
Tracheal stenosis as a complication of prolonged intubation in coronavirus disease 2019 (COVID-19) patients: a Peruvian cohort
Introduction
In December of 2019, a new coronavirus [coronavirus disease 2019 (COVID-19)] caused acute severe respiratory disease in China. World Health Organization (WHO) declared the novel coronavirus outbreak a public health emergency of international concern at the end of January 2020 (1). The most common and severe complication of patients with COVID-19 infection is a severe acute respiratory syndrome (SARS) requiring oxygen and ventilation therapies (2). According to some studies published until June 2020, the proportion of patients in intensive care unit (ICU) that required invasive mechanical ventilation (IMV) range between 29.1–64% among China population (3). In one study made in Wuhan, China, 26.1% of hospitalized patients were admitted to ICU because of complications that included SARS (61.1%), shock, and arrhythmias; and 47.2% of patients admitted to ICU required IMV (4). It has been demonstrated that COVID-19 patients had mean mechanical ventilation duration of 17 days and a high rate of reintubation (5,6).
Mortality among patients with IMV and treatment in the ICU has always been high. This rate increases if we add comorbidities and COVID-19 infection. In the UK, 65% of patients with COVID-19 in IMV died; and in the USA the number could go as high as 88% (3,7).
Tracheal stenosis (TS) is a complication that appears after prolonged intubation or performing a tracheostomy. The pathologic pathway of TS is due to prolonged ischemia that produces fibrotic tracheal scarring (8). For COVID-19 patients, where last up to as a mean of 17 days and many times requiring reintubation, TS after tracheostomy is around 1.5–2.6%, and 1–2% for cases with orotracheal intubation (9). Usually, these complications appear in patients with intubation or tracheostomy of more than 10 days (10). Factors that increase the risk of developing concentric TS are obesity, size of the endotracheal tube, the material of the endotracheal tube, and use of corticosteroids. The main symptoms are dyspnea, inspiratory stridor, and non-productive cough (11).
Peru is one of the most affected countries due to COVID-19 in all Latin America, with an incidence of 23.57 newly diagnosed cases per 1,000 population during the month September 2020 (12). It has; also, the highest mortality rate worldwide, 601.6 deaths per million population (13). This scenario is aggravated by the lack of healthcare providers and ICU providers, noting that Peru has the lowest indexes of ICU providers, ICU beds, and mechanical ventilators per 10,000 patients in South America (14).
So far, there have not been studies published that show the association between TS and COVID-19 infection. We present our case series and a revision of the literature of COVID-19 patients who developed TS after prolonged IMV and were managed in our hospital. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1721/rc).
Methods
Design, population, and sample size
The Service of Thoracic Surgery of Guillermo Almenara Irigoyen National Hospital is a national referral center for the surgical management of tracheal pathology. We developed a retrospective study of all the patients referred to our center with a diagnosis of TS, tracheoesophageal fistula (TEF), and tracheomalacia (TM) who were previously intubated due to COVID-19 infection and they underwent surgery between the months from June 2020 until May 2021.
Data collection and study variables
A total of 63 patients with TS, or other complications such as TEF and TM, were surgically managed. We obtained demographic data like age, sex, body mass index (BMI), previous pathologic history (arterial hypertension, diabetes mellitus, asthma), time of endotracheal intubation, time of hospitalization in ICU, functional class. Additionally, we obtained data of the TS: TS percentage, number of damaged rings, location of damaged rings, maturity of the mucosa from the TS, presence of subglottic involvement.
The maturity criteria of TS were established through bronchofibroscopy. Immature TS: erythema, granulomas, friability, ulcer, bleeding mucosa and mature TS: pale mucosa, established scar.
To define functional class, we have used the WHO scale “Classification of Functional State of the World Health Organization”:
WHO I: asymptomatic: ordinary physical activity does not cause symptoms.
WHO II: symptomatic on exertion: there is no discomfort at rest, but normal physical activity, causes increased symptoms.
WHO III: symptomatic with daily activity: there is no discomfort at rest, but less than ordinary activity causes increased symptoms.
WHO IV: symptomatic at rest: symptoms may be present at rest and are increased by almost any physical activity.
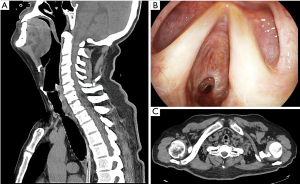
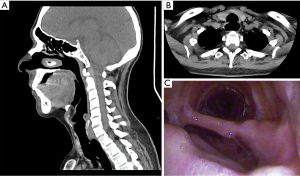
In respect of the presence of TEF, we obtained: the size of TEF, anatomic ubication, maturity. In respect of TM, localization and severity were obtained. Each patient was evaluated pre-surgically with a physical exam, lab analysis, antigenic test for COVID-19, cervicothoracic tomography with trachea-bronchial reconstruction, and fiberoptic bronchoscopy for evaluation of the mobility of vocal cords and characteristics of the TS such as severity, maturity, localization, size (Figure 1), and associated lesions like TEF and TM. When TEF was suspected, an upper endoscopic was made. We defined severe TS as the one occluding more than 50% of the tracheal lumen, or if it produced symptoms like dyspnea, laryngeal stridor. TEF was defined as the epithelized pathological communication between the trachea and esophagus, that was seen in the tomography, fiberoptic bronchoscopy, and confirmed with an upper endoscopy (Figure 2).
Ethical aspects
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Ethics Committee of NIT (No. 1141-2021-123), and individual consent for this retrospective analysis was waived.
Surgical technique
After pre-surgical evaluation, surgical options were tracheoplasty, Montgomery T tube placement (with or without tracheal resection), tracheostomy cannula placement (with or without tracheal resection) and TEF closure.
The choice was made considering the extent of injury (number of damaged rings), involvement of the subglottic region, the elasticity of the trachea, presence of associated lesions (TEF or TM), previous procedures in stenotic areas (dilatation, tracheostomy, laser) and the overall status of the patient.
The criteria for tracheal resection were extension of the stenosis <50% of the tracheal length, that the location of the stenosis does not cover the subglottic region, the elasticity of the trachea and functional class of the patient I–II according to WHO scale, however, the final decision was made intraoperatively.
The specific criteria for each type of surgical technique were the following:
Tracheostomy: patients admitted in poor general condition or functional class III–IV according to WHO scale.
T-tube placement: patients without criteria for tracheal resection, extensive and subglottic lesions.
Tracheal resection + tracheostomy: patients with ET + TEF with TS and poor general condition.
Tracheal resection + T-tube: patients with risk anastomosis, extension of lesion >50% of length.
Tracheoplasty: patients in good general condition, lesions <50% in length.
A T-shaped cervical incision was made. Infrahyoid muscles were separated from the midline. The anterior, posterior, and lateral part of the trachea is mobilized with blunt dissection and dissection with a scalpel for a better exposure and with care not to damage the recurrent laryngeal nerves.
Damaged tracheal rings are removed. Tracheal anastomosis was made with polyglycolic acid suture, stitches began in the lateral part of the cartilaginous trachea with 3/0 polyglycolic acid suture that allowed us proper tension. After that, stitches of 4/0 polyglycolic acid suture were placed on the opposite site of the surgeon with an interval of 3 mm.
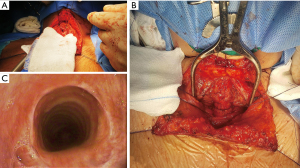
A nasogastric tube was introduced through the proximal trachea, and we asked the anesthesiologist to pass the endotracheal ringed tube for placement under direct vision below the anastomosis. Separate stitches were placed with 3/0 polyglycolic acid suture. Hemostasia was revisited, and a Jackson Pratt drainage was left on the pre-tracheal plane. (Figure 3).
In those patients where after tracheal ring resection, it was noticed inflammatory immature tissue, extensive damage, disease at the level of cricoid or subglottic, extensive damage of more than 50% of the trachea, we opted for the placement of a Montgomery T tube, verifying proper positioning in its proximal end and distal one, with intraoperative flexible fiberoptic bronchoscopy.
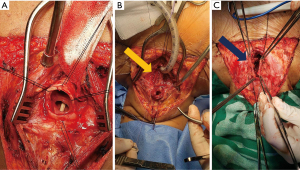
All TEF were managed through cervical T incision, the trachea was dissected circumferentially, the fistula was identified, separation of the trachea from the esophagus (Figure 4A) then two-layer planes are sutured: separate stitches by 3 mm with 4/0 polyglycolic acid suture in mucosae layer and then for the muscular layer invaginating stitches with 3/0 polyglycolic acid suture (Figure 4B), then muscle (sternocleidomastoid and/or prethyroid muscles) is placed between trachea and esophagus being sutured over the esophageal defect, later on the treatment of the tracheal defect is continued (Figure 4C).
Statistical analysis
A descriptive analysis was made. Continuous variables were shown as median and interquartile range, categoric variables were shown as percentages. Statistical analysis was made with Microsoft Excel 2016, STATA MP, and SPSS v25.
Results
Data from 63 patients diagnosed with TS due to prolonged intubation associated with COVID-19 infection were reviewed. The mean age was 49.8 (±10.4) years old, most diagnosed in men (74.6%). Mean BMI was 30.4 kg/m2, being grade I obesity the most frequent (39.7%). The main comorbidities were arterial hypertension (17.5%), diabetes mellitus (14.3%), asthma (3.2%). The meantime of hospitalization in the ICU was 30 days. The average orotracheal intubation time was 25 days. Most patients were classified as functional class II (50.8%) and functional class I (31.7%). Admission diagnoses were TS (42 patients), TS + TEF (15 patients), TS + TM (1 patients), TS + TEF + TM (5 patients) (Table 1).
Table 1
| Variables | N | Percentage (%) |
|---|---|---|
| Age (years) | 49.8 | (±10.4) |
| 30–39 | 10 | (15.9) |
| 40–49 | 22 | (34.9) |
| 50–59 | 19 | (30.2) |
| 60–69 | 10 | (15.9) |
| >70 | 2 | (3.2) |
| Gender | ||
| Male | 47 | (74.6) |
| Female | 16 | (25.4) |
| Comorbidities | ||
| Arterial hypertension | 11 | (17.5) |
| Diabetes mellitus | 9 | (14.3) |
| Asthma | 2 | (3.2) |
| BMI (kg/m2) | ||
| Normal (18.5–24.9) | 7 | (11.1) |
| Overweight (25–29.9) | 23 | (36.5) |
| Obesity I (30–34.9) | 25 | (39.7) |
| Obesity II (35–39.9) | 4 | (6.3) |
| Obesity III (>40) | 4 | (6.3) |
| Time in ICU (days) | 35.9 | (±22.3) |
| Intubation time (days) | 27.8 | (±15.8) |
| Time from hospital discharge to tracheal surgery | 3.4 | (±1.7) |
| WHO functional class | ||
| I | 20 | (31.7) |
| II | 32 | (50.8) |
| III | 8 | (12.7) |
| IV | 3 | (4.8) |
| Admission diagnosis | ||
| TS | 42 | (66.7) |
| TS + TEF | 15 | (23.8) |
| TS + TM | 1 | (1.6) |
| TEF + TS + TM | 5 | (7.9) |
| Vocal cord paralysis (diminished mobility) | ||
| Unilateral | 5 | 7.9 |
| Bilateral | 2 | 3.2 |
| Previous procedures | ||
| Dilatation | 1 | 1.6 |
| TCT | 21 | 33.3 |
| None | 41 | 65.1 |
COVID-19, coronavirus disease 2019; TS, tracheal stenosis; BMI, body mass index; ICU, intensive care unit; WHO, World Health Organization; TEF, tracheoesophageal fistula; TM, tracheomalacia; TCT, tracheostomy.
Regarding patients with TS, the most frequent anatomical localization was upper and middle third (55.6%), upper third (44.4%) (Figure 5). Fifty-three patients (84.1%) had a TS with a length of 1–4 cm, and ten patients (15.9%) had a TS with a length >4 cm. Most patients with TS were classified as Cotton-Myer grade III (88.9%). Mature stenosis was seen in 73% of patients with TS. Diminished unilateral mobility of the vocal cords was seen in 5 patients (7.9%), and it was bilateral in 2 patients (3.2%) (Table 2).
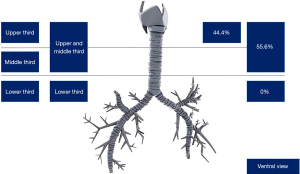
Table 2
| Variable | N | Percentage (%) |
|---|---|---|
| TS | 63 | |
| Localization (thirds) | 63 | 100.0 |
| Upper | 28 | 44.4 |
| Upper and middle | 35 | 55.6 |
| Vertical length (cm) | ||
| 1–4 | 53 | 84.1 |
| >4 | 10 | 15.9 |
| Cotton-Myer | ||
| I | 3 | 4.8 |
| II | 3 | 4.8 |
| III | 56 | 88.9 |
| IV | 1 | 1.6 |
| Mature | 46 | 73.0 |
| Immature | 17 | 27.3 |
| Subglottic | 7 | 11.1 |
| TEF | 20 | |
| Size (cm) | 3.2±1.5 | |
| Localization to the trachea | 20 | 100.0 |
| Upper | 17 | 85.0 |
| Middle | 2 | 10.0 |
| Upper middle | 1 | 5.0 |
| Mature | 15 | 75.0 |
| Immature | 5 | 25.0 |
| Nutrition | ||
| Gastrostomy | 4 | 6.3 |
| Jejunostomy | 5 | 7.9 |
| NJT | 12 | 19.0 |
| TM | 6 | |
| Localization to the trachea | 6 | 100.0 |
| Upper | 4 | 66.7 |
| Middle | 1 | 16.7 |
| Upper middle | 1 | 16.7 |
| Severity | 6 | 100.0 |
| Mild | 3 | 50.0 |
| Moderate | 1 | 16.7 |
| Severe | 2 | 33.3 |
| Surgical management | ||
| TS | 63 | 100.0 |
| Primary surgery | 30 | 47.6 |
| Ring resection + T tube | 25 | 39.7 |
| Ring resection + TCT | 4 | 6.3 |
| T tube insertion | 1 | 1.6 |
| TCT canula insertion | 3 | 4.8 |
| Resected tracheal rings | 6.9 | ±1.6 |
| TEF | 20 | 100.0 |
| Muscle interposition | 14 | 70.0 |
| Sternocleidomastoid | 1 | 5.0 |
| Sternothyroid | 6 | 30.0 |
| Sternohyoid | 2 | 10.0 |
| Omohyoid | 5 | 25.0 |
| Tracheomalacia | 6 | 100.0 |
| Plication | 3 | 50.0 |
COVID-19, coronavirus disease 2019; TS, tracheal stenosis; TEF, tracheoesophageal fistula; NJT, nasojejunal tube; TM, tracheomalacia; TCT, tracheostomy.
Regarding patients with TEF, the mean size was 3.2±1.5 cm, most of them were localized in the upper third (85%), with 75% of them being mature. Nutrition in these patients was made through a nasojejunal tube (19%). Regarding patients with TM, localization was in the upper third (66.7%), and 50% were mild.
Primary tracheoplasty was made in 30 patients (47.6%), ring resection plus insertion of Montgomery T tube in 25 patients (39.7%), ring resection plus insertion of tracheostomy cannula in 4 patients (6.3%), insertion of Montgomery T tube alone in 1 patient (1.6%) and insertion of tracheostomy cannula in 3 patients (4.8%). The average amount of tracheal rings removed was 6.9±1.6. In patients with TEF, full direct closure was made in all patients, in 14 of them (70%) direct closure plus muscular flap interposition, being the sternothyroid muscle the most frequently used. Additionally, repair of tracheomalacia was made in 3 patients.
Regarding immediate post-operative complications, 17 patients (27%) had superficial surgical site infection (SSI) and only 1 patient (1.6%) had a deep SSI. Two patients (3.2%) had partial dehiscence of the tracheoplasty suture, while 2 patients also (3.2%) had partial dehiscence of the suture that closed the TEF. We had 2 patients with non-severe anterior wall tracheal anastomosis dehiscence who were managed conservatively with observation and antibiotic therapy in both cases, and spontaneous closure was achieved.
Besides, there was two tracheoesophageal fistula closure dehiscence; one was mild that was managed with observation, antibiotic therapy, enteral nutrition, and spontaneous closure was achieved; and the second presented significant fistula dehiscence, a tracheostomy tube was placed and currently is awaiting corrective surgery.
Six patients (9.5%) had restenosis and seven patients (11.1%) presented T tube obstruction. Concerning the patients who presented restenosis, two patients underwent grade I Cotton-Myer restenosis in whom the management was conservative, three patients underwent grade II Cotton-Myer restenosis in which a T-tube was changed in one patient and a T-tube was placed in the other two patients; finally, one patient presented grade III Cotton-Myer restenosis in whom a T-tube was placed.
Of the 7 patients in whom T-tube obstruction was presented, fiberoptic bronchoscopy was performed in 5 patients, T-tube replacement in 1 patient, and placement of a tracheostomy cannula in 1 patient. Among other complications, 2 patients had hospital-acquired pneumonia (3.2%), 2 patients had bleeding (3.2%) and from all patients, only 1 patient (1.6%) died in the postoperative (Table 3). The deceased patient was a 61-year-old male, overweight, who had severe COVID-19 infection in March 2020, a patient referred from a less capacity hospital with diagnoses of TS and tracheoesophageal fistula.
Table 3
| Complications | N | Percentage (%) |
|---|---|---|
| Infection | ||
| Superficial | 17 | 27.0 |
| Deep | 1 | 1.6 |
| TS dehiscence | 2 | 3.2 |
| TEF dehiscence | 2 | 3.2 |
| Re-stenosis | 6 | 9.5 |
| Severity | ||
| Cotton-Myer | ||
| I | 2 | 3.2 |
| II | 3 | 4.8 |
| III | 1 | 1.6 |
| IV | 0 | 0 |
| Treatment | ||
| Observation | 2 | |
| T tube insertion | 3 | |
| T tube change | 1 | |
| T tube obstruction | 7 | 11.1 |
| Treatment | ||
| Fibro bronchoscopy | 5 | |
| T tube rechange | 1 | |
| TCT canula | 1 | |
| Other complications | ||
| Pneumonia | 2 | 3.2 |
| Bleeding | 2 | 3.2 |
| Death | 1 | 1.6 |
COVID-19, coronavirus disease 2019; TS, tracheal stenosis; TEF, tracheoesophageal fistula; TCT, tracheostomy.
He came emergency with stridor and in poor general condition and underwent emergency surgery resection of tracheal rings + closure of tracheoesophageal fistula and tracheostomy, awaiting definitive corrective surgery, after improving his clinical condition, he died of an acute myocardial infarction 2 months after.
Discussion
TS is a disease caused by ischemic necrosis associated with airway pressure. The main factor that causes TS is the pressure of the cuff in the tracheal mucosa; where a cuff pressure of more than 30 mmHg results in a decrease in capillary perfusion on the tracheal mucosa, which produces ischemia of the mucosa, inflammation of the tracheal cartilages; furthermore, more inflammation and fibrosis of several grades of circumferential distribution causes TS (15).
Before the COVID-19 pandemic it had been established that ischemic lesion on the tracheal mucosa could happen even in the next minutes after cuff insufflation with fibrotic changes around the 3–6 upcoming weeks. The incidence of TS after endotracheal intubation in the ICU is 6–21%, although only 1–2% of them are symptomatic (16). The time where symptoms initiated after TS goes from 28 days to 6 months according to previous cases (17-19). It should be suspected the risk of development of TS especially in patients that have been under mechanical ventilation for more than 10 days (7). Likewise, many risk factors associated with TS after endotracheal intubation have been studied like the size of the endotracheal tube, traumatic intubation, concomitant infection, female gender, estrogenic effect, obesity, and smoking (20,21).
Besides, various investigations were published evaluating patients with TS associated with prolonged intubation, which developed over more than 10 years, while our series presented a similar number of cases in 1 year (Table 4), which shows a significant increase in TS cases, highlighting the impact of COVID-19 infection on prolonged intubation time and the incidence of tracheal injuries (22-26).
Table 4
| Author* | Paris et al. | Rea et al. | Sarper et al. | Weindenbecher et al. | Andrilli et al. | Palacios et al. (our study) |
|---|---|---|---|---|---|---|
| Research | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort |
Retrospective cohort | Retrospective cohort |
| Year of publication | 1990 | 2002 | 2005 | 2007 | 2008 | 2022 |
| Range of years | 1973–1989 | 1991–2001 | 1985–2004 | 1985–2002 | 1991–2006 | 2020–2021 |
| Number of years | 16 years | 10 years | 19 years | 17 years | 16 years | 1 year |
| Number of patients | 112 | 65 | 45 | 101 | 35 | 63 |
| Male | 44 | 39 | 34 | 55 | 19 | 47 |
| Female | 68 | 26 | 11 | 46 | 16 | 16 |
| Ratio M/F | 0.65/1 | 1.5/1 | 3.1/1 | 1.2/1 | 1.2/1 | 2.93/1 |
| Age mean | 41 | 33 | 38 | NR | 43 | 49 |
| Age range (years) | 9–81 | 14–74 | 2–72 | 7–77 | 14–71 | 30–77 |
| IMV (days) | NR | NR | 8–11 | NR | NR | 25 |
| Tracheal lesions associated, n (%) | ||||||
| Tracheomalacia | 7 (6.3) | NR | NR | NR | NR | 6 (9.5) |
| Tracheoesophageal fistula | 3 (2.7) | NR | NR | NR | NR | 20 (31.7) |
| Vocal cord paralysis | NR | NR | NR | 12 (11.8) | NR | 7 (11.1) |
| Surgery technique | ||||||
| Resected tracheal length (cm) | 2.7 (1.5–7) | 2.5 (1.5–4) | 1.5–4 | 2–6 | 1.5–6 | 3.5 (2–5) |
| Previous treatment, n (%) | ||||||
| Tracheal dilatation | NR | NR | NR | NR | NR | 1 (1.6) |
| Tracheostomy | 28 (25.0) | 38 (38.5) | NR | NR | 13 (37.1) | 21 (33.3) |
| Tracheal complications, n (%) | ||||||
| Infection | NR | 5 (8.0) | 2 (6.0) | NR | NR | 3 (8.4) |
| Dehiscence | NR | 4 (6.0) | NR | NR | NR | NR |
| Granuloma | NR | 2 (3.0) | 3 (9.0) | NR | NR | NR |
| Death | NR | 1 (1.5) | 1 (3.0) | NR | NR | 1 (1.6) |
*, all the authors mentioned in the table have been cited in the article references. M, male; F, female; IMV, invasive mechanical ventilation; NR, no reported.
With the COVID-19 pandemic, where longer times of mechanical ventilation have been required, the use of prone position in a systematic way for the management of this kind of patients (27,28), and differed tracheostomies to more than 10–12 days (29), there has been a significant rise in the number of cases and complexity of tracheal pathology in patients with severe COVID-19 infection.
Recommendations regarding the time to perform either surgical or percutaneous tracheostomy in patients with COVID-19 infection varied widely. Many sources lacked a clear recommendation regarding time. When tracheostomy timing was established, it ranged from 3–4 to 21–28 days. Most protocols recommended a minimum of 14 days of MV for consideration of performing a tracheostomy. Likewise, some contraindications were considered, such as cardiac or respiratory instability, positive COVID-19 test, poor prognosis, and lack of clinical improvement (30). Subsequently, a study was performed where they proposed to delay the tracheostomy until the maximum of day 10 of MV, and it is considered only when patients show signs of clinical improvement (31).
In our study, we have evaluated clinical characteristics, anatomical distribution, and surgical management done in patients who developed TS after prolonged intubation. Some isolated case reports have been published, but to this date, there is not enough data or a big series that shows the association between TS and COVID-19 infection (9-11,32-35). Regarding TS findings, we had a mean age of 49 years old, being more frequently diagnosed in males (74.6%). Mean BMI was 30.4 kg/m2, being grade I obesity the most frequent (39.7%). The meantime of hospitalization in the ICU was 30 days. The mean time of orotracheal intubation was 25 days.
Our results show patients with TS developed more complex lesions, with longer stenosis percentages, more time hospitalized in the ICU, and more time with endotracheal intubation.
Comparing the incidence by gender, we found a male/female ratio of 2.93/1, which is higher than reported by D’Andrilli et al. (1.2/1) (22) and by Rea et al. (1.5/1) (25). This information could be due to the higher prevalence of severe COVID-19 in male patients. Paris et al. (26) conducted a retrospective cohort of patients with TS before the COVID-19 pandemic, where most patients were female (60.8%). The mean age found in our cohort was 49 years, which is consistent with the most frequent age range affected by COVID-19 infection (adult: 30–59 years).
TS associated with COVID-19 implies a longer IMV time; Sarper et al. published a series of cases with 45 patients with ST where the IMV interval was 8 to 11 days (24), which is shorter compared to our IMV range which was 27±15.8 days. This could also be due to the delayed tracheostomy time in patients with endotracheal intubation at the onset of the pandemic for up to >14 days.
París et al. reported 7 cases (6.3%) of tracheomalacia and 3 cases (2.7%) of tracheoesophageal fistula (26), unlike our cohort where we found 6 cases (9.5%) of associated tracheomalacia and 20 cases (31.7%) of associated tracheoesophageal fistula. We can observe more severe lesions in cases of post-intubation TS due to COVID-19, probably due to a longer intubation time, inflammatory process due to COVID-19 in the airway, poor care of the intubated patient, prone position, hypoxic mucosal damage, poor medical condition, excessive use of corticosteroids, history of diabetes and obesity. Knowing these findings, we propose to reinforce preventive measures for associated injuries such as proper care of the endotracheal tube, cuff pressure measurement (<30 mmHg), use of a soft silicone tube, chronic avoidance of systemic steroids, and early tracheostomy.
We present 7 cases (11.1%) of unilateral or bilateral vocal cord paralysis (decreased mobility), like that reported by Weidenbecher et al. (23). However, six patients presented FTE as an associated aggregate. The resected length in our cohort was 3.5 cm on average, greater than that reported by Rea et al. and París et al., with a length of 2.7 and 2.5 cm respectively (25,26); confirming the greater severity concerning the extension of the tracheal compromise, which leads to a lower probability of tracheal resection in patients with a history of COVID-19 infection. This is probably due to the inflammatory component of the tracheal mucosa exerted by viral invasion, as well as increased inflammation and fibrosis at the level of the tracheal cartilages.
In our cohort, a tracheostomy was performed as a treatment before tracheal reconstruction surgery in 21 patients (33.3%), like a pre-pandemic series report. D’Andrilli et al. and París et al. (22,26). Tracheostomies were performed in patients in poor general condition (WHO functional class III–IV), those with TEF to ensure airway, and some patients were referred to our center with tracheostomy for reconstructive surgery. Procedures before definitive surgery (CO2 laser, tracheal dilation, tracheostomy, stent placement) could decrease the probability of successful airway reconstruction.
Among the post-surgical complications, we found 18 cases (28.6%) of SSI, higher than that reported in other series with a range of 6–8% (22,24,25). However, of this group, 17 patients had superficial access which was resolved with antibiotic coverage and local cure. We only had one case of deep operative site infection that required surgical cleaning in the operating room. This high incidence of infection at the surgical site may be because they were patients with prolonged stay in the ICU, with bacterial colonization, and malnourished. Half of these cases occurred in patients with associated TEF.
The dehiscence rate in our study was 6.4%, like the pre-pandemic study by Rea et al. (6%) (25). These patients also had SSIs, which probably contributed to the development of dehiscence. This complication increases morbidity and hospital stay.
Regarding restenosis, it had a rate of 9.5%, probably due to a tension anastomosis, wide dissection due to an inflammatory process that caused devascularization of the anastomosis area, as well as the extension of the resected tracheal segment (3–4 cm).
In our study, natural ventilation, digestive tract restoration, and voice recovery were restored in 59 patients (93.7%) compared with other series published in the literature (good results reported between 87% and 100%) (22,24,25). Confirming the role of surgery as the treatment of choice in tracheal pathology. Furthermore, we only reported one patient who died (1.6%) due to a cause unrelated to the surgery.
TEF is pathologic communications between the esophagus and trachea, these can be congenital or acquired; the latter is the most frequent and is more commonly reported in patients with esophagus or lung cancer complications (36). Acquired TEF can also be formed due to prolonged intubations. In this setting, they are usually reported between 0.3% to 3% of patients (37). Is because of that, TEF can be developed in patients with COVID-19 and SARS who need mechanical ventilation (38). Symptoms are frequent cough after eating, bronchitis or purulent pneumonia, and recurrent aspiration; in COVID-19 patients with FET, gastric distention and sudden desaturation after extubating can be the first sign of suspicion (36,37).
TS, TEF, and other complications after endotracheal intubation are more frequent in COVID-19 patients probably because of prolonged intubation times, delay in performing a tracheostomy, lack of trained staff in ICU, prolonged prone position, and physiopathologic mechanisms of COVID-19 infection such as prothrombotic and antifibrinolytic status, high viral replication in the trachea epithelium that weakens its mucosa and high doses of systemic steroids that lead to mucosa atrophy (11,36).
Regarding patients that developed TEF, from 63 patients with TS, 20 patients develop TEF. The average size was 3.2±1.5 cm, the most common localization was upper third (85%) and most of them were mature TEF (75%). The nutrition of patients was possible through a nasojejunal tube (19%). Management was made by direct closure in all 20 patients, while in 14 patients (70%) a direct closure plus muscular flap interposition (with sternothyroid muscle). Two patients (3.2%) developed dehiscence of the surgery site.
Tracheal injuries due to endotracheal intubation in COVID-19 have different characteristics compared to patients with tracheal injuries due to intubation in the pre-COVID-19 era, such as an increase in the number of cases of tracheal injuries due to prolonged intubation in patients COVID-19, the highest prevalence in males, probably related to the greater distribution of severe COVID-19 in males. The average age of patients ranges between 30–51 years, which coincides with the age with the highest prevalence of severe COVID-19 infection. The lesions are more severe, with a higher prevalence of associated lesions such as TEF, tracheomalacia, and vocal cord paralysis. The extent of the lesion is on average greater than that reported in previous studies (3.5 cm).
For primary repair of the trachea, favorable prognostic factors are age, sex, size of the tracheal damage, trachea elasticity, presence of associated lesions (TEF, TM); general status of the patient and presence of diminished unilateral or bilateral mobility of vocal cords. It seems reasonable to predict that ventilation with positive pressure required in COVID-19 patients with the consequent prolonged intubation and late tracheostomy, in conditions of a COVID-ICU, along with airway inflammation by vasculitis phenomena, associated with the collapse of the health system all around Latin America and in our country, plus lack of ICU-trained staff are factors that increases the incidence of tracheal damage, not only in number but in complexity also with several associated lesions.
Limitations
The main limitations were the retrospective nature of our cohort, the absence of a control group of patients with tracheal pathology without COVID-19 infection, and the relatively short follow-up time after surgery.
Conclusions
TS is an airway pathology associated with both mechanical damages due to prolonged intubation and inflammatory damage due to COVID-19 infection. This condition can be associated with tracheoesophageal fistula, tracheomalacia, among others. We present a retrospective study of 63 patients who underwent corrective surgery: cervical tracheoplasty, Montgomery T-tube, or tracheostomy. This report is the first published cohort of patients with TS after COVID-19 infection in Peru; and one of the first series reported in Latin America, confirming the role of surgery as the treatment of choice in tracheal pathology.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1721/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1721/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1721/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1721/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). 2020.
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475-81. [Crossref] [PubMed]
- Wunsch H. Mechanical Ventilation in COVID-19: Interpreting the Current Epidemiology. Am J Respir Crit Care Med 2020;202:1-4. [Crossref] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [Crossref] [PubMed]
- Orebaugh S, Snyder JV. Direct laryngoscopy and endotracheal intubation in adults. UpToDate 2020.
- Möhlenkamp S, Thiele H. Ventilation of COVID-19 patients in intensive care units. Herz 2020;45:329-31. [Crossref] [PubMed]
- Zareifopoulos N, Lagadinou M, Karela A, et al. Intubation and mechanical ventilation of patients with COVID-19: what should we tell them? Monaldi Arch Chest Dis 2020; [Crossref] [PubMed]
- Mattioli F, Marchioni A, Andreani A, et al. Post-intubation tracheal stenosis in COVID-19 patients. Eur Arch Otorhinolaryngol 2021;278:847-8. [Crossref] [PubMed]
- Gervasio CF, Averono G, Robiolio L, et al. Tracheal Stenosis After Tracheostomy for Mechanical Ventilation in COVID-19 Pneumonia - A Report of 2 Cases from Northern Italy. Am J Case Rep 2020;21:e926731. [Crossref] [PubMed]
- Vasanthan R, Sorooshian P, Sri Shanmuganathan V, et al. Laryngotracheal stenosis following intubation and tracheostomy for COVID-19 pneumonia: a case report. J Surg Case Rep 2021;2021:rjaa569.
- Ramalingam H, Sharma A, Pathak V, et al. Delayed Diagnosis of Postintubation Tracheal Stenosis due to the Coronavirus Disease 2019 Pandemic: A Case Report. A A Pract 2020;14:e01269. [Crossref] [PubMed]
- MoHoP. National center for epidemiology, prevention, and control of diseases-Ministry of Health of Peru (MHP). Daily Report "COVID-19". 2020.
- Johns Hopkins University (JHU). COVID-19 dashboard by the center for Systems Science and Engineering (CSSE). 2020.
- OPS/OMS PSpAdS. Monitoring of the Response of South American Countries to the COVID-19 Pandemic. 2020.
- Grillo HC. Surgery of the trachea and bronchi. Shelton: PMPH USA, 2004.
- Dutau H, editor. Tracheal stenosis endoscopic treatment. In: Proceedings of the 12th world congress for Bronchology. Monduzzi Editore, 2002.
- Liu J, Zhang CP, Li Y, et al. Post-intubation tracheal stenosis after management of complicated aortic dissection: a case series. J Cardiothorac Surg 2015;10:148. [Crossref] [PubMed]
- Fernández Vaquero MA, Bartolomé Cela E, Villegas Fernández FR. Review of the post-intubation tracheal stenosis: a case report. Med Intensiva 2009;33:301-5. [PubMed]
- Terashima H, Sakurai T, Takahashi S, et al. Postintubation tracheal stenosis; problems associated with choice of management. Kyobu Geka 2002;55:837-42. [PubMed]
- Esteller-Moré E, Ibañez J, Matiñó E, et al. Prognostic factors in laryngotracheal injury following intubation and/or tracheotomy in ICU patients. Eur Arch Otorhinolaryngol 2005;262:880-3. [Crossref] [PubMed]
- Vandemoortele T, Laroumagne S, Bylicki O, et al. Endobronchial treatment of complete tracheal stenosis: report of 3 cases and description of an innovative technique. Ann Thorac Surg 2013;95:351-4. [Crossref] [PubMed]
- D'Andrilli A, Ciccone AM, Venuta F, et al. Long-term results of laryngotracheal resection for benign stenosis. Eur J Cardiothorac Surg 2008;33:440-3. [Crossref] [PubMed]
- Weidenbecher M Jr, Weidenbecher M, Iro H. Segmental tracheal resection for the treatment of tracheal stenoses. HNO 2007;55:21-8. [Crossref] [PubMed]
- Sarper A, Ayten A, Eser I, et al. Tracheal stenosis aftertracheostomy or intubation: review with special regard to cause and management. Tex Heart Inst J 2005;32:154-8. [PubMed]
- Rea F, Callegaro D, Loy M, et al. Benign tracheal and laryngotracheal stenosis: surgical treatment and results. Eur J Cardiothorac Surg 2002;22:352-6. [Crossref] [PubMed]
- París F, Borro JM, Tarrazona V, et al. Management of non-tumoral tracheal stenosis in 112 patients. Eur J Cardiothorac Surg 1990;4:265-8; discussion 268-9. [Crossref] [PubMed]
- Martín Delgado MC, Avilés-Jurado FX, Álvarez Escudero J, et al. Consensus document of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC), the Spanish Society of Otorhinolaryngology and Head and Neck Surgery (SEORL-CCC) and the Spanish Society of Anesthesiology and Resuscitation (SEDAR) on tracheotomy in patients with COVID-19 infection. Med intensiva 2020;44:493-9. [PubMed]
- Dubin A, Estenssoro E. Consideraciones sobre la traqueotomía en pacientes con COVID-19 en ventilación mecánica: momento de su indicación, técnicas, cuidados especiales. Sociedad Argentina de Terapia Intensiva 2020.
- Daniel Rappoport W, Tomás Gonzalez A, Felipe Capdeville F, et al. Traqueostomía en pacientes con COVID-19: Recomendaciones actuales. Revista de cirugía 2020;72:449-54.
- Bier-Laning C, Cramer JD, Roy S, et al. Tracheostomy During the COVID-19 Pandemic: Comparison of International Perioperative Care Protocols and Practices in 26 Countries. Otolaryngol Head Neck Surg 2021;164:1136-47. [Crossref] [PubMed]
- McGrath BA, Brenner MJ, Warrillow SJ, et al. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med 2020;8:717-25. [Crossref] [PubMed]
- Martínez-Téllez E, Orús Dotú C, Trujillo-Reyes JC, et al. Tracheotomy in patients COVID-19: A necessary high risk procedure. Two center experience. Arch Bronconeumol 2020;56:673-4. (Engl Ed). [Crossref] [PubMed]
- Lucchi M, Ambrogi M, Aprile V, et al. Laryngotracheal resection for a post-tracheotomy stenosis in a patient with coronavirus disease 2019 (COVID-19). JTCVS Tech 2020;4:360-4. [Crossref] [PubMed]
- Alturk A, Bara A, Darwish B. Post-intubation tracheal stenosis after severe COVID-19 infection: A report of two cases. Ann Med Surg (Lond) 2021;67:102468. [Crossref] [PubMed]
- Miwa M, Nakajima M, H, Kaszynski R, et al. Two Cases of Post-intubation Laryngotracheal Stenosis Occurring after Severe COVID-19. Intern Med 2021;60:473-7. [Crossref] [PubMed]
- Majid A, Kheir F. Tracheo-and broncho-esophageal fistulas in adults. UpToDate 2020.
- Roomi S, Talib U, Farooq S, et al. Tracheoesophageal fistula: a rare complication of prolonged intubation in COVID-19. Chest 2020;158:A2596-7. [Crossref]
- Fiacchini G, Tricò D, Ribechini A, et al. Evaluation of the Incidence and Potential Mechanisms of Tracheal Complications in Patients With COVID-19. JAMA Otolaryngol Head Neck Surg 2021;147:70-6. [Crossref] [PubMed]


