] [PubMed]
- Popova G, Boskovska K, Arnaudova-Danevska I, et al. Sputum Quality Assessment Regarding Sputum Culture for Diagnosing Lower Respiratory Tract Infections in Children. Open Access Maced J Med Sci 2019;7:1926-30. [Crossref] [PubMed]
- Paulsson M, Thelaus L, Riesbeck K, et al. Heparin-binding protein in lower airway samples as a biomarker for pneumonia. Respir Res 2021;22:174. [Crossref] [PubMed]
- Ayala-Lopez N, Peaper DR, Harb R. Procalcitonin Correlates With but Is Not Superior to Other Diagnostic Markers of Bacterial Pneumonia. Am J Clin Pathol 2021;155:537-46. [Crossref] [PubMed]
- Korppi M. Serum C-reactive protein is a useful tool for prediction of complicated course in children's pneumonia. Acta Paediatr 2021;110:1090-1. [Crossref] [PubMed]
- Isobe T. Guidelines for respiratory tract diseases. Nihon Naika Gakkai Zasshi 2009;98:2014-22. [Crossref] [PubMed]
- Miyashita N, Shimizu H, Ouchi K, et al. Assessment of the usefulness of sputum Gram stain and culture for diagnosis of community-acquired pneumonia requiring hospitalization. Med Sci Monit 2008;14:CR171-6. [PubMed]
- Viswanathan VK. Off-label abuse of antibiotics by bacteria. Gut Microbes 2014;5:3-4. [Crossref] [PubMed]
- Karakioulaki M, Stolz D. Biomarkers in Pneumonia-Beyond Procalcitonin. Int J Mol Sci 2019;20:2004. [Crossref] [PubMed]
- Póvoa P. C-reactive protein: a valuable marker of sepsis. Intensive Care Med 2002;28:235-43. [Crossref] [PubMed]
- Huttunen R, Syrjänen J. Heparin-binding protein: a potential biomarker in sepsis? Clin Infect Dis 2010;50:283-4; author reply 284-5. [Crossref] [PubMed]
- Linder A, Christensson B, Herwald H, et al. Heparin-binding protein: an early marker of circulatory failure in sepsis. Clin Infect Dis 2009;49:1044-50. [Crossref] [PubMed]
- Bentzer P, Fisher J, Kong HJ, et al. Heparin-binding protein is important for vascular leak in sepsis. Intensive Care Med Exp 2016;4:33. [Crossref] [PubMed]
- Fisher J, Linder A. Heparin-binding protein: a key player in the pathophysiology of organ dysfunction in sepsis. J Intern Med 2017;281:562-74. [Crossref] [PubMed]
- Linder A, Arnold R, Boyd JH, et al. Heparin-Binding Protein Measurement Improves the Prediction of Severe Infection With Organ Dysfunction in the Emergency Department. Crit Care Med 2015;43:2378-86. [Crossref] [PubMed]
- Kjölvmark C, Påhlman LI, Åkesson P, et al. Heparin-binding protein: a diagnostic biomarker of urinary tract infection in adults. Open Forum Infect Dis 2014;1:ofu004. [Crossref] [PubMed]
- Halldorsdottir HD, Eriksson J, Persson BP, et al. Heparin-binding protein as a biomarker of post-injury sepsis in trauma patients. Acta Anaesthesiol Scand 2018;62:962-73. [Crossref] [PubMed]
- Doseeva V, Colpitts T, Gao G, et al. Performance of a multiplexed dual analyte immunoassay for the early detection of non-small cell lung cancer. J Transl Med 2015;13:55. [Crossref] [PubMed]