Systematic review and meta-analysis of the correlation between plasma homocysteine levels and coronary heart disease
Introduction
In recent years, the incidence of coronary heart disease (CHD) has gradually increased. CHD seriously endangers human health, and its mortality rate has exceeded that of neoplastic diseases. Hyperlipidemia, smoking, diabetes and hypertension serve as traditional risk factors (1-3). However, 15–20% of patients with CHD still have no established risk factors and miss the opportunity for prevention (3). The relationship between plasma homocysteine (Hcy) and the prevalence of CHD has attracted attention, and it has been found that the level of plasma Hcy will have an impact on the incidence of CHD . Numerous studies have suggested that elevated Hcy may be an independent risk factor for CHD (4-6), but Armitage et al. (7) have a different view on this.
To reduce error of individual basic research, we summarized the data of plasma Hcy levels and the relationship between CHD using the method of meta-analysis. There have been relevant meta-analyses that have been previously reported, but studies in patients with known CHD may be affected by other risk factors, resulting in the inability to accurately reflect the direct relationship between Hcy and CHD. We included studies without confirmed CHD to objectively evaluate the relationship between the two conditions. We present the following article in accordance with the MOOSE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-78/rc).
Methods
Literature search strategy
Due to the relatively high level of relevant studies abroad, we searched the main foreign literature databases PubMed, Embase and Cochrane Library literature databases from the establishment of the database to October 2021. The search keywords were case-control studies, cohort studies, cardiovascular diseases, homocysteine, hyperhomocysteinemia and cystathionine beta-synthase. The search was performed by means of MeSH words combined with free words.
Literature screening
Inclusion criteria included cohort studies and case-control studies with a patient’s first diagnosis of CHD and documented plasma Hcy levels. The exclusion Criteria included case reports that were individual cases, duplicate data and studies, and studies in which participants were known to have definite CHD.
Risk of bias evaluation
The NOS scale was used to evaluate case-control studies and cohort studies, with a full score of 9 for 8 items. Including representativeness of the exposed cohort, selection of the nonexposed cohort, ascertainment of exposure, demonstration that outcome of interest was not present at start of start, comparability of cohorts on the basis of the design or analysis, assessment of outcome, was follow up long enough for outcomes to occur and adequacy of follow up of cohorts.
Data extraction
After the literature search, Endnote X9 software was used for unified processing. The duplicate check function was used to progressively screen all studies, which was independently performed by two evaluators. The titles and abstracts were read for preliminary screening, the full text was read after obtaining the original text, and the data from the literatures was extracted according to the inclusion and exclusion criteria. The extracted contents included the following: (I) basic information of the literature: title, author, publication date, contact address, and name of publication; (II) basic characteristics of study: study methods, total sample size of study, and number of groups; and (III) basic characteristics of participants: sex and age. In situations in which any dispute existed in the extraction of data and quality evaluation, the two evaluators negotiated or invited a third researcher to solve the difference in opinions.
Handling of missing data
If relevant data were not provided in the literature but could be obtained through calculation, they were retained. If there were no relevant data, the author could be contacted for acquisition of such data. If it was still unavailable, this literature was excluded.
Statistical analysis
Meta-analysis of the RR value and its 95% CI was performed by using Stata15.1 software to generate a forest plot. I² was used to evaluate the heterogeneity among the included studies. When I²>50%, it suggested that the results had great heterogeneity. A random effects model was used to perform sensitivity analysis and analyze the source of heterogeneity. When I²<50%, it suggested that the results had homogeneity. A fixed effects model was used. P values were used to determine whether the results had statistical differences, suggesting statistically significant when P<0.05 and homogeneous when P≥0.05. Sensitivity analysis was performed by excluding any literature one by one based on the effect of the pooled effect size to clarify the effect of the literature quality on heterogeneity factors. Publication bias was assessed using a funnel plot. To standardize the RRs, we estimated the RR value associated with an increase in Hcy by 5-µmol/L for each study.
Results
Literature search results
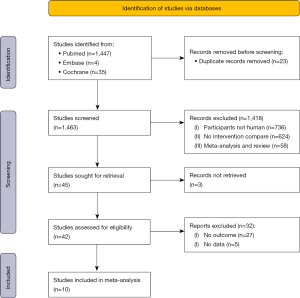
A total of 1,486 studies were searched using the above databases and search terms. A total of 1,463 studies were retrieved by Endnote X9 software. A total of 1,418 studies were excluded after reading the titles and abstracts, and 45 studies remained. Ten articles remained after full text reading and rescreening. Figure 1 shows the literature search results and screening process.
Basic characteristics of included literatures
According to the content correlation and the inclusion and exclusion criteria, 10 articles were finally included. A total of 10,103 subjects we included in these articles. After quality evaluation, all articles were of high quality according to the NOS scoring scale. The basic information of the literature is shown in Table 1.
Table 1
| Author | Year | Region | Sample size | Case/control | Follow-up time (y) | NOS score (pts) |
|---|---|---|---|---|---|---|
| Arnesen E (8) | 1995 | Norway | 600 | 122/478 | 4 | 6 |
| de Bree A (9) | 2003 | Ireland | 919 | 170/749 | 10.3 | 7 |
| Zylberstein DE (10) | 2004 | Sweden | 1,368 | – | 24 | 7 |
| Knekt P (11) | 2001 | Finland | 224 | 75/149 | 13 | 8 |
| Knekt P (12) | 2001 | Finland | 796 | 272/524 | 13 | 6 |
| Cui R (13) | 2008 | China | 444 | 134/310 | 10 | 6 |
| Ridker PM (14) | 2000 | Boston | 366 | 122/244 | 3 | 7 |
| Shai I (15) | 2004 | USA | 695 | 237/458 | 8 | 7 |
| Voutilainen S (16) | 2004 | USA | 2,682 | – | 8 | 6 |
| Sun Y (17) | 2009 | China | 2,009 | – | 12 | 7 |
NOS, Newcastle-Ottawa Scale.
Combined results of the RR value and its 95% CI
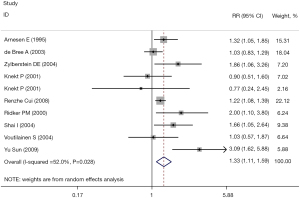
All studies reported the RR value and 95% CI of the correlation between the plasma Hcy level and CHD. The combined results showed that there was a significant correlation between the plasma Hcy level and the incidence of CHD (RR =1.33, 95% CI: 1.11, 1.59, I2=52.0%). The fixed effect model was selected (P=0.002), and the difference was statistically significant. The results are shown in Figure 2.
Sensitivity analyses
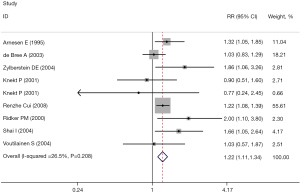
Sensitivity analysis was performed on the results, and the literature was excluded one by one. The heterogeneity was significantly reduced after excluding the literature by Yu Sun et al. (17) (RR =1.22, 95% CI: 1.11, 1.34, I2=26.5%; P<0.001), and the difference was statistically significant. These results are shown in Figure 3.
Risk of bias evaluation
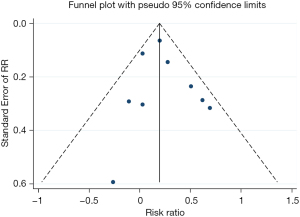
The funnel plot showed no significant publication bias in the results, as shown in Figure 4.
Discussion
Hcy is a hydroxy-containing amino acid generated by the demethylation of methionine in the liver, muscle and certain other tissues. It is mainly metabolized by the kidney and liver through two pathways: methylation or sulfation to methionine. The normal plasma Hcy concentration is 5–15 µmol/L, of which approximately 70–80% is bound to plasma proteins (mainly albumin), approximately 1% exists in the circulating blood as free thiol, and approximately 20–30% binds to the Hcy dimer by itself or binds to mixed disulfides of other thiols, such as cysteine to form Hcy-Cys. At present, it has been clarified that increasing age, low estrogen levels, deficiency in vitamins (VitB6, VitB12, folic acid) that act as coenzymes in the Hcy pathway, long-term smoking, alcohol consumption, renal insufficiency, hypothyroidism, tumors and drug reactions can lead to increased plasma Hcy levels. The Chinese Guidelines for the Prevention and Control of Hypertension, revised in 2018 (18), use total fasting plasma Hcy ≥15 µmol/L as the diagnostic criteria for hyperhomocysteinemia (HHcy). According to the degree of Hcy elevation, mild HHcy (Hcy 15–30 µmol/L), moderate HHcy (Hcy 31–100 µmol/L) and severe HHcy (Hcy >100 µmol/L) were classified.
The pathological basis of CHD is intimal injury of the coronary artery, endothelial cell barrier function damage after deposition, and smooth muscle proliferation. Furthermore, growth factors promote a chronic inflammatory response that leads to the final formation of an atheromatous plate. The pathogenesis of CHD induced by Hcy has not been fully elucidated, and there are several possible mechanisms. The first is through vascular endothelial cell injury and apoptosis. Intravascular cells are the barrier between smooth muscles and the blood, and they can also regulate local blood vessels by secreting vasoconstrictor and vasodilator substances. Nitric oxide is an important vasodilator secreted by endothelial cells. Elevated Hcy can promote hydrogen peroxide production, and peroxide accumulation inhibits the activity of nitric oxide lyase. This reduces NO production and impairs diastolic function, which causes endothelial cell function damage (19). Endothelial progenitor cells are precursor cells of vascular endothelial cells and contribute to the course of neovascularization and repair after vascular injury. Hcy inhibits the proliferation of endothelial progenitor cells by promoting the hypomethylation of cyclin A and inhibiting the expression of cyclin A. This leads to endothelial dysfunction and promotes the formation of atherosclerosis (20). The second possible mechanism involves smooth muscle cells (SMCs). SMCs are the main cells that constitute the tissue structure of the vascular wall and maintain tension. SMC proliferation leads to vascular wall thickening, structural wall destruction, and weakened diastolic function, which constitute the pathological basis of atherosclerosis. The third possible mechanism involves lipid metabolism disorders. Lipid metabolism disorders are important risk factors for atherosclerosis, of which an increase in low-density lipoprotein cholesterol (LDL-C) is the main factor causing atherosclerosis. At present, oxidized low-density lipoprotein (ox-LDL) is considered to be the most important atherosclerotic factor. High-density lipoprotein cholesterol (HDL-C) has a preventive effect on atherosclerosis. The fourth possible mechanism involves platelet adhesion. Platelets pretreated with Hcy and thiolactone had increased adhesion to collagen and fibrin and increased platelet adhesion (21). Platelets are activated after adhesion, followed by a release reaction and an adhesion reaction, accelerating thrombosis. For the fifth possible mechanism, Hcy can stimulate high-sensitivity C-reactive protein production by affecting the N-methyl-D-aspartate receptor-ROS-extracellular signal-regulated protein kinase 1/2 p38-nuclear factor κB pathway (22). C-reactive protein is one of the most important inflammatory mediators leading to atherosclerosis, and it can promote the progression of atherosclerosis by mediating the inflammatory response, stimulating endothelial cells to express adhesion molecules, and inhibiting NO production to damage endothelial cells. High levels of Hcy can promote the formation of early atherosclerosis by activating splenic T cells, increasing the secretion of related proinflammatory factors, and reducing the production of cytokines (23). The sixth possible mechanism involves Hcy-induced oxidative stress. This is an important factor in Hcy-induced coronary atherosclerosis, which is mainly caused by the imbalance of oxidative and antioxidant functions in organisms. It is speculated that Hcy is mainly involved in the formation of atherosclerosis through oxidative stress.
This meta-analysis showed the relationship between different levels of Hcy and CHD. This study showed that for every 5 µmol/L increase in Hcy, the risk of CHD-related events increased by approximately 20%. Because the quality of the included studies was high, it was considered that it could accurately measure the risk of CHD. There are still some limitations in the meta-analysis. We only rely on published data, which may only bias positive findings more. However, funnel plots do not show this bias and can only represent published data. Another bias is that we do not standardize the results evaluated in the study, which may lead to some bias.
Conclusions
In summary, elevated Hcy levels increase the risk of CHD by approximately 20%. Therefore, the subsample size is limited, and studies on the correlation between plasma Hcy levels and CHD still need to be conducted by including more studies with large sample sizes and better quality.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-78/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-78/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang S, Xie J, Xu Y, et al. Progress in the markers of sputum stasis in the field of TCM. Chinese Journal of Basic Medicine of Traditional Chinese Medicine 2021;27:1525-31.
- Huang Y, Yao S, Liu J, et al. Predictive value of non-fasting blood lipids for the diagnosis of CHD. The Chinese Medical Journal of the Coal Industry 2021;25:627-31.
- Smith SC Jr. Current and future directions of cardiovascular risk prediction. Am J Cardiol 2006;97:28A-32A. [Crossref] [PubMed]
- Humphrey LL, Fu R, Rogers K, et al. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin Proc 2008;83:1203-12. [Crossref] [PubMed]
- Liu L. Association of plasma homocysteine with coronary heart disease and its risk factors. Changchun: Jilin University; 2016.
- Li C. Correlation of CHD and homocysteine was explored. Chinese Journal of Metallurgical Industry Medicine 2019;36:308-9.
- Armitage JM, Bowman L, Clarke RJ, et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA 2010;303:2486-94. [Crossref] [PubMed]
- Arnesen E, Refsum H, Bønaa KH, et al. Serum total homocysteine and coronary heart disease. Int J Epidemiol 1995;24:704-9. [Crossref] [PubMed]
- de Bree A, Verschuren WM, Blom HJ, et al. Coronary heart disease mortality, plasma homocysteine, and B-vitamins: a prospective study. Atherosclerosis 2003;166:369-77. [Crossref] [PubMed]
- Zylberstein DE, Bengtsson C, Björkelund C, et al. Serum homocysteine in relation to mortality and morbidity from coronary heart disease: a 24-year follow-up of the population study of women in Gothenburg. Circulation 2004;109:601-6. [Crossref] [PubMed]
- Knekt P, Alfthan G, Aromaa A, et al. Homocysteine and major coronary events: a prospective population study amongst women. J Intern Med 2001;249:461-5. [Crossref] [PubMed]
- Knekt P, Reunanen A, Alfthan G, et al. Hyperhomocystinemia: a risk factor or a consequence of coronary heart disease? Arch Intern Med 2001;161:1589-94. [Crossref] [PubMed]
- Cui R, Moriyama Y, Koike KA, et al. Serum total homocysteine concentrations and risk of mortality from stroke and coronary heart disease in Japanese: The JACC study. Atherosclerosis 2008;198:412-8. [Crossref] [PubMed]
- Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836-43. [Crossref] [PubMed]
- Shai I, Stampfer MJ, Ma J, et al. Homocysteine as a risk factor for coronary heart diseases and its association with inflammatory biomarkers, lipids and dietary factors. Atherosclerosis 2004;177:375-81. [Crossref] [PubMed]
- Voutilainen S, Virtanen JK, Rissanen TH, et al. Serum folate and homocysteine and the incidence of acute coronary events: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 2004;80:317-23. [Crossref] [PubMed]
- Sun Y, Chien KL, Hsu HC, et al. Use of serum homocysteine to predict stroke, coronary heart disease and death in ethnic Chinese. 12-year prospective cohort study. Circ J 2009;73:1423-30. [Crossref] [PubMed]
- Revision Committee of the Chinese Guidelines for the Prevention and Control of Hypertension. Liu LS. Chinese Guidelines for the Prevention and Treatment of Hypertension (revised 2018). Prevention and Treatment of Cardiovascular Disease 2019;19:1-44.
- Esse R, Barroso M, Tavares de Almeida I, et al. The Contribution of Homocysteine Metabolism Disruption to Endothelial Dysfunction: State-of-the-Art. Int J Mol Sci 2019;20:867. [Crossref] [PubMed]
- Yang S. Role of TRPV1 in high homocysteine-promoting apoptosis in endothelial cells. Yinchuan: Ningxia Medical University; 2017.
- Malinowska J, Tomczynska M, Olas B. Changes of blood platelet adhesion to collagen and fibrinogen induced by homocysteine and its thiolactone. Clin Biochem 2012;45:1225-8. [Crossref] [PubMed]
- Pang X, Liu J, Zhao J, et al. Homocysteine induces the expression of C-reactive protein via NMDAr-ROS-MAPK-NF-κB signal pathway in rat vascular smooth muscle cells. Atherosclerosis 2014;236:73-81. [Crossref] [PubMed]
- Zhang Z, Wang L. Homocysteine was associated with CHD. China Cycle Magazine 2016;31:405-7.