Years of sorafenib investigation in advanced non-small cell lung cancer: is there a ‘NExUS’ linking an unsuccessful treatment and a potentially active one?
Sorafenib, a multi-targeted receptor tyrosine-kinase inhibitor (TKI) with a supposedly predominant anti-angiogenic activity (inhibition of vascular endothelial growth factor receptor-2, -3 and platelet-derived growth factor ß), has been the subject of extensive clinical research in advanced non-small cell lung cancer (NSCLC). Unfortunately, randomized phase II and III trials, in which the drug was tested in combination with chemotherapy versus chemotherapy alone for either chemonaïve and pretreated non-squamous NSCLC patients, failed to show any survival improvement for the sorafenib experimental arms (1-3). Among these studies, the most recently published phase III ‘NExUS’ (NSCLC research Experience Utilizing Sorafenib) trial confirmed no survival prolongation with the addition of sorafenib to platinum-based chemotherapy, namely cisplatin/gemcitabine, as front-line therapy for patients with non-squamous advanced NSCLC (HR=0.98) (2). Undoubtedly, such a finding couples with that coming from the ‘ESCAPE’ (Evaluation of Sorafenib, CArboplatin and Paclitaxel Efficacy in NSCLC) trial in which sorafenib failed to improve survival when added to carboplatin/paclitaxel in a similar patient population (HR=1.15) (1). On the other hand, the ‘ESCAPE’ trial reported a detrimental effect on survival for the sorafenib arm in patients with squamous cell cancer (HR=1.85), which had a relevant impact on the ongoing ‘NExUS’ trial; in fact, the enrollment of patients with squamous cell histology was halted in the ‘NExUS’, thus leading to the exclusion from the primary efficacy population a total of 132 randomized patients (2). Unfortunately, two years and 772 patients later, the results of the ‘NExUS’ trial added little extra information, besides the notion that sorafenib does not improve survival in non-squamous cancer patients regardless of the platinum-based chemotherapy backbone with which it is combined (2).
Nevertheless, the ‘NExUS’ trial still showed a clinically modest but statistically significant prolongation in time to progression (HR=0.73) and progression-free survival (HR=0.83) for the sorafenib plus cisplatin/gemcitabine arm; of note, both curves clearly separated past six months since treatment initiation, namely at a time when non-progressive patients were continuing on maintenance sorafenib (2). Therefore, although the skepticals may argue that in the ‘NExUS’ trial only approximately half of patients scans underwent central radiologic review, it appeared as if sorafenib given as single-agent was associated with a certain degree of clinical activity; nevertheless, some activity was also shown in earlier phase II studies of sorafenib monotherapy as well as in the recently presented phase III ‘MISSION’ (Monotherapy administration of Sorafenib in patients with non-small cell lung cancer) trial, in which patients who had received at least two but no more than three previous lines of therapy were randomized to either sorafenib or placebo (4-6). However, given the invariably negative results demonstrated by the randomized phase III trials in terms of the primary survival endpoint, the question as to whether sorafenib investigation in NSCLC should be definitively abandoned because of lack of efficacy is very relevant (1,2,6). Certainly, in the absence of a validated predictive biomarker, we can conclude that we do not need more clinical studies of sorafenib in unselected patients. On the other hand, years of trials of anti-angiogenic agents in advanced NSCLC have returned so far no reliable biomarker of clinical activity, which does not necessarily mean that a therapy associated with a small benefit in a large patient population may not be highly beneficial for a selected group of individuals. Against this scenario, the most rational way to build future sorafenib trials would be that of learning from past results.
Firstly, ever since the early clinical development of the reversible epidermal growth factor receptor (EGFR)-TKIs gefitinib and erlotinib for advanced NSCLC, we learned that the concomitant administration of TKIs with chemotherapy is probably not an optimal strategy (7). In fact, similarly to EGFR-TKIs, sorafenib inhibits tumor growth by inducing G1 cell cycle arrest, thus potentially interfering with the cycle-dependent toxicity of chemotherapy when this is administered concomitantly (8). Therefore, pharmacodynamic separation achieved by intermittent delivery of sorafenib intercalated with chemotherapy as well as its sequential administration following front-line induction chemotherapy seem to be two reasonable therapeutic strategies that certainly need to be tested in future clinical trials.
Secondly, due to its BRAF inhibitory effect, it has been postulated that sorafenib treatment might be particularly beneficial for patients with a hyperactivation of the Ras/Raf/MEK/ERK prosurvival/anti-apoptotic signaling pathway, which might be the case of tumors harboring a KRAS and/or BRAF mutation (approximately 1/4 of all NSCLCs) (9). Consistently, studies attempting at relating the activity of sorafenib to KRAS mutation have documented an encouraging degree of clinical activity in the so biologically selected group of patients (Table 1). In fact, with the exception of the MISSION trial, in which the small number of tumor tissues collected for KRAS analysis (only 10% of the total) might have influenced significantly the reliability of the data on biomarker analysis, sorafenib has been shown to provide a disease control rate in 53% to 90% of advanced NSCLC patients with aberrant KRAS activation (Table 1). Importantly. these results are significantly superior to those obtained with approved second- or third-line therapies (i.e., erlotinib or docetaxel) in an analogous KRAS-mutant population of patients (17,18). On the other hand, EGFR mutation positive NSCLCs should be excluded from further sorafenib testing, since EGFR-mutant disease is best targeted by EGFR-inhibitors. In fact, due to its EGFR ‘oncogene addiction’, EGFR-mutant NSCLC is highly dependent on EGFR signaling even at later stages of the disease and despite progression on treatment with EGFR-TKIs (19,20). In addition, a recent study evaluating first-line erlotinib plus sorafenib suggested no benefit from the combination in the EGFR-mutant population compared with what it could have been expected with erlotinib alone (Table 2), thus confirming in the clinic the lack of synergistic activity of the dual blockade of EGFR and angiogenesis in EGFR-mutants (15,16,21).
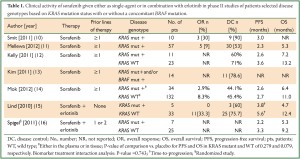
Full table
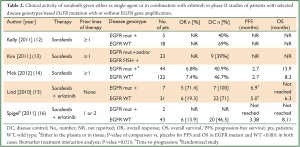
Full table
Finally, since in most cases a single drug cannot achieve the optimal inhibitory concentrations for all the targets, combination strategies seem to be the best way to proceed in order to maximize antitumor activity and prevent escape mechanisms. As for sorafenib, preclinical studies, strongly support its use in association with drugs aimed at targeting the Ras/Raf/MEK/ERK pathway, and clinical trials should soon be initiated in order to test this hypothesis (22-24).
In conclusion, similarly to what has been observed with other multi-targeted receptor TKIs, the achievement of positive clinical data with sorafenib in NSCLC has been hampered by the fact that no biomarker of sensitivity has been identified. However, just by learning from the past, besides implementing correlative studies either in plasma and in tumor tissue, future sorafenib studies should be limited to non-oncogene addicted NSCLC, possibly testing sorafenib either intercalated or sequentially to chemotherapy. Also, biological combination strategies with sorafenib and other KRAS-targeting agents should be pursued given the solid preclinical rationale showing that a more complete blockade of the Ras/Raf/MEK/ERK pathway may result into increased anti-tumor activity and prevention of resistance mechanisms.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol 2010;28:1835-42.
- Paz-Ares LG, Biesma B, Heigener D, et al. Phase III, Randomized, Double-Blind, Placebo-Controlled Trial of Gemcitabine/Cisplatin Alone or With Sorafenib for the First-Line Treatment of Advanced, Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2012;30:3084-92.
- Molina JR, Dy GK, Foster NR, et al. A randomized phase II study of pemetrexed (PEM) with or without sorafenib (S) as second-line therapy in advanced non-small cell lung cancer (NSCLC) of nonsquamous histology: NCCTG N0626 study. J Clin Oncol 2011;29:abstr 7513.
- Blumenschein GR Jr, Gatzemeier U, Fossella F, et al. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol 2009;27:4274-80.
- Wakelee HA, Lee JW, Hanna NH, et al. A Double-Blind Randomized Discontinuation Phase-II Study of Sorafenib (BAY 43-9006) in Previously Treated Non-Small-Cell Lung Cancer Patients: Eastern Cooperative Oncology Group Study E2501. J Thorac Oncol 2012;7:1574-82.
- Paz-Ares L, Hirsh V, Zhang L, et al. Monotherapy administration of sorafenib in patients with non-small cell lung cancer: phase III, randomized, double-blind, placebo-controlled MISSION trial. Ann Oncol 2012;23:abstr 33.
- Davies AM, Ho C, Lara PN Jr, et al. Pharmacodynamic separation of epidermal growth factor receptor tyrosine kinase inhibitors and chemotherapy in non-small-cell lung cancer. Clin Lung Cancer 2006;7:385-8.
- Takezawa K, Okamoto I, Yonesaka K, et al. Sorafenib inhibits non-small cell lung cancer cell growth by targeting B-RAF in KRAS wild-type cells and C-RAF in KRAS mutant cells. Cancer Res 2009;69:6515-21.
- Sequist LV, Heist RS, Shaw AT, et al. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann Oncol 2011;22:2616-24.
- Smit EF, Dingemans AM, Thunnissen FB, et al. Sorafenib in patients with advanced non-small cell lung cancer that harbor K-ras mutations: a brief report. J Thorac Oncol 2010;5:719-20.
- Presented at: the 2012 AACR-IASLC Joint Conference on Molecular Origins of Lung Cancer: Biology, Therapy and Personalized Medicine; Jan 8-11, 2012; San Diego#PR5.
- Kelly RJ, Rajan A, Force J, et al. Evaluation of KRAS mutations, angiogenic biomarkers, and DCE-MRI in patients with advanced non-small-cell lung cancer receiving sorafenib. Clin Cancer Res 2011;17:1190-9.
- Kim ES, Herbst RS, Wistuba II, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov 2011;1:44-53.
- Mok TSK, Paz-Ares L, Wu YL, et al. Association between tumor EGFR and KRAS mutation status and clinical outcomes in NSCLC patients randomized to sorafenib plus best supportive care (BSC) or BSC alone: Subanalysis of the phase III MISSION trial. Ann Oncol 2012;23:abstr 9.
- Lind JS, Dingemans AM, Groen HJ, et al. A multicenter phase II study of erlotinib and sorafenib in chemotherapy-naive patients with advanced non-small cell lung cancer. Clin Cancer Res 2010;16:3078-87.
- Spigel DR, Burris HA 3rd, Greco FA, et al. Randomized, double-blind, placebo-controlled, phase II trial of sorafenib and erlotinib or erlotinib alone in previously treated advanced non-small-cell lung cancer. J Clin Oncol 2011;29:2582-9.
- Mao C, Qiu LX, Liao RY, et al. KRAS mutations and resistance to EGFR-TKIs treatment in patients with non-small cell lung cancer: a meta-analysis of 22 studies. Lung Cancer 2010;69:272-8.
- Janne PA, Shaw AT, Pereira JR, et al. Phase II double-blind, randomized study of selumetinib (SEL) plus docetaxel (DOC) versus DOC plus placebo as second-line treatment for advanced KRAS mutant non-small cell lung cancer (NSCLC). J Clin Oncol 2012;30:abstr 7503.
- Metro G, Crinò L. Advances on EGFR mutation for lung cancer. Transl Lung Cancer Res 2012;1:5-13.
- Janjigian YY, Smit EF, Horn L, et al. Activity of Afatinib/Cetuximab in patients (pts) with EGFR mutant non-small cell lung cancer (NSCLC) and acquired resistance (AR) to EGFR inhibitors. Ann Oncol 2012;23:abstr 1227.
- Thomas M, Reuss A, Fischer JR, et al. Innovations: Randomized phase II trial of erlotinib (E)/bevacizumab (B) compared with cisplatin (P)/gemcitabine (G) plus B in first-line treatment of advanced nonsquamous (NS) non-small cell lung cancer (NSCLC). J Clin Oncol 2011;29:abstr 7504.
- Morgillo F, Cascone T, D’Aiuto E, et al. Antitumour efficacy of MEK inhibitors in human lung cancer cells and their derivatives with acquired resistance to different tyrosine kinase inhibitors. Br J Cancer 2011;105:382-92.
- Yuen JS, Sim MY, Sim HG, et al. Combination of the ERK inhibitor AZD6244 and low-dose sorafenib in a xenograft model of human renal cell carcinoma. Int J Oncol 2012;41:712-20.
- Koh YW, Shah MH, Agarwal K, et al. Sorafenib and Mek inhibition is synergistic in medullary thyroid carcinoma in vitro. Endocr Relat Cancer 2012;19:29-38.


