
Partial upper sternotomy for aortic valve replacement provides similar mid-term outcomes as the full sternotomy
Introduction
The surgical aortic valve replacement through the upper partial sternotomy (MiniAVR) is a safe and effective alternative to the standard full sternotomy (FS) approach (1). It can be the procedure of choice not only for all primary isolated aortic valve operations but also for the combined surgical procedures on the ascending aorta and aortic arch (2). This approach can be used for elderly patients; it is generally suitable for obese patients and provides preferred access for the implantation of sutureless aortic valves (3,4). Many studies have demonstrated the short-term advantages of MiniAVR, the most important being less ventilation time, shorter hospital stay, less postoperative bleeding and lower consumption of catecholamines among patients operated via partial sternotomy (5-7). Preservation of the chest wall integrity with this type of approach should not be underestimated, as it provides a positive effect on the respiratory dynamics, a satisfactory cosmetic result, and an earlier return to normal activity after discharge, amongst other benefits to the patient (8,9). Although this minimally invasive approach should be counted among the basic skills every cardiac surgeon should possess, some authors point out the potential negative aspects of this approach: limited access used during aortic valve replacement may result in longer cardio-pulmonary bypass (CPB) and aortic cross-clamp times, with a potential negative effect on neurological and renal outcomes. The resulting longer surgical time could also have an adverse effect on certain subgroups of high-risk patients (10,11). However, the majority of the published mid- and long-term results focus on survival only (12), omitting the other aspects of the postoperative phase such as long-term morbidity expressed by the hospital readmission rate in patients undergoing aortic valve surgery. The advantages of the minimally invasive approach early on in the postoperative phase should be reflected in the mid-term results as well.
The aim of our study was to analyze the short- and mid-term results of MiniAVR versus FS with particular regard to the combined incidence of postoperative complications. A primary combined end-point was defined as: death, stroke and re-hospitalization due to any cause 3 years postoperatively. The rehospitalization rate due to any cause for the next 3 years after surgery was chosen as a secondary end-point. A subgroup of the rehospitalizations was identified as cardiovascular (CV) readmissions to the hospital (myocardial infarction, stroke, peripheral vascular disorder, valve-related pathology, dysrhythmias). We hypothesize, that MiniAVR should be associated with similar or lower incidence of primary combined end-point. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1494/rc).
Methods
Study population and clinical data
Between February 2014 and July 2016, two hundred consecutive patients underwent isolated aortic valve replacement with biological prosthesis at the Department of Cardiac Surgery, Kralovske Vinohrady University Hospital, Prague, Czech Republic. One hundred patients underwent surgery using the minimally invasive upper partial sternotomy technique (MiniAVR group) and 100 patients underwent a full sternotomy (FS group). Excluded from this study are patients who underwent a combined procedure, infective endocarditis with confirmed annular abscess, were operated on using any other minimally invasive approach, and all reoperations. All patients were operated on by experienced staff surgeons (J.H., P.B.).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by University Hospital Kralovske Vinohrady Institutional Ethics Committee (No. EK-VP/4010120) and informed consent was taken from all the patients. All data sources are subjected to the General Data Protect Regulation of the European Union.
The perioperative data was obtained from medical records. Clinical and echocardiographic follow-ups were completed in 97.5% of patients (median 589 days, IQR 906 days). The total clinical follow-up was 297 patient-years. The administrative follow-up was performed after 3 years in 100% of patients, based on information acquired from National Cardiac Surgery Registry and insurance companies. Only the patients who died during this period have the administrative follow-up shortened. As a result, the median follow-up in both groups was 3 years (mean 2.68 and 2.84 years in MiniAVR and FS group, respectively; range, 5–1,095 days). The total administrative follow-up was 551 patient-years. The first rehospitalization has been defined as the first rehospitalization after discharge from the hospital and the 3rd year after surgery. The 2nd rehospitalisation is the rehospitalization following the first-one but happened before the 3rd year after surgery. The second rehospitalization could happen only among the patients that have had the first-one. It is analogous to any further rehospitalization.
Surgical technique
After induction of general anesthesia, partial upper J-ministernotomy to the right 3rd or 4th intercostal space was performed through a 7–10 cm long skin incision using an oscillation saw, cutting the bone in two stages and taking care to avoid injury of the right internal thoracic artery. The selection of the intercostal space was based on the standard preoperative chest X-ray, performed in all cases. This position does not exclude the incision in the 3th intercostal space, if the aortic annulus is not situated more than 3 cm under the level of planed cut (13). For better exposure, the thymus fat tissue was removed, and pericardial suspension stitches were placed under the retractor. After administration of a full dose of heparin, direct aortic cannulation of the ascending aorta or proximal aortic arch with Select Series Angled TipTM arterial cannula (Medtronic, Inc., Minneapolis, MN, USA) and venous cannulation of the right atrial appendage with oval double-stage cannula VC2TM (Medtronic, Inc., Minneapolis, MN, USA) was performed. To prevent increased blood return from the pulmonary circulation the pulmonary vent is always used, placed at the root of the artery. After cross-clamping the ascending aorta, St. Thomas cardioplegic solution was administered: non-selective into the aortic root in case of aortic stenosis or selective directly into the coronary ostia in the setting of relevant aortic insufficiency. Three types of stented bioprostheses (Perimount®, Edwards Lifesciences Corp., Irvine, CA, USA; Trifecta®, Abbott Park, IL, USA, Mitroflow®, LivaNova, UK) were implanted with running monofilament polypropylene 3/0 suture. The sutureless prostheses (3F Enable®, Medtronic Inc., Minneapolis, MN, USA; and Perceval®, LivaNova, UK) were implanted as recommended by the company. All patients were operated in normothermic conditions. Epicardial pace-maker electrodes were placed on the surface of the right ventricle before removing the cross clamp to gain access to the right ventricle and to prevent its injury. Atrial pacing has not been routinely used. The pericardial space in patients with partial upper sternotomy was drained with Blake drains® (Ethicon, Somerville, NJ, USA), which were placed circumferentially behind the left ventricle with its tip ending at the root of pulmonary artery.
Statistical analysis and outcomes definitions
Valve-related outcomes were defined per published guidelines (14). Continuous and discrete variables are expressed as mean ± SD or median and range for data not normally distributed. Since the Shapiro-Wilk test showed the distribution of the specified continuous variables is not normal, they are characterized by the median and the interquartile range (IQR). Categorical and ordinal variables are expressed by number and percentage of observations. Continuous and discrete variables were compared using a two-sample t-test or Mann-Whitney test, where appropriate. Categorical and ordinal variables were compared using Pearson’s Chi-squared test or Fischer’s exact test, where appropriate. The rehospitalizations were analyzed by Prentice, Williams, and Peterson [1981], presuming that a subject is not at risk of a second event until the first event has occurred and so on. The chosen statistical model is a natural generalization of the Cox’s proportional hazards model. The variation of the model with time to each event measured from the previous event (gap time) was used. Unlike other survival analysis models used for recurrent events, this model assumes that events within subjects are correlated (15). Data was limited to five rehospitalizations in order to keep the risk set large enough. Competing risks regression model according to the method of Fine and Gray [1999] was used to compare cardiologic indications occurrence for rehospitalization in the presence of other competing indications (16). Software syntax described in Amorim et al. was used (17). The probability of freedom from event was calculated according to the Kaplan–Meier method. Freedom-from-event curves were compared by log-rank test. A P value <0.05 was considered to indicate statistical significance. Data were analyzed using a statistical software Stata, release 14.2 (StataCorp LP, College Station, TX, USA). The patients, among them the partial sternotomy had to be extended to the 4. intercostal space, were not marked as a “conversion”.
Results
Patients
Between February 2014 and July 2016, two hundred consecutive patients underwent isolated aortic valve replacement with a biological prosthesis. One hundred patients were operated with minimally invasive technique, and 100 patients operated with a full sternotomy (FS group). Data and preoperative clinical characteristics are summarized in Table 1. Overall MiniAVR group has less often arterial hypertension (73% vs. 90%, P=0.003) and 2.65% lower ejection fraction on average (P=0.05). Other categories have no statistically significant differences.
Table 1
| Patients characteristics | MiniAVR (n=100) | FS (n=100) | P value |
|---|---|---|---|
| Age (years), median (IQR) | 73.5 (12.0) | 72.0 (9.0) | 0.353 |
| Gender (female), n (%) | 52 (52.0) | 52 (52.0) | 1 |
| BMI (kg/m2), median (IQR) | 29.1 (6.2) | 29.7 (8.6) | 0.489 |
| IHD, n (%) | 22 (22.0) | 21 (21.0) | 1 |
| EF, mean (SD) | 58.55 (8.51) | 55.90 (10.38) | 0.050 |
| MI, n (%) | 4 (4.0) | 9 (9.0) | 0.251 |
| AH, n (%) | 73 (73.0) | 90 (90.0) | 0.003 |
| IDDM, n (%) | 6 (6.0) | 6 (6.0) | 1 |
| Stroke, n (%) | 14 (14.0) | 7 (7.0) | 0.165 |
| COPD, n (%) | 14 (14.0) | 13 (13.0) | 1 |
| EuroScore II, median (IQR) | 1.48 (1.25) | 1.17 (0.92) | 0.146 |
MiniAVR, upper partial sternotomy; FS, full sternotomy group; IQR, interquartile range; BMI, body mass index; IHD, ischemic heart disease; EF, ejection fraction of the left ventricle; SD, standard deviation; MI, myocardial infarction; AH, arterial hypertension; IDDM, insuline dependent diabetes mellitus; COPD, chronic obstructive pulmonary disease.
Perioperative and early postoperative outcomes
The intraoperative characteristics of both groups are provided in the Table 2. The cross-clamp and bypass times were longer in MiniAVR group, despite the fact, that in FS group no sutureless valves were used. The total operative time was also longer in the MiniAVR group, but this was not statistically significant. The size and type of biological prostheses used is provided in Table 3. We opted for an unplanned extension to the 4th ICS in 5 patients because of very long aorta, compressing the right atrium, which prevented safe venous cannulation. There were 2 conversions from the MiniAVR to the FS group because of periprocedural bleeding from the right ventricle, injured during pace-maker electrode implantation and during the insertion of the pericardial drainage. These patients were included in the MiniAVR group, and the conversion was rated among the complications. The patients operated using the minimally invasive technique showed significantly lower need for inotropes. The incidence of trivial paravalvular leak (PVL), not requiring reintervention and diagnosed at discharge, was observed also less often in MiniAVR group. The average postoperative bleeding was statistically significant lower in the MiniAVR group: mean 389 mL (SD 209.5) vs. 412 mL (SD 322), median 300 mL (IQR, 290) and 365 mL (IQR, 207), P=0.031 (Mann-Whitney test). On the other hand, the median number of the transfusion units applied did not differ relevant between the groups. Other serious postoperative complications such as stroke, myocardial infarction, early re-exploration surgery because of severe bleeding or heart tamponade, or new onset of continuous hemodialysis were statistically insignificant (Table 4). High-grade atrioventricular block requiring pacemaker (PM) implantation immediately after surgery or until day 30 after surgery did not reach statistical significance. Furthermore, later in the postoperative phase (longer than 30 POD) the surgical approach has no influence on the new PM implantation (1% vs. 2%). Severe pericardial effusion occurred in 7 cases, 2 in the MiniAVR group, 5 in the full sternotomy group (P=0.445) respectively, requiring CT- or TTE-controlled puncture.
Table 2
| Operative times | MiniAVR, median (IQR) | FS, median (IQR) | P value |
|---|---|---|---|
| Total surgery time (min) | 135.0 (27.5) | 131.5 (25.0) | 0.094 |
| CPB time (min) | 58.0 (20.0) | 49.5 (13.5) | 0.002 |
| Cross-clamp time (min) | 46.5 (15.0) | 40.0 (10.5) | 0.005 |
MiniAVR, upper partial sternotomy; IQR, interquartile range; FS, full sternotomy; CPB, cardio-pulmonary bypass.
Table 3
| Trademark of the valve | MiniAVR | FS |
|---|---|---|
| Enable® | 16 | 0 |
| Mitroflow® | 55 | 24 |
| Perceval® | 20 | 0 |
| Perimount® | 0 | 29 |
| Trifecta® | 9 | 47 |
| Size | ||
| Median (IQR) | 23.0 (2.0) | 23.0 (4.0) |
| P value | 0.312 | |
MiniAVR, upper partial sternotomy; FS, full sternotomy; IQR, interquartile range.
Table 4
| Complication | MiniAVR (%) | FS (%) | P value |
|---|---|---|---|
| Death | 2 | 2 | 1 |
| Stroke | 5 | 4 | 1 |
| Myocardial infarction | 1 | 0 | 1 |
| New CVVHD | 4 | 0 | 0.121 |
| New atrial fibrillation | 53 | 54 | 1 |
| Median blood loss (mL) | 300 | 365 | 0.031 |
| Median PRBCs (units) | 2.0 | 2.0 | 0.247 |
| Need of inotropes | 21 | 47 | 0.001 |
| Wound healing disturbance | 0 | 3 | 0.246 |
| New pacemaker | 5 | 2 | 0.445 |
| Re-thoracotomy (bleeding/heart tamponade) | 6 | 6 | 1 |
| Pleural puncture | 9 | 11 | 0.814 |
| Pericardial effusion | 21 | 18 | 0.721 |
| Trivial PVL | 4 | 13 | 0.040 |
| Sepsis | 3 | 0 | 0.246 |
| Median length of stay | 7 | 7 | 0.680 |
MiniAVR, upper partial sternotomy; FS, full sternotomy; CVVHD, continuous veno-venous hemodialysis; PRBCs, packed red blood cells; PVL, paravalvular leak.
Primary outcomes
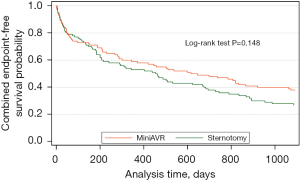
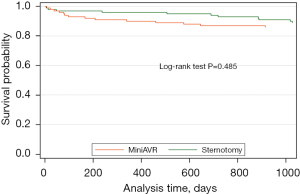
The primary combined end-point occurred in 62% patients in MiniAVR group and in 73% patients in FS group (HR =0.78, 95% CI: 0.55–1.09, P=0.148) (Figure 1). Mid-term mortality according to the type of operation is shown in Figure 2. The 3-year estimated survival was 86% and 89% for MiniAVR and FS group (P=0.485) (Figure 2), respectively.
At least one re-admission to the hospital has been reported in 70% of patients with FS and 58% from the MiniAVR group. The total number of rehospitalizations in FS group was 165 and 160 among patients operated using partial upper sternotomy. Consequently, the mean number of readmissions per capita was almost the same in both groups (1.65 vs. 1.60, P=0.836). The median time between the operation and the first rehospitalization is distinctly longer among the patients with limited surgical access compared to the FS group [736 days (95% CI: 446–1,072) vs. 469 days (95% CI: 240–694) respectively] although formally does not reach the statistical significance (P=0.096). Based on the results of competing risk analysis, there is no statistically significant difference in cardiologic indications for the first rehospitalization between the groups (i.e., MiniAVR vs. FS), after adjusting for indications of other types (subhazard ratio 0.90, P=0.693). Analysis of cumulative incidence functions suggests that there is no substantial difference even for further rehospitalizations. Both probability and the time distribution of readmission due to any reason during the next 3 years after aortic valve replacement were not influenced by surgical strategy (Figure 3). For clarity, only the first three rehospitalizations are provided.
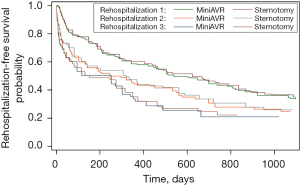
Only one readmission due to valve prosthesis complications was observed in each group (P=1). In both cases: prosthetic thrombosis occurred, were successfully treated pharmacologically, and did not require any surgical re-intervention. There was only one late deep wound infection observed after day 30 among the patients operated with partial sternotomy, resulting in the unfortunate death of the patient. No late deep wound infection occurred in the FS group.
Discussion
In this retrospective study, we compared short- and mid-term results in patients undergoing aortic valve replacement via partial upper ministernotomy versus full sternotomy. In comparison to other studies, we focused on morbidity leading to rehospitalization. There is a lot of evidence concerning mortality after AVR. Far less attention has been paid to the morbidity among patients successfully treated and discharged from hospital. We hypothesize, that upper ministernotomy is associated with the same or lower long-term morbidity expressed by the incidence of hospital readmissions in the next 3 years after primary aortic valve surgery. We compared two similar groups of patients operated using two different surgical approaches (MiniAVR vs. FS). The high volume of octogenarians (19% vs. 28%) and women (both 52%) represents the “real-world surgery”. We decided for a retrospective analysis of the patients requesting biological aortic valve prosthesis, because the implantation of the mechanical ones is indicated for a limited group of younger patients and associated with specific complications related to the long-term coumarin administration. Following the meta-analysis published by Brown et al., we were also able to show a significant reduction of cross-clamp and CPB-time in the FS group, however, the reduction of 8.5 and 6.5 min, respectively seems to be clinically unimportant (5). Despite the use of rapid deployment valve prostheses (RDPV) (Perceval®- LivaNova, United Kingdom and 3F Enable®-Medtronic Inc., Minneapolis, MN, USA) in the MiniAVR group only, the bypass time was longer among these patients and are in line with most of the published studies (5,18,19). The learning curve in RDPV-implantation could negatively influence final cross-clamp times. Considerably shorter bypass and cross-clamp time compared to other studies (12,20,21) has been reached by using of monofilament 3/0 running suture by implantation of stented biological prostheses (22). Furthermore, the implantation of the ATS 3f Enable® (also defined as RDPV) in the minimally invasive group did not really reduce the time of implantation compared to our results, as a multicentric study in 10 European centres published by Martens et al. has shown: “mean aortic cross-clamp and cardiopulmonary bypass times were noted as 58.1±25.1 and 84.9±34.2 min, respectively” (23). Szwerc et al. published in their study higher use of inotropes in the MiniAVR group, explaining that fact with an imperfect local cooling (24). Our findings do not support this. In our cohort the higher use of inotropes in FS group could be associated with a lower ejection fraction of the left ventricle observed among these patients (58.55% vs. 55.90%, P=0.050). However, the 2.65% of average difference does not fully explain the need for inotropic support. This study did not prove any reduction of severe postoperative events among patients operated through partial upper sternotomy, in contrast to the meta-analysis carried out by Phan et al., identifying 50 comparative studies with total of 12,786 patients, demonstrating significant lower incidence of renal failure (25). However, authors of this study acknowledge that the various surveys used might not have been that statistically robust. The length of total hospital stay was not reduced in the group of partial upper sternotomy in agreement with Kirmani et al. (26), influenced most probably by our department specific practice. Thus, in our retrospective analysis, we compared a long-term effect of the partial upper J-shaped right-sided sternotomy from the morbidity point of view, assuming that long-term mortality per se cannot be the only indicator of the risks (17). The 3-years mortality of 14% and 11%, respectively among patients with a mean age of 73 years is expected and could be only marginally compared to other studies, including patients more than 10 years younger (12,27). Furthermore, as Doll et al. have established, when other risk factors are considered such as age, hypertension, and CPB time, minimally invasive access cannot be considered as a predictor of survival any longer (20). The quality of life after minimally invasive aortic valve replacement has been previously classified in a questionnaire (8-QOL, EQ-5D), based on the subjective evaluation of patients with potential for substantial bias (12,27,28). The frequency of readmissions in the early and mid-term period after surgery can be better reflected in the problems faced by the patients. As mentioned above, the surgical strategy has no impact on the number of rehospitalizations three years after surgery (Figure 3). In addition, the combination of death and stroke within the same period did achieve the level of significant difference (P=0.146). However, there is an obvious tendency to detect better results in the MiniAVR group of patients. The median time of the first re-admission almost nine months in some, seems to be a benefit resulting from the minimally invasive approach. Especially on the assumption that the longer from the hospital discharge the most likely the readmission is not related to the surgery. Nevertheless, our sub-analysis of the first readmissions from cardiovascular (CV) and other reasons cannot prove the higher risk of CV-related rehospitalizations in either FS or MiniAVR patients. Furthermore, our retrospective analysis confirms published results by Detter et al., providing a very low incidence of valve-related complications after aortic valve surgery in the mid-term perspective, regardless of the used technique (27).
Limitations
There are several limitations of this study: this was a retrospective, non-randomized, observational, single center study and all inherent disadvantages apply. Next, the study is based on a single-center experience, which can influence some results by local clinical practice. Finally, the number of patients in both groups was limited and the use of self-expandable sutureless valves was limited to the mini sternotomy group. This may lead to misinterpretation of calculated results.
Conclusions
This study confirmed the effectiveness and safety of aortic valve replacement through upper partial sternotomy, bringing some partial advantages in the early postoperative course, such as a lower use of transfusions and the generally low rate of complications. The minimally invasive surgical access in aortic valve replacement is associated with similar mid-term mortality and morbidity in terms of the rehospitalization rate. The results suggest that the minimally invasive aortic valve replacement tends to have better results 3 years postoperatively. Further studies with a larger patient population, randomized controlled design and long-term follow-up are needed to further elucidate this important issue.
Acknowledgments
Many thanks to Dr. Marie-Claire Smulova, who, as a native speaker, proofread the whole article.
Funding: The article was supported by Charles University Program (UNCE MED 02 and PROGRES Q38).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1494/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1494/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1494/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1494/coif). PT reports that he is supported by the scientific grant program of the Charles University Prague, Czech Republic (UNCE MED 02 and PROGRES Q38) and in the past received a part-time salary for lectures and presentations for the firms, Medtronic, B Braun and Abiomed. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by University Hospital Kralovske Vinohrady Institutional Ethics Committee (No. EK-VP/4010120) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raja SG, Benedetto U, Amrani M. Aortic valve replacement through J-shaped partial upper sternotomy. J Thorac Dis 2013;5:S662-8. [PubMed]
- Deschka H, Erler S, Machner M, et al. Surgery of the ascending aorta, root remodelling and aortic arch surgery with circulatory arrest through partial upper sternotomy: results of 50 consecutive cases. Eur J Cardiothorac Surg 2013;43:580-4. [Crossref] [PubMed]
- Martens S, Zierer A, Ploss A, et al. Sutureless Aortic Valve Replacement via Partial Sternotomy. Innovations (Phila) 2010;5:12-5. [Crossref] [PubMed]
- Welp HA, Herlemann I, Martens S, et al. Outcomes of aortic valve replacement via partial upper sternotomy versus conventional aortic valve replacement in obese patients. Interact Cardiovasc Thorac Surg 2018;27:481-6. [Crossref] [PubMed]
- Brown ML, McKellar SH, Sundt TM, et al. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2009;137:670-679.e5. [Crossref] [PubMed]
- Murtuza B, Pepper JR, Stanbridge RD, et al. Minimal access aortic valve replacement: is it worth it? Ann Thorac Surg 2008;85:1121-31. [Crossref] [PubMed]
- Cosgrove DM 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596-7. [Crossref] [PubMed]
- Walther T, Falk V, Metz S, et al. Pain and quality of life after minimally invasive versus conventional cardiac surgery. Ann Thorac Surg 1999;67:1643-7. [Crossref] [PubMed]
- Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997;226:421-6; discussion 427-8. [Crossref] [PubMed]
- Malaisrie SC, Barnhart GR, Farivar RS, et al. Current era minimally invasive aortic valve replacement: techniques and practice. J Thorac Cardiovasc Surg 2014;147:6-14. [Crossref] [PubMed]
- Walther T, Falk V, Mohr FW. Minimally invasive surgery for valve disease. Curr Probl Cardiol 2006;31:399-437. [Crossref] [PubMed]
- Aliahmed HMA, Karalius R, Valaika A, et al. Efficacy of Aortic Valve Replacement through Full Sternotomy and Minimal Invasion (Ministernotomy). Medicina (Kaunas) 2018;54:26. [Crossref] [PubMed]
- Klein P, Klop IDG, Kloppenburg GLT, et al. Planning for minimally invasive aortic valve replacement: key steps for patient assessment. Eur J Cardiothorac Surg 2018;53:ii3-8. [Crossref] [PubMed]
- Akins CW, Miller DC, Turina MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. J Thorac Cardiovasc Surg 2008;135:732-8. [Crossref] [PubMed]
- Yadav CP, Srenivas V, Khan MA, et al. An Overview of Statistical Models for Recurrent Event Analysis: A Review. Epidemiology (Sunnyvale) 2018;8:354. [Crossref]
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509. [Crossref]
- Amorim LD, Cai J. Modelling recurrent events: a tutorial for analysis in epidemiology. Int J Epidemiol 2015;44:324-33. [Crossref] [PubMed]
- Glauber M, Ferrarini M, Miceli A. Minimally invasive aortic valve surgery: state of the art and future directions. Ann Cardiothorac Surg 2015;4:26-32. [PubMed]
- Fudulu D, Lewis H, Benedetto U, et al. Minimally invasive aortic valve replacement in high risk patient groups. J Thorac Dis 2017;9:1672-96. [Crossref] [PubMed]
- Doll N, Borger MA, Hain J, et al. Minimal access aortic valve replacement: effects on morbidity and resource utilization. Ann Thorac Surg 2002;74:S1318-22. [Crossref] [PubMed]
- Gilmanov D, Bevilacqua S, Murzi M, et al. Minimally invasive and conventional aortic valve replacement: a propensity score analysis. Ann Thorac Surg 2013;96:837-43. [Crossref] [PubMed]
- Kitamura T, Edwards J, Miyaji K. Continuous Suture Technique for Aortic Valve Replacement Shortens Cross-Clamp and Bypass Times. Tex Heart Inst J 2017;44:390-4. [Crossref] [PubMed]
- Martens S, Sadowski J, Eckstein FS, et al. Clinical experience with the ATS 3f Enable® Sutureless Bioprosthesis. Eur J Cardiothorac Surg 2011;40:749-55. [Crossref] [PubMed]
- Szwerc MF, Benckart DH, Wiechmann RJ, et al. Partial versus full sternotomy for aortic valve replacement. Ann Thorac Surg 1999;68:2209-13; discussion 2213-4. [Crossref] [PubMed]
- Phan K, Xie A, Di Eusanio M, et al. A meta-analysis of minimally invasive versus conventional sternotomy for aortic valve replacement. Ann Thorac Surg 2014;98:1499-511. [Crossref] [PubMed]
- Kirmani BH, Jones SG, Malaisrie SC, et al. Limited versus full sternotomy for aortic valve replacement. Cochrane Database Syst Rev 2017;4:CD011793. [Crossref] [PubMed]
- Detter C, Deuse T, Boehm DH, et al. Midterm results and quality of life after minimally invasive vs. conventional aortic valve replacement. Thorac Cardiovasc Surg 2002;50:337-41. [Crossref] [PubMed]
- Borger MA, Moustafine V, Conradi L, et al. A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. Ann Thorac Surg 2015;99:17-25. [Crossref] [PubMed]


