
Interstitial lung disease and wedge resection are poor prognostic factors for non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide, with non-small cell lung cancer (NSCLC) accounting for more than 80% of all cases (1). Although the standard treatment for early-stage NSCLC is lobectomy combined with systematic lymph node dissection, some reports showed that sublobar resection for early-stage NSCLC has a more favorable prognosis than lobectomy (2,3).
Video-assisted thoracic surgery (VATS) for patients with NSCLC has been widely adopted, and various studies have reported the advantages of this approach (4-7). These reports revealed that VATS is associated with minimal pain, a shorter hospital stay, less-marked reduction in the inflammatory immune response, and better maintenance of the postoperative respiratory function than thoracotomy. However, the relationship between the prognosis and operative approaches, such as VATS, in NSCLC patients undergo pulmonary resection has not been elucidated.
Several prognostic factors in patients with early-stage NSCLC have been reported (8-14). Although the prognosis of stage IA NSCLC is considered good compared with the advanced stage, the age, gender, carcinoembryonic antigen (CEA), tumor size, operative procedure, surgical margin, pleural invasion, lymphatic invasion, histological type, and presence of combined pulmonary fibrosis and emphysema (CPFE) have been reported as prognostic factors in patients with early-stage NSCLC. Furthermore, the risk factors are varied and not consistent among reports.
In the present study, we retrospectively evaluated the prognostic factors in pathological stage IA NCSLC patients who underwent pulmonary resection. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1757/rc).
Methods
Patients
Nine hundred and fifty-six NSCLC patients who underwent pulmonary resection at Kanazawa Medical University between January 2002 and March 2020 were identified. Among these, 8 patients were lost to follow-up and 5 patients had insufficient data, and then, 540 patients who had sufficient data with pathological stage IA were enrolled in this retrospective study.
Data, including clinicopathological factors, such as the gender, age, comorbidities, smoking history, respiratory function, CEA, lung cancer lobe involvement, histological type, lymphatic invasion, vascular invasion, and differentiation, were collected. Comorbidities were divided into three categories: interstitial lung disease, malignant disease, and angina pectoris. The smoking history was evaluated using the Brinkman index, which is calculated by multiplying the number of cigarettes smoked per day by the number of years the subject has been smoking. Respiratory function parameters, such as the percent-predicted vital capacity (%VC) and forced expiratory volume in 1 second as a percentage of forced vital capacity (FEV1%), were collected.
Operative factors
The operative approach was divided into four categories: complete VATS (C-VATS, surgery performed only to provide a monitoring view), hybrid VATS (H-VATS, surgery combined with direct vision without rib spreading), robot-assisted thoracic surgery (RATS), and open thoracotomy. The operative procedure was stratified into three categories: wedge resection, segmentectomy, and lobectomy.
Postoperative complications
Postoperative complications were categorized into five grades according to the Clavien-Dindo classification system (15). The Clavien-Dindo classification was established in 1992. It is a simple and feasible grading system for all types of postoperative complications (16). In 2004, it was modified to allow grading of life-threatening complications and long-term disability caused by a complication (17). This revised version has defined five grades of severity with subgrades (grades I, II, IIIa, IIIb, IVa, IVb, and V), and the suffix “d” (for “disability”) is used to denote any postoperative impairment. This modified version of the Clavien-Dindo classification has been widely used in clinical practice.
Statistical analyses
Pearson’s chi-squared test of independence was used to compare the frequencies of the variables. The cumulative survival rates were calculated by the Kaplan-Meier method, and survival curves were compared using a log-rank test. The risk factors related to postoperative complications were analyzed using logistic regression analysis. All statistical analyses were two-sided and statistical significance was set at P<0.05. Statistical analyses were conducted using the JMP software program, version 13.2 (SAS Institute Inc., Cary, NC, USA).
Declaration and ethical statement
The present study was conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Kanazawa Medical University approved the protocol (No. I392) and written informed consent was obtained from all patients.
Results
Patient characteristics
The clinicopathological characteristics of the 540 NSCLC patients with pathological stage IA disease are listed in Table 1. Among these, 310 (57.4%) were men, and the median age was 70.6 years old. The median Brinkman index was 400. Altogether, 319 (59.1%) patients had comorbidities, including 7 (1.3%) with interstitial lung disease, 107 (19.8%) with malignant disease, and 35 (6.5%) with angina pectoris. The median CEA was 3.0 ng/mL, the median %VC was 100.7%, and the median FEV1% was 73.9%. The pulmonary lobes resected for NSCLC included the right upper lobe in 165 (30.6%) patients, right middle lobe in 33 (6.1%), right lower lobe in 118 (21.9%), left upper lobe in 130 (24.1%), and left lower lobe in 94 (17.4%) patients.
Table 1
| Characteristics | Value |
|---|---|
| Gender (male/female), n | 310/230 |
| Age (years), median [range] | 70.6 [22–92] |
| Comorbidity, n (%) | 319 (59.1) |
| Interstitial lung disease | 7 (1.3) |
| Malignant disease | 107 (19.8) |
| Angina pectoris | 35 (6.5) |
| Smoking index, median [range] | 400 [0–3,250] |
| CEA (ng/mL), median [range] | 3.0 [0.5–44.0] |
| %VC, median [range] | 100.7 [53.4–177.7] |
| FEV1%, median [range] | 73.9 [30.5–99.4] |
| Lobe of lung cancer (RU/RM/RL/LU/LL), n | 165/33/118/130/94 |
| Operative approach (RATS/C-VATS/H-VATS/open), n | 10/244/267/19 |
| Operative procedure (Wedge/Seg/Lob), n | 131/57/352 |
| Histology (Ad/Sq/LCNEC/Pleo/AdSq/large/carcinoid), n | 454/60/13/3/4/1/5 |
| Lymphatic invasion (present/absent), n | 92/448 |
| Vascular invasion (present/absent), n | 136/404 |
| Differentiation (G1/2/3/4), n | 240/243/45/12 |
| Postoperative complication, n (%) | 127 (23.5) |
| Clavien-Dindo grade (0/1/2/3a/3b), n | 413/0/48/76/3 |
| Thirty-day mortality, n (%) | 1 (0.2) |
| Ninety-day mortality, n (%) | 1 (0.2) |
| Postoperative hospital-stay (days), median [range] | 10 [3–89] |
CEA, carcinoembryonic antigen; %VC, percent-predicted vital capacity; FEV1%, forced expiratory volume % in one second; RU, right upper; RM, right middle; RL, right lower; LU, left upper; LL, left lower; RATS, robot-assisted thoracic surgery; C, complete; VATS, video-assisted thoracic surgery; H, hybrid; Wedge, wedge resection; Seg, segmentectomy; Lob, lobectomy; Ad, adenocarcinoma; Sq, squamous cell carcinoma; LCNEC, large cell neuroendocrine carcinoma; Pleo, pleomorphic carcinoma; AdSq, adenosquamous cell carcinoma; Large, large cell carcinoma; G, grade.
Operative factors
C-VATS was performed in 244 (45.2%) patients, H-VATS in 267 (49.4%) patients, RATS in 10 (1.9%) patients, and open thoracotomy in 19 (3.5%) patients. Wedge resection was performed in 131 (24.3%) patients, segmentectomy in 57 (10.6%), and lobectomy in 352 (65.2%).
Pathological factors
Histological types were divided into 7 types as follows: 454 (84.1%) patients were diagnosed with adenocarcinoma, 60 (11.1%) patients with squamous cell carcinoma, 13 (2.4%) with large-cell neuroendocrine carcinoma, 3 (0.6%) with pleomorphic carcinoma, 4 (0.7%) with adenosquamous cell carcinoma, 1 (0.2%) with large cell carcinoma, and 5 (0.9%) with carcinoid. Lymphatic invasion was present in 92 (17.0%) patients, and vascular invasion was present in 136 (25.2%) patients. The differentiation grade was divided into 4 categories: G1 in 240 (44.4%) patients, G2 in 243 (45.0%) patients, G3 in 45 (8.3%) patients, and G4 in 12 (2.2%) patients.
Postoperative complications
Postoperative complications were observed in 127 (23.5%) patients. Clavien-Dindo grade II complications were noted in 48 (8.9%) patients, grade IIIa in 76 (14.1%), and grade IIIb in 3 (0.6%). Major postoperative complications included air leakage in 65 (12.0%) patients, arrhythmia in 21 (3.9%) [atrial fibrillation in 19 (3.5%) patients and paroxysmal supraventricular tachycardia in 2 (0.4%) patients], atelectasis in 16 (3.0%), pneumonia in 12 (22.2%), a fever in 4 (0.7%), chylothorax in 2 (0.4%), and cerebral infarction in 2 (0.4%). Serious postoperative complications included postoperative bleeding in one patient, postoperative respiratory failure in one patient, and right middle lobe congestion in one patient. Two patients improved with surgery, and one patient was ameliorated by a ventilator. The median duration of postoperative hospital stay was 10 days, and the 30- and 90-day mortality rates were 0.05% and 0.05%, respectively.
Survival analyses
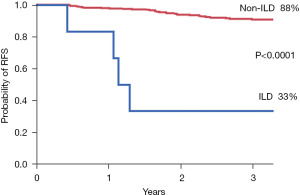
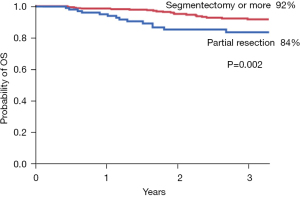
The median relapse-free survival (RFS) time is 765 days (range, 5– 6,330 days) and the median overall survival (OS) time is 839 days (range, 5–6,330 days). The RFS is shown in Figure 1. There were significant prognostic differences depending on the presence of interstitial lung disease (P<0.0001), CEA level (P=0.007), and wedge resection (P=0.002). The OS is shown in Figure 2. There were significant prognostic differences depending on the presence interstitial lung disease (P=0.0015), CEA (P<0.0001) and the smoking history (P=0.0003).
Univariate and multivariate analyses
The relationship between clinicopathological patient characteristics or operative factors and the RFS was analyzed (Table 2). The presence of interstitial lung disease (P<0.0001), CEA level (P=0.006), and operative procedure (P=0.002) showed significant differences in the univariate analysis. Furthermore, the presence of interstitial lung disease [hazard ratio (HR): 7.725, 95% confidence interval (CI): 2.144–21.776, P=0.003], CEA level (HR: 1.923, 95% CI: 1.015–3.518, P=0.045), and operative procedure (HR: 2.086, 95% CI: 1.100–3.825, P=0.025) were considered risk factors for the RFS in the multivariate analysis.
Table 2
| Variables | 5-year RFS (%) | Univariate P value | Multivariate analysis | ||
|---|---|---|---|---|---|
| HR | 95% CI | P value | |||
| Gender | 0.676 | – | – | – | |
| Male | 86.3 | ||||
| Female | 88.9 | ||||
| Age | 0.175 | – | – | – | |
| ≤75 years | 85.7 | ||||
| >75 years | 90.8 | ||||
| Comorbidity | – | – | – | ||
| Malignant disease | 0.913 | ||||
| Present | 90.5 | ||||
| Absent | 86.8 | ||||
| Interstitial lung disease | <0.0001 | 7.725 | 2.144–21.776 | 0.003 | |
| Present | 33.3 | ||||
| Absent | 88.2 | ||||
| Angina pectoris | 0.651 | – | – | – | |
| Present | 88.7 | ||||
| Absent | 87.3 | ||||
| Smoking history | 0.140 | – | – | – | |
| Present | 86.3 | ||||
| Absent | 88.7 | ||||
| %VC | 0.611 | – | – | – | |
| <80 | 92.9 | ||||
| ≥80 | 87.0 | ||||
| FEV1% | 0.209 | – | – | – | |
| <70 | 85.5 | ||||
| ≥70 | 88.2 | ||||
| CEA | 0.006 | 1.923 | 1.015–3.518 | 0.045 | |
| >5 ng/mL | 79.0 | ||||
| ≤5 ng/mL | 89.9 | ||||
| Operative approach | 0.331 | – | – | – | |
| C-VATS or RATS | 82.4 | ||||
| H-VATS or Open | 88.7 | ||||
| Operative procedure | 0.002 | 2.086 | 1.100–3.825 | 0.025 | |
| Wedge resection | 81.1 | ||||
| Segmentectomy or more | 89.2 | ||||
| Postoperative complication | 0.641 | – | – | – | |
| Present | 84.6 | ||||
| Absent | 88.1 | ||||
| Lymphatic invasion | 0.086 | – | – | – | |
| Present | 81.9 | ||||
| Absent | 88.6 | ||||
| Vascular invasion | 0.120 | – | – | – | |
| Present | 82.3 | ||||
| Absent | 89.2 | ||||
| Differentiation | 0.101 | – | – | – | |
| G1 or 2 | 85.4 | ||||
| G3 or 4 | 82.6 | ||||
NSCLC, non-small cell lung cancer; RFS, relapse-free survival; HR, hazard ratio; CI, confidence interval; %VC, percent-predicted vital capacity; FEV1%, forced expiratory volume % in one second; CEA, carcinoembryonic antigen; C, complete; VATS, video-assisted thoracic surgery; RATS, robot-assisted thoracic surgery; H, hybrid; G, grade.
The relationship between clinicopathological patient characteristics or operative factors and the OS was analyzed (Table 3). The presence of interstitial lung disease (P=0.0015), smoking history (P=0.0003), and CEA level (P<0.0001) showed significant differences in the univariate analysis. Furthermore, the smoking history (HR: 2.539, 95% CI: 1.372–5.002, P=0.002) and CEA level (HR: 2.464, 95% CI: 1.389–4.317, P=0.002) were considered risk factors for the OS in the multivariate analysis.
Table 3
| Variables | 5-year OS (%) | Univariate P value | Multivariate analysis | ||
|---|---|---|---|---|---|
| HR | 95% CI | P value | |||
| Gender | 0.344 | – | – | – | |
| Male | 82.0 | ||||
| Female | 86.6 | ||||
| Age | 0.276 | – | – | – | |
| ≤75 years | 82.1 | ||||
| >75 years | 87.2 | ||||
| Comorbidity | |||||
| Malignant disease | 0.913 | – | – | – | |
| Present | 83.1 | ||||
| Absent | 83.7 | ||||
| Interstitial lung disease | 0.0015 | 3.431 | 0.54–11.931 | 0.158 | |
| Present | 62.5 | ||||
| Absent | 84.2 | ||||
| Angina pectoris | 0.575 | – | – | – | |
| Present | 91.4 | ||||
| Absent | 83.4 | ||||
| Smoking history | 0.0003 | 2.539 | 1.372–5.002 | 0.002 | |
| Present | 77.2 | ||||
| Absent | 91.6 | ||||
| %VC | 0.969 | – | – | – | |
| <80 | 89.6 | ||||
| ≥80 | 83.4 | ||||
| FEV1% | 0.293 | – | – | – | |
| <70 | 81.6 | ||||
| ≥70 | 84.7 | ||||
| CEA | <0.0001 | 2.464 | 1.389–4.317 | 0.002 | |
| >5 ng/mL | 68.6 | ||||
| ≤5 ng/mL | 88.5 | ||||
| Operative approach | 0.122 | – | – | – | |
| C-VATS or RATS | 78.1 | ||||
| H-VATS or Open | 85.4 | ||||
| Operative procedure | 0.942 | – | – | – | |
| Wedge resection | 90.0 | ||||
| Segmentectomy or more | 82.7 | ||||
| Postoperative complication | 0.098 | – | – | – | |
| Present | 75.6 | ||||
| Absent | 86.3 | ||||
| Lymphatic invasion | 0.661 | – | – | – | |
| Present | 76.8 | ||||
| Absent | 85.5 | ||||
| Vascular invasion | 0.499 | – | – | – | |
| Present | 78.6 | ||||
| Absent | 85.6 | ||||
| Differentiation | 0.201 | – | – | – | |
| G1 or 2 | 84.5 | ||||
| G3 or 4 | 78.0 | ||||
NSCLC, non-small cell lung cancer; OS, overall survival; HR, hazard ratio; CI, confidence interval; %VC, percent-predicted vital capacity; FEV1%, forced expiratory volume % in one second; CEA, carcinoembryonic antigen; C, complete; H, hybrid; VATS, video-assisted thoracic surgery; G, grade; RATS, robot-assisted thoracic surgery.
Discussion
In the present study, we analyzed the prognostic factors for inpatients who underwent pulmonary resection for pathological stage IA NSCLC. This study demonstrated that interstitial lung disease, CEA, and partial resection were significant prognostic factors for the RFS, whereas CEA and smoking history were significant prognostic factors for the OS. Although the survival rate has been similar between patients treated with limited resection and patients receiving lobectomy in some reports (18,19), wedge resection was reported to be a risk factor for locoregional recurrence in other reports (8, 20-22). Because these reports showed a trend toward a higher locoregional recurrence rate in patients who underwent wedge resection than in those receiving segmentectomy (8,20-22), segmentectomy appears to be the more suitable surgical procedure in patients being considered for sublobar resection.
We previously reported that the presence of CPFE was a statistically significant predictor of recurrence for patients with clinical stage I NSCLC (12). Furthermore, lung cancer patients with idiopathic pulmonary fibrosis (IPF) showed a significantly worse mortality rate than lung cancer patients without IPF (23). IPF is the most frequent and severe type of idiopathic interstitial pneumonia and has a median survival of approximately 3 years after the diagnosis (24). In the treatment of lung cancer patients with IPF, physicians are reluctant to treat lung cancer because of the poor prognosis (25). The GAP [gender (G), age (A), and two lung physiology variables (P)] staging system has been used to predict the mortality and timing of lung transplantation in IPF patients (26). Although no beneficial treatment modalities were determined for GAP stage II/III, active therapies, such as surgery, for lung cancer patients with IPF in GAP stage I were recommended in a previous study (27). Therefore, the benefit of surgery for NSCLC patients with interstitial lung disease should be carefully evaluated using the GAP staging system.
Limitations
Several limitations associated with the present study warrant mention. First, the study was retrospective in nature and potentially involved unobserved cofounding and selection biases. Second, the present study was performed at a single institution, and the study population was relatively small.
In conclusion, our findings described the prognostic factors in pathological stage IA NCSLC patients who underwent pulmonary resection. This study revealed that the presence of interstitial lung disease, CEA level, and operative procedure were risk factors for the RFS, while the smoking history and CEA level were risk factors for the OS. These results suggest that segmentectomy is a more suitable surgical procedure than wedge resection for patients who are being considered for sublobar resection. Furthermore, the benefit of surgery for NSCLC patients with interstitial lung disease should be carefully evaluated.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1757/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1757/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1757/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1757/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Kanazawa Medical University approved the protocol (No. I392) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Berfield KS, Wood DE. Sublobar resection for stage IA non-small cell lung cancer. J Thorac Dis 2017;9:S208-10. [Crossref] [PubMed]
- Sakurai H, Asamura H. Sublobar resection for early-stage lung cancer. Transl Lung Cancer Res 2014;3:164-72. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8. [Crossref] [PubMed]
- Sakuraba M, Miyamoto H, Oh S, et al. Video-assisted thoracoscopic lobectomy vs. conventional lobectomy via open thoracotomy in patients with clinical stage IA non-small cell lung carcinoma. Interact Cardiovasc Thorac Surg 2007;6:614-7. [Crossref] [PubMed]
- Koike T, Koike T, Yoshiya K, et al. Risk factor analysis of locoregional recurrence after sublobar resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;146:372-8. [Crossref] [PubMed]
- Shimada Y, Yoshida J, Hishida T, et al. Predictive factors of pathologically proven noninvasive tumor characteristics in T1aN0M0 peripheral non-small cell lung cancer. Chest 2012;141:1003-9. [Crossref] [PubMed]
- Koike T, Koike T, Yamato Y, et al. Predictive risk factors for mediastinal lymph node metastasis in clinical stage IA non-small-cell lung cancer patients. J Thorac Oncol 2012;7:1246-51. [Crossref] [PubMed]
- Koike T, Tsuchiya R, Goya T, et al. Prognostic factors in 3315 completely resected cases of clinical stage I non-small cell lung cancer in Japan. J Thorac Oncol 2007;2:408-13. [Crossref] [PubMed]
- Maeda R, Funasaki A, Motono N, et al. Combined pulmonary fibrosis and emphysema predicts recurrence following surgery in patients with stage I non-small cell lung cancer. Med Oncol 2018;35:31. [Crossref] [PubMed]
- Tas F, Ciftci R, Kilic L, et al. Age is a prognostic factor affecting survival in lung cancer patients. Oncol Lett 2013;6:1507-13. [Crossref] [PubMed]
- Sayar A, Turna A, Solak O, et al. Nonanatomic prognostic factors in resected nonsmall cell lung carcinoma: the importance of perineural invasion as a new prognostic marker. Ann Thorac Surg 2004;77:421-5. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Prognostic factors in clinical stage I non-small cell lung cancer. Ann Thorac Surg 1999;67:927-32. [Crossref] [PubMed]
- Rena O, Oliaro A, Cavallo A, et al. Stage I non-small cell lung carcinoma: really an early stage? Eur J Cardiothorac Surg 2002;21:514-9. [Crossref] [PubMed]
- Hung JJ, Wang CY, Huang MH, et al. Prognostic factors in resected stage I non-small cell lung cancer with a diameter of 3 cm or less: visceral pleural invasion did not influence overall and disease-free survival. J Thorac Cardiovasc Surg 2007;134:638-43. [Crossref] [PubMed]
- Nakamura H, Kawasaki N, Taguchi M, et al. Survival following lobectomy vs limited resection for stage I lung cancer: a meta-analysis. Br J Cancer 2005;92:1033-7. [Crossref] [PubMed]
- Griffin JP, Eastridge CE, Tolley EA, et al. Wedge resection for non-small cell lung cancer in patients with pulmonary insufficiency: prospective ten-year survival. J Thorac Oncol 2006;1:960-4. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87-93. [Crossref] [PubMed]
- El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol 2007;14:2400-5. [Crossref] [PubMed]
- Yoon JH, Nouraie M, Chen X, et al. Characteristics of lung cancer among patients with idiopathic pulmonary fibrosis and interstitial lung disease - analysis of institutional and population data. Respir Res 2018;19:195. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Barczi E, Nagy T, Starobinski L, et al. Impact of interstitial lung disease and simultaneous lung cancer on therapeutic possibilities and survival. Thorac Cancer 2020;11:1911-7. [Crossref] [PubMed]
- Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684-91. [Crossref] [PubMed]
- Han S, Lee YJ, Park JS, et al. Prognosis of non-small-cell lung cancer in patients with idiopathic pulmonary fibrosis. Sci Rep 2019;9:12561. [Crossref] [PubMed]


