The efficacy of endobronchial valves for the treatment of bronchopleural fistula: a single-arm clinical trial
Introduction
A bronchopleural fistula (BPF) is defined as communication between the bronchus and pleural cavity, and it is a dreaded complication of severe pulmonary disease. Lung resection remains the leading cause of BPF with a prolonged pulmonary air leak, although refinements in surgical techniques have substantially reduced the incidence of BPF (1-3). Followed by invasive infectious or inflammatory etiologies, include aspergillus, tuberculosis, additional etiologies. Other causes of BPF including mechanical ventilation, pulmonary trauma, spontaneous pneumothorax, acute respiratory distress syndrome (ARDS), radiofrequency ablation, iatrogenic injuries, radiation (3,4). BPF is a relatively rare but worrying complication of several pulmonary diseases. BPF has high incidence and mortality rates and is associated with long-term hospitalization and high resource utilization. The treatment of BPF includes surgical procedures, medical therapy, and the interventional therapy with bronchoscopy. However, the success of existing treatments has been uneven, and the lack of consensus indicates that there is no optimal treatment available. The current interventions seem to be complementary and therapy should be individualized to improve success rates (4). The treatment of BPF remains a challenging and frustrating problem.
Surgical intervention, pleurodesis, and prolonged chest tube drainage have several disadvantages, and patients are not always suitable for major thoracic surgery because of various reason, therefore a variety of bronchoscopic methods have been tried to improve air leakage (5). Many bronchoscopic attempts have been made to treat BPF, including fibrin or tissue glue, spigots, silver nitrate, stents, vascular coils, gel foam, and an autologous endobronchial blood patch (6-12). To treat air leaks, the use of an endobronchial one-way valve (EBV) has been proposed.
In the presence of persisting peripheral air leaks, an EBV can be inserted. These valves were originally developed to reduce the bronchoscopic lung-volume in patients with emphysema. Because of these valves are one-way airway blockers, EBVs prevent air from entering into the affected segmental bronchus and allow mucus to be discharge. Therefore we thought this may be a promising minimal-invasive procedure that can treat peripheral BPF (13). We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-258/rc).
Methods
We retrospectively analyzed the data of 26 patients with a mean age of 54 years (range, 28–86 years) who were treated for BPF using an EBV between August 2017 and October 2020. This sample constitutes all patients treated in our hospital (Shanghai Pulmonary Hospital, Tongji University School of Medicine) for this condition with this intervention during this timeframe. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The need for ethical approval was waived by the Institutional Review Board of Shanghai Pulmonary Hospital as it is a retrospective observational study. Informed consent was previously obtained from each patient before any invasive procedures were performed. The outcomes of endobronchial valve placement for patients with BPF are summarized in Table 1.
Table 1
| Patient | Sex/age | Etiology | Chest tube duration before valve placement (days) | Endobronchial valve placement location | Chest tube duration after valve placement (days) | Complications | Prognosis of BPF |
|---|---|---|---|---|---|---|---|
| P1 | M/60 | NTM, bronchiectasis | 25 | LUL ×1; LUL ×1 | 5 | Granulation proliferation | Improved |
| P2 | M/59 | NTM | N/A | LUL ×1 | N/A | No | Improved |
| P3 | M/30 | Tuberculosis | 187 | LUL ×1; lingula ×1; LUL ×1; lingula ×1 | 30 | Valve displacement | Improved |
| P4 | F/56 | NTM | 35 | LUL ×2; RLL ×1 | 7 | No | Unhealed |
| P5 | M/42 | Aspergilloma; pulmonary lobectomy; bronchopleural fistula; postoperative empyema | 65 | RLL ×1; RLL ×1 | 2 | No | Improved |
| P6 | F/42 | Aspergilloma; pulmonary fenestration | N/A | LUL ×1; LLL ×1 | N/A | No | Improved |
| P7 | M/73 | Severe pneumonia; tuberculosis; aspergilloma | 104 | RLL ×1 | 90 | Hemoptysis | Unhealed |
| P8 | M/28 | Bronchiectasis; pulmonary lobectomy | N/A | LLL ×1 | N/A | No | Unhealed |
| P9 | F/76 | Bronchogastric fistula; partial gastrectomy | N/A | LLL ×1 | N/A | No | Improved |
| P10 | M/50 | Bronchoesophageal fistula; esophagectomy | N/A | RLL ×1 | N/A | No | Improved |
| P11 | F/32 | Pulmonary abscess; pulmonary lobectomy; bronchiectasis | N/A | RLL ×1; RLL ×1 | N/A | No | Improved |
| P12 | M/63 | Carcinoma in situ of lung; pulmonary lobectomy | 70 | RLL ×1 | 30 | No | Improved |
| P13 | M/43 | Postoperative empyema; obsolete pulmonary tuberculosis | N/A | RLL ×2 | N/A | No | Improved |
| P14 | F/59 | NTM; bronchiectasis | N/A | RUL ×3 | N/A | No | Improved |
| P15 | M/53 | Spontaneous pneumothorax; COPD | 49 | LUL ×3; lingula ×1 | 6 | No | Improved |
| P16 | M/86 | Hydropneumothorax; COPD; Mediastinal emphysema, subcutaneous emphysema | 26 | LUL ×1 | 4 | No | Improved |
| P17 | M/74 | Hydropneumothorax; COPD; postoperative lung cancer | 15 | LUL ×2 | 5 | No | Unhealed (bullectomy of lung) |
| P18 | M/69 | Spontaneous pneumothorax; COPD; severe pneumonia | 14 | RLL ×2 | 7 | No | Improved |
| P19 | M/73 | Bronchogastric fistula; partial gastrectomy | N/A | LLL ×1 | N/A | No | Improved |
| P20 | M/51 | Tuberculosis; pulmonary lobectomy | 86 | RLL ×1 | 4 | No | Unhealed (pulmonary fenestration) |
| P21 | F/33 | Tuberculosis; tuberculosis destroyed lung | N/A | LUL ×1; lingula ×1 | N/A | No | Unhealed |
| P22 | M/68 | Carcinoma in situ of lung; pulmonary lobectomy | 99 | Lingula ×1 | 9 | No | Improved |
| P23 | M/43 | NTM | 151 | LLL ×2 | 80 | No | Unhealed |
| P24 | M/52 | Lung malignant tumor; neoadjuvant posterior lobectomy +radical resection | 58 | RML ×1 | 36 | No | Improved |
| P25 | M/34 | Tuberculous emphysema; exploratory thoracotomy and repair of visceral pleural leakage; tuberculosis | 202 | RLL ×1 | 98 | No | Improved |
| P26 | F/54 | Pulmonary cyst with infection; excision of pulmonary cyst | 222 | LUL ×2 | 38 | No | Improved |
BPF, bronchopleural fistula; LLL, left lower lobe; LUL, left upper lobe; N/A, not applicable; RML, right middle lobe; RUL, right upper lobe; RLL, right lower lobe; NTM, non-tuberculosis mycobacteria; COPD, chronic obstructive pulmonary disease.
Preoperative preparation and anesthesia
Patients were asked to fast (without eating or drinking water) for at least six hours before undergoing bronchoscopy. Patients with diabetes were asked to skip a dose of hypoglycemic drugs or insulin, and patients with hypertension were asked to take hypotensive drugs. The bronchoscopy was performed with general anesthesia. Patients were placed in the supine position on the table after entering the operating room. ECG (Electrocardiogram), pulse oxygen saturation, and blood pressure were monitored, and a peripheral or central venous access was established. Succinylcholine (1–1.5 mg/kg), sufentanil (0.4–0.6 µg/kg), and propofol (1.5–2 mg/kg) were intravenously injected to induce general anesthesia. After 1 minute, a laryngeal mask was placed on the patient (size number 5 was used for male patients, while size number 4 was used for female patients used). Mechanical ventilation was administered during navigation, and succinylcholine (3–5 µg/kg/min) and propofol (25–75 µg/kg/min) maintained anesthesia. Naloxone (0.2–0.4 mg) was administered at the end of the bronchoscopy, and the laryngeal mask was removed after the patient was completely awake (14).
Equipment
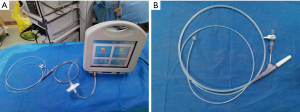
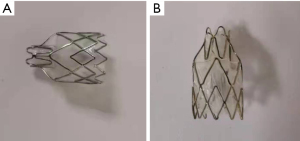
During EBV placement, a CLV-290SL Xenon light source (Olympus Corporation, Japan), a CLV-290SL image processing station (Olympus Corporation, Japan), and a BF-1TQ290 flexible bronchoscope (Olympus Corporation, Japan) were used. The location of the BPF was identified using the Chartis system (Pulmonx SARL, CHARTIS CONSOLE, Switzerland; Figure 1) and a digital thoracic drainage system (Medela AG, Switzerland). The Emphasys Zephyr endobronchial valve (ZEBV, Pulmonx Inc. Neuchatel, Switzerland) was used; it is a second-generation device which is composed of a silicone ‘duck bill’ attached to a nitinol skeleton (Figure 2).
Endobronchial valve placement technique
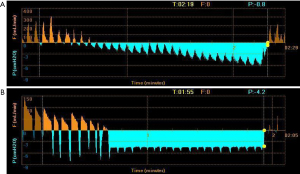
The first step in the placement of an EBV was to localize the source of the BPF. Results of computed tomography (CT) needed to be analyzed before any intervention took place. Then, routine bronchoscopy was performed to evaluate the airway condition. The Chartis assessment system consists of two parts. The first part is a balloon catheter which is inserted through the working channel of the bronchoscope. Balloon inflation blocks the airway, blocking the flow of inhaled air, so that the air can only flow out through the central lumen of the catheter. The second part is a console, which is used to display the flow and pressure readings in real time. If there is no collateral ventilation (CV) to supply the airway distal to the balloon, air flow from the lobe will gradually decrease. A continuous flow reading indicates the presence of CV in the target lobe (Figure 3) (15). A digital thoracic drainage system was also used to locate the site of BPF. When the balloon blocked the lobe, there was an immediate significant reduction in airflow visualized on the digital screen and flow-time graph. The processes were repeated to determine the segmental or subsegmental airway or airways that, when blocked, offered the greatest reduction in air leak rate. Then these airways were used as target airway for valve implantation
Then, the EBVs were delivered to the target airway using a flexible catheter. A valve loader provided with the system compressed the valves into the distal tip of the delivery catheter. The delivery catheter was then passed via the working channel of a bronchoscope (3.0 mm inner diameter) and guided to the target airway. After placement in place, the valve was unfolded (16). The leak then decreased after placement of the valves.
Generally, the air leak of the lung lobe was detected first, followed by air leaks of lung segments. EBVs were first placed in segments that were difficult to access, then into segments that were easier to access. The fewer valves placed, the better.
Statistical analysis
We used IBM SPSS Statistics 24 to perform all analyses. The measurement data conforming to the normal distribution shall be expressed as the mean, and those not conforming to the normal distribution shall be expressed as the median. The counting data is expressed by rate or composition ratio.
Results
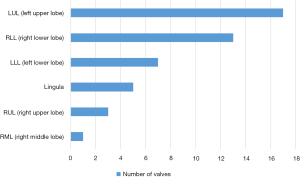
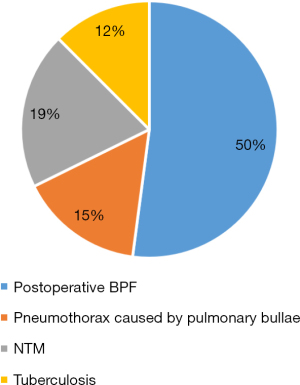
A total of 26 patients underwent EBV placement procedures in the study; 19 were men, and 7 were women. The median age of all patients was 53.5 years (mean, 54 years; range, 28–86 years). A total of 46 valves were placed, with a median of 2 valves per procedure (mean, 1.8 valves; range, 1 to 4 valves). Left upper lobe (LUL) was the most common lobe in which valves were placed (Figure 4). The underlying etiologies for BPF were postoperative BPF (50%; n=14), pneumothorax caused by pulmonary bullae (15%; n=4), non-tuberculosis mycobacteria (NTM) (19%; n=5), and tuberculosis (12%; n=3). Previous postoperative BPF for the group as a whole included lobectomy (n=8), pulmonary fenestration (n=1), exploratory thoracotomy and repair of visceral pleural leakage (n=1), partial gastrectomy (n=2), esophagectomy (n=1), and excision of pulmonary cyst (n=1; Figure 5).
A total of 16 patients underwent chest tube insertion. Before receiving EBVs, the average chest tube duration in the group of patients was 88 days (median, 61.5 days; range, 14–222 days). The average duration after which patients had the chest tube removed was 28.2 days after EBVs placement (median, 7 days; range, 2–98 days). At the time of chest tube removal, close drainage equipment demonstrated zero flow in these patients. The effective rate EBV for the treatment of BPF was 73.1%, and the condition of 19 patients with BPF was improved after treatment.
Patients were followed up for an average of 3–6 months after treatment. There were no adverse events related to the valve implantation procedure reported at the follow-ups. Six patients underwent valve removal using flexible bronchoscopy and grasping forceps under sedation and with spontaneous breathing, and there were no any further complications, while 20 cases did not have their valves removed. During bronchoscopy when valve removal happened, 1 patient had granulation tissue, 1 patient had valve migration, and 1 patient had a bronchial hemorrhage, which was improved after treatment with bronchial artery embolization. Patients for whom the valves were not removed, there were no valve related complications.
In addition to the placement of one-way valves, 5 patients also underwent other bronchoscopic procedures including the use of a silicone plug, lauryl alcohol, argon laser plasma coagulation, ventricular septal occluder, and umbrella occlude at a different time. After the poor effect of EBV, and remove the valve before surgeries, one patient underwent bronchial stump ligation and pulmonary fenestration, and one patient underwent a bullectomy of the lung.
Discussion
BPF represents abnormal communication between the pleural cavity and bronchial tree, which significantly increases incidence rate, mortality, and length of hospital stay. In this study, we reported on a consecutive series of 26 cases of EBV placement in a range of clinical scenarios for postoperative BPF, pneumothorax, NTM, and tuberculosis. Postoperative complications for pulmonary resection were the most common cause of BPF reported in our study. The underlying etiologies for BPF were postoperative BPF (50%; n=14) in our study. Tuberculosis and NTM are generally less common than BPF (4), but the two etiologies were frequent in our sample, which may be because our hospital is a pulmonary specialist hospital. The most common site of BPF was LUL, followed by RLL, and LLL, and the RML was the least common. This result may be related to the predilection sites of primary diseases.
Treatment of BPF includes surgical procedures and medical therapy, and a variety of interventional therapy with bronchoscopy have been previously reported, including instillation of ethanol, antibiotics, lead shots, albumin glutaraldehyde tissue adhesive and fibrin glue, and these endoscopic techniques were always for the treatment of long-term air leaks (15). Unfortunately, the infusion of these substances is often related to irreversible obstruction of target airways, significant foreign body reaction, or both. They play a role as airway or bronchial blockers. Furthermore, according to a recent report, they are also related to the risk of spigot migration and the incidence rate of lung disease, including atelectasis, lung abscess, and pneumonia (11).
The use of EBV placement for BPF is an increasingly recognized technique. EBVs were initially developed for lung volume reduction surgery in patients with severe emphysema. Research has confirmed the efficacy of endobronchial valves to manage a persistent air, including the management of a postoperative air leak and other clinical scenarios in which EBVs were effectively used to eliminate airflow from specific regions of the lung (17-30). In retrospect, the duration of chest tube placement, the length of stay, mortality, and anxiety of patients might have been reduced if EBV placement was considered earlier. EBV placement is a relatively mature procedure, which is safe and effective, and generates less trauma and fewer complications. Based on these advantages, an increasing number of doctors tend to choose this method to treat BPF. In our study, the effective rate of EBV for the treatment of BPF was 73.1%. There was only 1 case who had the complication of a bronchial hemorrhage, but this was improved after treatment with bronchial artery embolization. The primary disadvantage of an EBV lies in its cost. However, the cost can be offset by the multiple advantages, including that the treatment can be reversed by removing the EBV and that EBV placement is safe for critically ill patients who are unable to undergo surgical intervention (28).
There was 1 patient who underwent EBV placement for BPF, but for which the purpose of the EBV was also to reduce their lung volume. In addition to one-way valve placement, 5 patients underwent other bronchoscopic procedures, including use of a silicone plug, lauryl alcohol, argon laser plasma coagulation, and ventricular septal occlude. One patient also underwent bronchial stump ligation and pulmonary fenestration.
To be selected to undergo EBV placement, patients had to demonstrate a persistent continuous air leak, which was defined as an intrathoracic chest tube duration of more than 2 weeks even though they received conservative therapy, surgical therapy, or both. Postoperative BPF patients were also considered to be good candidates, but not all such patients were suitable for EBVs placement. Their suitability depended on the length and diameter of the fistula. EBV placement was considered suitable if the surgical stump was long enough and the diameter of fistula was ≥3 and ≤8 mm.
The study is limited by its retrospective nature, the small sample size, and the lack of a control group. It is hoped that a large sample and multi-center research can be carried out in the future to further summarize indications of EBV placement and increase the success rate of this intervention.
Conclusions
The placement of EBVs is a simple, effective, and safe treatment of BPF in our study, and this intervention may be suitable for wide application in clinical practice.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-258/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-258/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-258/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The need for ethical approval was waived by the Institutional Review Board of Shanghai Pulmonary Hospital as it is a retrospective observational study. The written informed consent was obtained from each individual.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shekar K, Foot C, Fraser J, et al. Bronchopleural fistula: an update for intensivists. J Crit Care 2010;25:47-55. [Crossref] [PubMed]
- Asamura H, Kondo H, Tsuchiya R. Management of the bronchial stump in pulmonary resections: a review of 533 consecutive recent bronchial closures. Eur J Cardiothorac Surg 2000;17:106-10. [Crossref] [PubMed]
- Grotberg JC, Hyzy RC, De Cardenas J, et al. Bronchopleural Fistula in the Mechanically Ventilated Patient: A Concise Review. Crit Care Med 2021;49:292-301. [Crossref] [PubMed]
- Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest 2005;128:3955-65. [Crossref] [PubMed]
- Liberman M, Muzikansky A, Wright CD, et al. Incidence and risk factors of persistent air leak after major pulmonary resection and use of chemical pleurodesis. Ann Thorac Surg 2010;89:891-7; discussion 897-8. [Crossref] [PubMed]
- Venuta F, Rendina EA, De Giacomo T, et al. Postoperative strategies to treat permanent air leaks. Thorac Surg Clin 2010;20:391-7. [Crossref] [PubMed]
- Sivrikoz CM, Kaya T, Tulay CM, et al. Effective approach for the treatment of bronchopleural fistula: application of endovascular metallic ring-shaped coil in combination with fibrin glue. Ann Thorac Surg 2007;83:2199-201. [Crossref] [PubMed]
- Watanabe S, Watanabe T, Urayama H. Endobronchial occlusion method of bronchopleural fistula with metallic coils and glue. Thorac Cardiovasc Surg 2003;51:106-8. [Crossref] [PubMed]
- Fruchter O, Kramer MR, Dagan T, et al. Endobronchial closure of bronchopleural fistulae using amplatzer devices: our experience and literature review. Chest 2011;139:682-7. [Crossref] [PubMed]
- Andreetti C, D'Andrilli A, Ibrahim M, et al. Effective treatment of post-pneumonectomy bronchopleural fistula by conical fully covered self-expandable stent. Interact Cardiovasc Thorac Surg 2012;14:420-3. [Crossref] [PubMed]
- Sasada S, Tamura K, Chang YS, et al. Clinical evaluation of endoscopic bronchial occlusion with silicone spigots for the management of persistent pulmonary air leaks. Intern Med 2011;50:1169-73. [Crossref] [PubMed]
- Giddings O, Kuhn J, Akulian J. Endobronchial valve placement for the treatment of bronchopleural fistula: a review of the current literature. Curr Opin Pulm Med 2014;20:347-51. [Crossref] [PubMed]
- Schweigert M, Kraus D, Ficker JH, et al. Closure of persisting air leaks in patients with severe pleural empyema--use of endoscopic one-way endobronchial valve. Eur J Cardiothorac Surg 2011;39:401-3. [Crossref] [PubMed]
- Gu Y, Wu C, Yu F, et al. Application of endobronchial ultrasonography using a guide sheath and electromagnetic navigation bronchoscopy in the diagnosis of atypical bacteriologically-negative pulmonary tuberculosis. Ann Transl Med 2019;7:567. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Gompelmann D, et al. Radiological and clinical outcomes of using Chartis™ to plan endobronchial valve treatment. Eur Respir J 2013;41:302-8. [Crossref] [PubMed]
- Saettele TM, Jimenez CA. Digital quantification of air leak to identify the location of an alveolopleural fistula. Ann Am Thorac Soc 2014;11:1152-4. [Crossref] [PubMed]
- Firlinger I, Stubenberger E, Müller MR, et al. Endoscopic one-way valve implantation in patients with prolonged air leak and the use of digital air leak monitoring. Ann Thorac Surg 2013;95:1243-9. [Crossref] [PubMed]
- Toma TP, Kon OM, Oldfield W, et al. Reduction of persistent air leak with endoscopic valve implants. Thorax 2007;62:830-33. [Crossref] [PubMed]
- Dooms CA, Decaluwe H, Yserbyt J, et al. Bronchial valve treatment for pulmonary air leak after anatomical lung resection for cancer. Eur Respir J 2014;43:1142-8. [Crossref] [PubMed]
- Bronstein ME, Koo DC, Weigel TL. Management of air leaks post-surgical lung resection. Ann Transl Med 2019;7:361. [Crossref] [PubMed]
- Criss CN, Barbaro R, Bauman KA, et al. Selective Management of Multiple Bronchopleural Fistulae in a Pediatric Patient on Extracorporeal Membrane Oxygenation: A Multidisciplinary Approach. J Laparoendosc Adv Surg Tech A 2018;28:1271-4. [Crossref] [PubMed]
- Kalatoudis H, Nikhil M, Zeid F, et al. Bronchopleural Fistula Resolution with Endobronchial Valve Placement and Liberation from Mechanical Ventilation in Acute Respiratory Distress Syndrome: A Case Series. Case Rep Crit Care 2017;2017:3092457. [Crossref] [PubMed]
- Gaspard D, Bartter T, Boujaoude Z, et al. Endobronchial valves for bronchopleural fistula: pitfalls and principles. Ther Adv Respir Dis 2017;11:3-8. [Crossref] [PubMed]
- Dugan KC, Laxmanan B, Murgu S, et al. Management of Persistent Air Leaks. Chest 2017;152:417-23. [Crossref] [PubMed]
- Toth JW, Podany AB, Reed MF, et al. Endobronchial occlusion with one-way endobronchial valves: a novel technique for persistent air leaks in children. J Pediatr Surg 2015;50:82-5. [Crossref] [PubMed]
- Reed MF, Gilbert CR, Taylor MD, et al. Endobronchial Valves for Challenging Air Leaks. Ann Thorac Surg 2015;100:1181-6. [Crossref] [PubMed]
- Alexander ES, Healey TT, Martin DW, et al. Use of endobronchial valves for the treatment of bronchopleural fistulas after thermal ablation of lung neoplasms. J Vasc Interv Radiol 2012;23:1236-40. [Crossref] [PubMed]
- Abu-Hijleh M, Blundin M. Emergency use of an endobronchial one-way valve in the management of severe air leak and massive subcutaneous emphysema. Lung 2010;188:253-7. [Crossref] [PubMed]
- Mitchell KM, Boley TM, Hazelrigg SR. Endobronchial valves for treatment of bronchopleural fistula. Ann Thorac Surg 2006;81:1129-31. [Crossref] [PubMed]
- Hance JM, Martin JT, Mullett TW. Endobronchial Valves in the Treatment of Persistent Air Leaks. Ann Thorac Surg 2015;100:1780-5; discussion 1785-6. [Crossref] [PubMed]