
Does needle-type increase the diagnostic yield of malignancies in endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA)?—a prospective comparative study
Introduction
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a commonly used minimally invasive technique. It emerged as the top-choice diagnostic procedure for benign and malignant lesions of the mediastinum and lung parenchyma (1-4).
It allows for simultaneous, accurate localization and sampling of the mediastinal and hilar lymph nodes with high accuracy and minimal complications (5-8). During the EBUS procedure, a biopsy is performed using commercially available needles inserted through the scope’s working channel. Many practitioners use larger bore needles or needles of a different design to obtain more tissue volume and improve the procedure’s effectiveness. However, there is mixed evidence regarding other needle types’ diagnostic accuracy or sensitivity (9-14). Furthermore, the EBUS-TBNA is constantly developed, and new solutions are studied to optimize the diagnostic yield. Recent papers have examined, among other things, the impact of the number of needle passes (2), use of rapid on-site evaluation (ROSE) (15) or suction syringe on the quality of specimens and a diagnostic value (16).
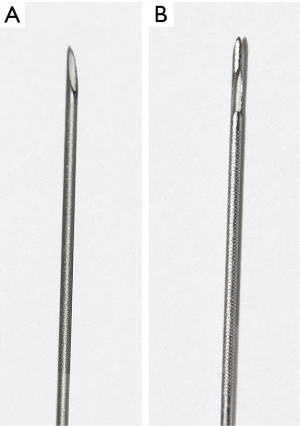
The ProCore® needle is an innovative histology needle used in EBUS-TBNA. Its notable technical features include its slenderness and the side-cutting window, which allows the tissue to enter the needle’s lumen and cut a complete tissue sample. Finally, this needle potentially obtains a better-quality core tissue by retaining its morphological architecture, thus increasing the overall diagnostic accuracy (2,17).
This study aimed to evaluate the potential advantage of the ProCore® needle compared with the standard needles in terms of diagnostic yield. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1594/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Bioethics Committee of the Poznan University of Medical Sciences (No. 688/20) and informed consent was obtained from all participants.
Patients
From 2017 to 2020, a total of 1,992 patients underwent EBUS-TBNA due to various indications. The study included patients aged 18 or above, where EBUS-TBNA was performed to establish the diagnosis of malignancy with the exclusion of malignant lymphoma. Patients with the suspicion of sarcoidosis, tuberculosis or requiring EBUS-TBNA due to preoperative staging were also excluded from the study.
Finally, 363 patients were enrolled in the study; 125 (34.4%) were female, and 238 (65.6%) were male. The mean age was 64.9±8.2 years. A total of 51 patients were included in the ProCore® group, whereas 312 patients were included in the standard needle group (Figure 1). They underwent prior clinical and radiological examination of the mediastinum to confirm enlarged hilar or mediastinal lymph nodes. Biopsy was performed in case of any of the following indications: disseminated neoplastic disease, suspicion of metastasis other than lung cancer, and suspicion of neoplastic disease recurrence or N2/N3 lung cancer diagnosis. These indications were followed across both groups. The details are presented in Table 1.
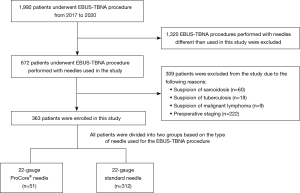
Table 1
| Indication | Number of patients | Standard needles | ProCore® needles |
|---|---|---|---|
| Disseminated neoplastic disease | 19 | 16 | 3 |
| Metastasis other than lung cancer | 66 | 52 | 14 |
| Suspicion of recurrence | 16 | 13 | 3 |
| N2 and/or N3 lung cancer | 262 | 231 | 31 |
Procedural technique
Before EBUS-TBNA, radiological imaging was reviewed to precisely locate the affected mediastinal lymph nodes. Patients were administered topical anesthesia with 1% lidocaine and conscious sedation. All the procedures were performed by two experienced endoscopists (performing >100 EBUS-TBNA per year). PENTAX EBUS endoscope (EB-1970UK; Pentax Medical, Tokyo, Japan) was used.
A diagnostic procedure was performed on patients using one of the needles: standard (22-gauge SonoTip®, Medi-Globe GmbH, Achenmühle, Germany) or ProCore® (22-gauge EchoTip ProCore® Endobronchial HD Ultrasound Biopsy Needle, Cook Medical, Bloomington, IN, USA). Basic characteristics of the two needles used in our study are presented in Table 2, Figures 2,3. According to the protocol, each lymph node was biopsied three times using the same needle. The cytology specimens were fixated using 95% ethyl alcohol and subsequently processed with Tissue-Tek Prisma (Sakura Finetek USA, Inc., Torrance, CA, USA). The cell-block fixative was formalin. If the final diagnosis was not established via EBUS-TBNA, either mediastinoscopy or video-assisted thoracic surgery (VATS) biopsy was performed according to the guidelines (18). The diagnosis was established either with cytology smears on the pathology slides and/or via the cell block technique. Then, the multidisciplinary cancer team discussed the diagnosis in terms of further treatment or diagnostics.
Table 2
| Type | SonoTip® | ProCore® |
|---|---|---|
| Size | 22, 25 | 22, 25 |
| Minimum accessory channel (mm) | 2.8 | 2 |
| Stylet tip | Beveled | Recessed ball tip |
| Technical features | – | Side cutting window |
| Amount of tissue | Small sample | Larger sample |
| Sample | Cytological | Tissue core, better quality |
The data was analyzed to calculate the sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV), and diagnostic accuracy of EBUS-TBNA performed using ProCore® or the standard needle.
Statistical analysis
Unpaired t-test was employed to analyze the data with normal distribution and homogeneous variances. The normality of the distribution was tested using the Shapiro-Wilk test, and the equality of variances was checked using Levene’s test. The data that did not follow a Gaussian distribution was analyzed using the Mann-Whitney U test. Categorical data were analyzed using either the χ2 test or the Fisher-Freeman-Halton test. We calculated the sensitivity, specificity, PPV and NPV of the biopsy for all cases as well as for different types, sizes of needles, and indications. We compared the correct outcome ratio (sum of true positive and true negative results) between the groups using the χ2 test. All results with a P value of <0.05 were considered significant. Statistical calculations were performed using the Statistica 12.0 PL software (StatSoft Polska, Kraków, Poland) or StatXact 9.0 (Cytel Inc., Cambridge, MA, USA).
Results
We performed 1,089 lymph node diagnostic biopsies via EBUS-TBNA in 363 patients, with 51 examined using the ProCore® needle and 312 using the standard needle. We were able to establish a final diagnosis using EBUS-TBNA in 306 patients (84.3%) in both groups. However, a diagnosis could not be made via EBUS in 57 (15.7%) patients; thus, mediastinoscopy or VATS biopsy was required. The number of patients who required additional surgical procedures did not differ significantly between the two needles [ProCore® (4 patients, 7.8%) vs. standard (53 patients, 17%), P=0.26]. There were no complications after the EBUS-TBNA procedure in either group.
For both groups of patients who underwent EBUS-TBNA, the overall sensitivity was 80.6%; specificity, 96.4%; NPV, 60.0%; and PPV, 98.7%. With the ProCore® needle, the sensitivity was 89.2%, and the specificity was 100.0%, whereas for the standard needle, these were 79.3% and 95.7%, respectively. However, these values were not significantly different between the needles (P=0.14). The percentages of true positives for the individual needles are presented in Table 3.
Table 3
| Type of needle | Proper diagnosis, n (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Proper diagnosis (P value) |
|---|---|---|---|---|---|---|
| ProCore® | 47 (92.2) | 89.2 | 100.0 | 100.0 | 77.8 | 0.14 |
| Standard | 259 (83.0) | 79.3 | 95.7 | 98.5 | 57.3 | |
| All | 306 (84.3) | 80.6 | 96.4 | 98.7 | 60.0 |
PPV, positive predictive value; NPV, negative predictive value.
The number of accurate diagnoses (both true positive and true negative) did not differ significantly between the needle types and sizes in the whole group (P=0.6). The needle sensitivity for the individual indications also did not significantly differ (P=0.71 for ProCore® and P=0.43 for the standard needle). These data are illustrated in Table 4.
Table 4
| Needle type | Indication | Proper diagnosis, n (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NNV (%) | Proper diagnosis (P value) |
|---|---|---|---|---|---|---|---|
| ProCore® | Non-lung cancer metastases | 12 (85.7) | 75.0 | 100.0 | 100.0 | 75.0 | 0.71 |
| N2/N3 lung cancer diagnosis | 29 (93.5) | 91.3 | 100.0 | 100.0 | 80.0 | ||
| Disseminated neoplastic disease | 3 (100.0) | 100.0 | – | 100.0 | – | ||
| Recurrence | 3 (100.0) | 100.0 | – | 100.0 | – | ||
| Standard | Non-lung cancer metastases | 40 (76.9) | 59.3 | 96.0 | 94.1 | 68.6 | 0.43 |
| N2/N3 lung cancer diagnosis | 193 (83.5) | 80.6 | 97.5 | 99.4 | 51.3 | ||
| Disseminated neoplastic disease | 15 (93.8) | 100.0 | 66.7 | 92.9 | 100.0 | ||
| Recurrence | 11 (84.6) | 81.8 | 100.0 | 100.0 | 50.0 |
PPV, positive predictive value; NPV, negative predictive value.
Discussion
The diagnostic yields of various needles have been relatively well described for transesophageal endoscopic ultrasound-guided fine needle aspiration (EUS-FNA), especially in gastrointestinal tract tumor biopsies. However, there is still a paucity of data with regard to which of the EBUS needles has higher sensitivity and should be recommended for performing biopsy (15,19,20). Miyazaki et al. (21) performed a prospective interventional study using 22- and 25-gauge ProCore® needles. They managed to obtain the excellent quality of the samples and establish the diagnosis in the majority of cases. They concluded that the ProCore® needle is worth using in the histologic evaluation of mediastinal lymphadenopathy. However, their paper is a single institution study that lacks a randomized comparison standard needle with the Procore® needle.
Studies reporting a benefit of the ProCore® needle are limited by the lack of randomization, retrospective nature, unexpected and unexplained low sensitivities with the standard needle and lack of on-site cytology, the presence of which can direct site of sampling on the lymph node (22,23). One of the few randomized studies comparing 22-gauge ProCore® and the 22-gauge standard (Vizishot, Olympus, Tokyo, Japan) needle in the diagnosis of sarcoidosis did not find a difference in the sensitivity, specimen adequacy, or safety of EBUS-TBNA when performed with the ProCore® or the Olympus needle in subjects with sarcoidosis (24).
It is worth emphasizing that obtaining a diagnosis with EBUS-TBNA may eliminate the need for mediastinoscopy or surgical biopsy (9,10). Compared with mediastinoscopy, EBUS-TBNA has a similar diagnostic rate but is much less invasive, causing less discomfort and fewer complications (25-27).
Our prospective analysis reveals no statistically significant differences in the diagnostic yield between the ProCore® and standard needles. Regardless of the needle’s type and size, a high percentage of proper diagnoses was still obtained. In our study, the percentage of patients requiring mediastinoscopy or VATS was double in the standard needle group (16%) compared to the ProCore® group (8%). This appears to have not met statistical significance but is a large clinical difference in our opinion. This is a tangible outcome that affects patient care and if the ProCore® needle truly reduces the number of patients requiring an invasive surgical procedure, it would be a superior needle even if the diagnostic yield and sensitivities are similar.
Yang et al. (2) revealed similar results. In their paper, the mRNA’s diagnostic adequacy and concentration were identical for the 25-gauge ProCore® and standard 22-gauge needles. However, the use of the ProCore® needle decreased puncture time and frequency. Furthermore, they also observed better elasticity and precision when puncturing using the ProCore® needle, which easily reached the lymph nodes. However, this study only analyzed a small group of 39 patients.
The lack of significant differences between the ProCore® and standard FNA needles in the accuracy of the sample’s diagnosis and adequacy were reported in a previous meta-analysis (20). Nevertheless, it is worth mentioning that in this particular study, the ProCore® needle was able to finalize a diagnosis with fewer passes (20). According to Oh et al. (28), the core and standard fine needles are comparable in terms of diagnostic accuracy, technical performance, and safety profile.
A relevant aspect to consider is that in a few studies, the ProCore® needle was able to establish a final diagnosis with faster and less frequent puncturing compared with the standard needle (2,20). Yang et al. (2) emphasized that the ProCore® needle was able to obtain sufficient samples for diagnosis even after a single-pass puncture.
McCracken et al. (14) compared the ProCore® needle with the standard fine needle in a group of 235 patients and observed a statistically significant difference in terms of diagnostic sensitivity. They also found that significantly fewer patients required further diagnostic procedures. Berger et al. (29) demonstrated that the ProCore® needle could possibly have a higher diagnostic yield owing to its larger gauge compared with the standard needle. However, this study only assessed two groups of 24 patients.
Our study involves a prospective analysis of a large group of 363 patients. Also, we ensured the validity of our findings by having the same two experienced providers perform all of the proceedings in our high-volume center. To the best of our knowledge, this study represents the most extensive series reporting the use of EBUS-TBNA needles in malignant lesions. High sensitivity, specificity, and diagnostic yield allow the endoscopists to select a preferred needle type without compromising the quality of diagnostics. All the collected specimens were of high quality, which led to accurate diagnoses and, consequently, avoided further invasive surgical procedures for most patients.
However, the authors acknowledge several limitations of this study. First, although our study is a prospective one, the needle type group sizes are not comparable, and there is a large disproportion between the groups. Needles were not always available in the clinic in equal amounts during the study period, which resulted in limitations in recruiting patients. Moreover, our study is not a randomized trial. Randomization was challenging to achieve due to the limited availability of needles. We also reduced the number of patients with a disseminated neoplastic process, which constituted a minority among them. In their case, making a quick diagnosis was crucial; patients often presented with a poor general condition, which caused limitations during the recruiting process.
Our study demonstrates that all needle types yield high diagnostic accuracy in obtaining the tissue core, which allowed us to sample good-quality specimens. Future studies should compare prospective data with that from a randomized controlled trial.
Conclusions
The needles commonly used in EBUS-TBNA have high diagnostic efficacy and can be successfully used to diagnose mediastinal pathologies. The ProCore® needle had no significant advantage over other standard needle types.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1594/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1594/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1594/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Bioethics Committee of the Poznan University of Medical Sciences (No. 688/20) and informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dincer HE, Andrade R, Zamora F, et al. A new needle on the block: EchoTip ProCore endobronchial ultrasound needle. Med Devices (Auckl) 2016;9:467-73. [Crossref] [PubMed]
- Yang L, Gu Y, Wang H, et al. Novel ProCore 25-gauge needle for endobronchial ultrasound-guided transbronchial needle aspiration reduces the puncture time and frequency, with comparable diagnostic rate for mediastinal and hilar lymphadenopathy. Thorac Cancer 2020;11:748-53. [Crossref] [PubMed]
- Vaidya PJ, Kate AH, Yasufuku K, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in lung cancer diagnosis and staging. Expert Rev Respir Med 2015;9:45-53. [Crossref] [PubMed]
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736-55. [PubMed]
- Chhajed PN, Odermatt R, von Garnier C, et al. Endobronchial ultrasound in hilar and conventional TBNA-negative/inconclusive mediastinal lymphadenopathy. J Cancer Res Ther 2011;7:148-51. [Crossref] [PubMed]
- Hürter T, Hanrath P. Endobronchial sonography: feasibility and preliminary results. Thorax 1992;47:565-7. [Crossref] [PubMed]
- Kurimoto N, Murayama M, Yoshioka S, et al. Assessment of usefulness of endobronchial ultrasonography in determination of depth of tracheobronchial tumor invasion. Chest 1999;115:1500-6. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Koh E, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer 2005;50:347-54. [Crossref] [PubMed]
- Saji J, Kurimoto N, Morita K, et al. Comparison of 21-gauge and 22-gauge Needles for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration of Mediastinal and Hilar Lymph Nodes. J Bronchology Interv Pulmonol 2011;18:239-46. [Crossref] [PubMed]
- Yarmus LB, Akulian J, Lechtzin N, et al. Comparison of 21-gauge and 22-gauge aspiration needle in endobronchial ultrasound-guided transbronchial needle aspiration: results of the American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation Registry. Chest 2013;143:1036-43. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Takahashi R, et al. Comparison of 21-gauge and 22-gauge aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. Respirology 2011;16:90-4. [Crossref] [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Randomized Study of 21-gauge Versus 22-gauge Endobronchial Ultrasound-guided Transbronchial Needle Aspiration Needles for Sampling Histology Specimens. J Bronchology Interv Pulmonol 2011;18:306-10. [Crossref] [PubMed]
- Sterlacci W, Sioulas AD, Veits L, et al. 22-gauge core vs 22-gauge aspiration needle for endoscopic ultrasound-guided sampling of abdominal masses. World J Gastroenterol 2016;22:8820-30. [Crossref] [PubMed]
- McCracken DJ, Bailey M, McDermott MT, et al. A retrospective analysis comparing the use of ProCore® with standard fine needle aspiration in endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). Ir J Med Sci 2019;188:85-8. [Crossref] [PubMed]
- Fabbri C, Fuccio L, Fornelli A, et al. The presence of rapid on-site evaluation did not increase the adequacy and diagnostic accuracy of endoscopic ultrasound-guided tissue acquisition of solid pancreatic lesions with core needle. Surg Endosc 2017;31:225-30. [Crossref] [PubMed]
- Jagan N, Landeen CA, Moore DR, et al. Waste not, want not: diagnostic material found in suction syringe aspirate during endobronchial ultrasound guided transbronchial needle aspiration. J Thorac Dis 2019;11:3270-5. [Crossref] [PubMed]
- Jhala N, Jhala D. Definitions in tissue acquisition: core biopsy, cell block, and beyond. Gastrointest Endosc Clin N Am 2014;24:19-27. [Crossref] [PubMed]
- Tournoy KG, Rintoul RC, van Meerbeeck JP, et al. EBUS-TBNA for the diagnosis of central parenchymal lung lesions not visible at routine bronchoscopy. Lung Cancer 2009;63:45-9. [Crossref] [PubMed]
- Iwashita T, Nakai Y, Samarasena JB, et al. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc 2013;77:909-15. [Crossref] [PubMed]
- Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy 2016;48:339-49. [PubMed]
- Miyazaki K, Hirasawa Y, Aga M, et al. Examination of endobronchial ultrasound-guided transbronchial needle aspiration using a puncture needle with a side trap. Sci Rep 2021;11:9789. [Crossref] [PubMed]
- Dong Z, Li H, Jiang H, et al. Evaluation of cytology in lung cancer diagnosis based on EBUS-TBNA. J Cytol 2017;34:73-7. [Crossref] [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing lung cancer: a randomized study. Respiration 2013;85:486-92. [Crossref] [PubMed]
- Dhooria S, Sehgal IS, Prasad KT, et al. Diagnostic yield and safety of the ProCore versus the standard EBUS-TBNA needle in subjects with suspected sarcoidosis. Expert Rev Med Devices 2021;18:211-6. [Crossref] [PubMed]
- Jeyabalan A, Shelley-Fraser G, Medford AR. Impact of needle gauge on characterization of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) histology samples. Respirology 2014;19:735-9. [Crossref] [PubMed]
- Hashimi H, Cooke DT, David EA, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of non-small cell lung cancer. J Vis Surg 2018;4:37. [Crossref] [PubMed]
- Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest 2013;143:1044-53. [Crossref] [PubMed]
- Oh HC, Kang H, Lee JY, et al. Diagnostic accuracy of 22/25-gauge core needle in endoscopic ultrasound-guided sampling: systematic review and meta-analysis. Korean J Intern Med 2016;31:1073-83. [Crossref] [PubMed]
- Berger PA, Nazir U, Amos L, et al. A Comparison of the 22-Gauge Medi-Globe Sonotip II Transbronchial Aspiration Needle with the 25-Gauge Cook Echotip Ultra Transbronchial Aspiration Needle, and the 25-Gauge Cook Echotip Procore Core Biopsy Needle During Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration, and Core Biopsy. Am J Respir Crit Care Med 2015;191:A3737.



