
Association of marital status with cardiovascular outcome in patients with breast cancer
Introduction
Breast cancer is the most common carcinoma among women, accounting for approximately 24.2% of all female cancer cases, which has become the leading cause of cancer death in female patients (1). Death causes of breast cancer patients include cancer-related causes and non-cancer-related causes. Cardiovascular death, as the major cause of non-cancer-related morbidities, serves as the major contribution to mortality in patients with breast cancer (2), indicating that the prevention and control of cardiovascular death may contribute to the survival improvement in breast cancer patients. The risk factor identification of cardiovascular death is one of important strategies to prevent cardiovascular death in patients with breast cancer.
Previous studies mostly focused on the relationship between anticancer treatment and cardiovascular death in breast cancer patients (3-5). Besides cardiovascular death risk originating from anti-tumor therapy, social and psychological factors were emphasized in cardio-oncology (6,7). Marital status is one of ignored but important social and psychological factors (8), however, the relationship between marital status and cardiovascular death in breast cancer patients was still unknown. Marital status was evidently relevant to overall survival and cancer-caused special survival in cancer patients (9,10). Married status was related to lower cardiovascular events risk in general population (8,11), and married patients had lower risk of adverse cardiovascular events (12). These findings suggested marital status was playing a significant role in cardiovascular diseases in patients with breast cancer. Furthermore, previous studies reported that unmarried cancer patients were at a higher risk of stroke related death (6) and disease-specific mortality (7). However, these studies only provided limited evidence because of the limited types of unknown marital status and univariate analyses (6,7). The influence of different marital status (e.g., married, single, separation, divorce and widow) on cardiovascular death in breast cancer patients remained unclear. Hence, the present population-based, propensity-matched study was conducted to arrest the association between marital status and cardiovascular death risk, and to further explore the impact of different marital status on cardiovascular death in patients with breast cancer based on the Surveillance, Epidemiology, and End Results (SEER) database. We presented the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1261/rc).
Methods
The source of data
We extracted the data from the SEER*Stat software (version 8.3.6) with access to the SEER database, an authoritative shared database in the U.S., which had been widely used for cardio-oncology studies. Publicly available information in SEER did not require ethical approval. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study population and design
The female patients with pathological diagnosis of breast cancer were identified in the SEER database from 2010 to 2014. The patients were selected if meeting the following criteria: (I) case selection (site and morphology, primary site-labeled) being ‘C50.x’; (II) age at diagnosis greater than or equal to 25 years; (III) known marital status; (IV) female; (V) clear cause of death. The exclusion criteria were as follows: (I) without pathological diagnosis; (II) individuals with multiple primary tumors; (III) either autopsy only or death certificate only; (IV) missing race record; (V) unclear grade; (VI) unclear stage [breast cancer Adjusted the American Joint Committee on Cancer (AJCC) staging system stage Cancer Staging Manual 6th Edition]; (VII) unknown estrogen receptor (ER) status or progesterone receptor (PR) status or human epidermal receptor 2 (HER2) status. A total of 182,666 breast cancer patients were divided into two groups: married (legal marriage) and unmarried (single, separated, divorced, widowed and domestic partners) (9), which were used to explore the association of marital status with cardiovascular death in breast cancer patients. Domestic partners were then excluded due to small sample size (N=493). In total, 182,173 breast cancer patients were classified as five subgroups as follows: married (N=107,043), single (N=28,246), separated (N=2070), divorced (N=20,656) and widowed (N=24,158). We further quantitatively evaluated the impact of different marital status on cardiovascular death in breast cancer patients. Categorical variables included marital status (married and unmarried), age at diagnosis (25–60 and >60 years) (13), race (white, black and other), income (low and high) (14), grade (low and high), ER status (positive and negative), PR status (positive and negative), HER2 status (positive and negative), AJCC stage (I, II, III and IV), surgery (yes and no evidence), chemotherapy (yes and no evidence) and radiotherapy (yes and no evidence). As for the income, low household income represented the worst 50% among all breast cancer patients, and high household income represented the best 50% among all breast cancer patients (14). Low grade included well differentiated (Grade I) and moderately differentiated (Grade II), while high grade included poorly differentiated (Grade III) and undifferentiated (Grade IV).
Propensity score matching (PSM)
A 1:1 PSM was utilized to balance the bias between the married and unmarried groups referring to our previous project (15,16). Logistic regression was used to estimate propensity scores. The nearest neighbor algorithm with caliper width of 0.02 was applied to perform matching. All the variables in the baseline were enrolled into the propensity score calculation. When the P values were higher than 0.05, we considered that the two groups reached a balance.
Study endpoint
The primary endpoint was cardiovascular death. According to the International Classification of Diseases-10 (ICD-10) codes (17), it was defined as death attributable to cardiovascular diseases including disease of heart, atherosclerosis, cerebrovascular disease, aortic aneurysm and dissection, other diseases of arteries, arterioles and capillaries, and hypertension without heart disease. The follow-up time was counted as the period from first diagnosis with breast cancer to the date of death or last follow-up. The deadline date was December 31, 2015.
Statistical analysis
The statistical analyses were conducted by SPSS version 25.0 (SPSS, Chicago, IL, USA), R software version 3.6.1, and Stata version 15 (StataCorp, College Station, USA). Categorical variables were presented as frequencies. χ2 test was used to analyze the associations between marital status and these categorical variables at baseline. Given that non-cardiovascular death was a competing risk, univariate competing-risks models were applied to evaluate the relationship between marital status (married and unmarried) and cardiovascular death risk in patients with breast cancer. Multivariate competing-risks regression analyses were further performed to avoid false-positive results (18). Further subgroup analyses were used to evaluate the associations between different marital status (married, single, separated, divorced and widowed) and cardiovascular death risk in patients with breast cancer. Statistical significance was defined by a two-tailed P value less than 0.05.
Results
Baseline characteristic in study participants
As shown in Table 1, a total of 182,666 patients with breast cancer were enrolled to present study before PSM. There were significant differences on age at diagnosis (P<0.001), race (P<0.001), income (P<0.001), grade (P<0.001), ER status (P<0.001), PR status (P<0.001), HER2 status (P=0.001), AJCC stage (P<0.001), surgery (P<0.001), chemotherapy (P<0.001) and radiotherapy (P<0.001) between the two groups. After PSM, 141,302 breast cancer patients were eventually identified. There were no significant differences on the above-mentioned variables between the two groups, except race (P<0.001), surgery (P=0.014), PR status (P=0.008) and ER status (P=0.028), whereas PR status and ER status were not related to the cardiovascular death risk in patients with breast cancer (Table S1).
Table 1
| Variables | Before PSM, N (%) | After PSM, N (%) | |||||
|---|---|---|---|---|---|---|---|
| Married | Unmarried | P | Married | Unmarried | P | ||
| N | 107,043 | 75,623 | 70,651 | 70,651 | |||
| Age at diagnosis | <0.001 | 0.970 | |||||
| 25–60 years | 61,453 (57.4) | 33,290 (44.0) | 33,073 (46.8) | 33,066 (46.8) | |||
| >60 years | 45,590 (42.6) | 42,333 (56.0) | 37,578 (53.2) | 37,585 (53.2) | |||
| Race | <0.001 | <0.001 | |||||
| White | 87,807 (82.0) | 57,037 (75.4) | 56,836 (80.4) | 53,779 (76.1) | |||
| Black | 7,486 (7.0) | 12,841 (17.0) | 11,601 (16.4) | 5,515 (7.8) | |||
| Other& | 11,750 (11.0) | 5745 (7.6) | 5,271 (7.5) | 8,300 (11.7) | |||
| Income* | <0.001 | 0.412 | |||||
| Low | 51,709 (48.3) | 40,042 (52.9) | 36,413 (51.5) | 36,567 (51.8) | |||
| High | 55,334 (51.7) | 35,581 (47.1) | 34,238 (48.5) | 34,084 (48.2) | |||
| Grade# | <0.001 | 0.096 | |||||
| Low | 71,224 (66.5) | 48,979 (64.8) | 46,318 (65.6) | 46,020 (65.1) | |||
| High | 35,819 (33.5) | 26,644 (35.2) | 24,333 (34.4) | 24,631 (34.9) | |||
| ER status | <0.001 | 0.028 | |||||
| Positive | 88,774 (82.9) | 61,909 (81.9) | 58,061 (82.2) | 57,744 (81.7) | |||
| Negative | 18,269 (17.1) | 13,714 (18.1) | 12,590 (17.8) | 12,907 (18.3) | |||
| PR status | <0.001 | 0.008 | |||||
| Positive | 78,232 (73.1) | 53,786 (71.1) | 50,822 (71.9) | 50,373 (71.3) | |||
| Negative | 28,811 (26.9) | 21,837 (28.9) | 19,829 (28.1) | 20,278 (28.7) | |||
| HER2 status | 0.001 | 0.441 | |||||
| Positive | 16,813 (15.7) | 11,464 (15.2) | 10,808 (15.3) | 10,704 (15.2) | |||
| Negative | 90,230 (84.3) | 64,159 (84.8) | 59,843 (84.7) | 59,947 (84.8) | |||
| AJCC stage | <0.001 | 0.136 | |||||
| I | 55,868 (52.2) | 35,212 (46.6) | 34,673 (49.1) | 34,462 (48.8) | |||
| II | 35,919 (33.6) | 26,335 (34.8) | 24,414 (34.2) | 24,136 (34.2) | |||
| III | 11,504 (10.7) | 9,693 (12.8) | 8,383 (11.9) | 8,658 (12.3) | |||
| IV | 3,752 (3.5) | 4,383 (5.8) | 3,451 (4.9) | 3,395 (4.8) | |||
| Surgery | <0.001 | 0.014 | |||||
| Yes | 102,342 (95.6) | 69,275 (91.6) | 66,210 (93.7) | 66,453 (94.1) | |||
| No evidence | 4,701 (4.0) | 6,348 (8.4) | 4,441 (6.3) | 4198 (5.9) | |||
| Chemotherapy | <0.001 | 0.833 | |||||
| Yes | 58,144 (54.3) | 45,706 (60.4) | 41,304 (58.5) | 41,265 (58.4) | |||
| No evidence | 48,899 (45.7) | 29,917 (39.6) | 29,347 (41.5) | 29,386 (41.6) | |||
| Radiotherapy | <0.001 | 0.953 | |||||
| Yes | 47,017 (43.9) | 38,047 (50.3) | 34,066 (48.2) | 34,055 (48.2) | |||
| No evidence | 60,026 (56.1) | 37,576 (49.7) | 36,585 (51.8) | 36,596 (51.8) | |||
&, other includes American Indian/Alaska Native and Asian/Pacific Islander; *, low (median household income was the worst 50% among all breast cancer patients) and high (median household income was the best 50%); #, low (Grade I: well-differentiated and Grade II: moderately differentiated) and high (Grade III: poorly differentiated and Grade IV: undifferentiated). PSM, propensity-score matching; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal receptor 2; AJCC, the American Joint Committee on Cancer.
Association of marital status with cardiovascular death risk in patients with breast cancer
Before PSM, unmarried condition was associated with increased cardiovascular death risk [unadjusted hazard ratio (HR) =3.085, 95% confidence interval (CI): 2.831–3.361, P<0.001] compared to their married counterparts in patients with breast cancer, as shown in Figure 1A, Table 2, and Table S1. To correct for confounding bias, multivariate analysis further demonstrated that marital status was an independent predictor for cardiovascular death in breast cancer patients, as shown in Table 2. Adjustment for confounding covariates (Model 1: all covariates in the baseline) showed that unmarried condition was still associated with increased cardiovascular death risk (HR =2.043, 95% CI: 1.871–2.230, P<0.001). After adjustment (Model 2: it was same as Model 1, and also including ER status and PR status; Model 3: it was the same as Model 2, and also included race and surgery), it indicated a robust adjusted HR on cardiovascular death risk (Model 2: HR =2.039, 95% CI: 1.867–2.226, P<0.001; Model 3: HR =1.971, 95% CI: 1.802–2.155, P<0.001)

Table 2
| Variables | Before PSM | After PSM | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Unadjusted HR | |||||
| Married | Reference | Reference | |||
| Unmarried | 3.085 (2.831–3.361) | <0.001 | 2.012 (1.835–2.208) | <0.001 | |
| Model 1a | |||||
| Married | Reference | Reference | |||
| Unmarried | 2.043 (1.871–2.230) | <0.001 | 1.958 (1.785–2.148) | <0.001 | |
| Model 2b | |||||
| Married | Reference | Reference | |||
| Unmarried | 2.039 (1.867–2.226) | <0.001 | 1.954 (1.781–2.144) | <0.001 | |
| Model 3c | |||||
| Married | Reference | Reference | |||
| Unmarried | 1.971 (1.802–2.155) | <0.001 | 1.920 (1.748–2.107) | <0.001 | |
a, Model 1: HR was adjusted for statistically significant factors according to univariate analysis (age at diagnosis, income, grade, HER2 status, AJCC stage, chemotherapy and radiotherapy); b, Model 2: it was the same as Model 1, and also included ER status and PR status; c, Model 3: It is the same as Model 2, and also includes race and surgery. PSM, propensity-score matching; HR, hazard ratio; CI, confidence interval; HER2, human epidermal receptor 2; AJCC, the American Joint Committee on Cancer; ER, estrogen receptor; PR, progesterone receptor.
After PSM, similar phenomenon was observed, and unmarried breast cancer patients still had higher cardiovascular death risk in unadjusted model: HR =2.012, 95% CI: 1.835–2.208, P<0.001), Model 1 (HR =1.958, 95% CI: 1.785–2.148, P<0.001), Model 2 (HR =1.954, 95% CI: 1.781–2.144, P<0.001), and Model 3 (HR =1.920, 95% CI: 1.748–2.107, P<0.001), as shown in Figure 1B, Table 2, and Tables S1-S4.
Association of different marital status with cardiovascular death risk in patients with breast cancer
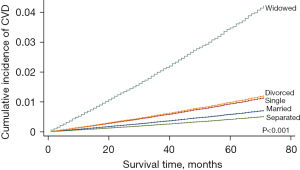
As shown in Table 3, the separated condition was not correlated with cardiovascular death risk before PSM (HR =0.711, 95% CI: 0.381–1.327, P=0.284) and after PSM (HR =0.886, 95% CI: 0.474–1.658, P=0.705). With the exception of separated condition, the other three unmarried conditions were linked to higher cardiovascular death risk as follows: single (adjusted HR =1.623, 95% CI: 1.421–1.853, P<0.001), divorced (adjusted HR =1.394, 95% CI: 1.209–1.608, P<0.001), and widowed (adjusted HR =2.460, 95% CI: 2.227–2.717, P<0.001) (Table 3 and Table S5). In particularly, widowed condition had the highest cardiovascular death risk in all 4 unmarried subgroups (Figure 2, Table 3 and Table S5).
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI)* | P value | ||
| Marital status | |||||
| Married | Reference | Reference | |||
| Single | 1.617 (1.420–1.842) | <0.001 | 1.623 (1.421–1.853) | <0.001 | |
| Separated | 0.711 (0.381–1.327) | 0.284 | 0.886 (0.474–1.658) | 0.705 | |
| Divorced | 1.712 (1.486–1.973) | <0.001 | 1.394 (1.209–1.608) | <0.001 | |
| Widowed | 6.208 (5.658–6.811) | <0.001 | 2.460 (2.227–2.717) | <0.001 | |
*, HR was adjusted for statistically significant factors according to univariate analysis (age at diagnosis, race, income, grade, HER2 status, AJCC stage, surgery, chemotherapy and radiotherapy). HR, hazard ratio; CI, confidence interval; HER2, human epidermal receptor 2; AJCC, the American Joint Committee on Cancer.
Discussion
Marriage, one of the important social and psychological factors, has been proven closely related with the cardiovascular prognosis of patients with cancer. Breast cancer is one of the mostly common forms of cancer worldwide. The association of marital status (married vs. unmarried) with cardiovascular death risk of patients with breast cancer is still ambiguous. Previous studies found that marital status was associated with cardiovascular death risk of patients with breast cancer, and unmarried patients suffered from higher cardiovascular death risk. However, these findings may be confounded by three factors as follows: firstly, unlike the various classifications of marital status, the limited type of “unknown marital status” may limit their results. Secondly, these studies were restricted to univariate analysis and could not exclude possible false-positive data. Lastly, these studies neglected the competing risk of non-cardiovascular death. Hence, by reducing inter-group bias via PSM and correcting for potential confounding factors with multivariate competitive risk model analysis, the present study found that unmarried condition was obviously associated with increased cardiovascular death risk (approximately by 1-fold) in patients with breast cancer. It was partially consistent with a prospective cohort study by Schultz et al. (8), who reported that the cardiovascular mortality risk in 1,963 unmarried patients increased by 24%, a observational study by Zaorsky et al. who found that the stroke death risk of 2,895,946 unmarried cancer patients also increased by 1.15-fold (6), and an investigation by Stoltzfus et al. who reported that the risk of fatal heart disease in 2,895,946 unmarried cancer patients increased by 1.23-fold (7). Similarly, Onwudiwe et al. (19) further confirmed that cardiovascular event-free survival in 30,239 unmarried patients with breast cancer raised by 1.22-fold after adjusting baseline cardiovascular risk factors.
As far as the cardiovascular benefit of marriage was concerned, it was owing to social and emotional support, especially spousal support (11,20) and supervision (21,22). Firstly, spousal support and supervision promoted healthy lifestyle changes (defined as primary prevention of cardiovascular disease) for breast cancer survivors (21,22). Since being diagnosed with breast cancer, married female was more likely to cultivate a healthy lifestyle due to the supervision of spouse. Conversely, unmarried breast cancer patients, who lacked the support and care from spouse, were more likely to suffer from psychological distress and become addicted to bad lifestyle (e.g., smoking and excessive drinking) (23-26). Secondly, supervision and care of spouse usually promoted early detection, early diagnosis and early treatment (defined as secondary prevention of cardiovascular disease for breast cancer survivors) (11,20). Support from spouse improved adherence to treatments in breast cancer patients with cardiovascular complications, while unmarried ones were possibly non-adherent to their prescribed medications, thus related to higher cardiovascular death risk (27).
Besides social and emotional support, other potential explanations might be related to physiological factors. The cancer diagnosis brought out varying degrees of psychological stress, such as cancer-related distress, depression and anxiety, which were considered as risk factors increasing the cardiovascular death risk in breast cancer patients (28,29). The married breast cancer patients suffered from less psychological burden, because the spouse shared the burden of negative emotions and offered positive emotional support (30). Unmarried patients may tend to suffer more negative emotions. Physiological factors (e.g., perceived stress and chronicity of stress) mediated the harmful influence of marital status on cardiovascular events among breast cancer patients via higher oxidative stress, lower telomerase activity, and shorter telomere length (31). Oxidative stress and shortened telomeres might result in higher rate of cardiovascular death in unmarried patients. A Swedish population-based cohort study reported that single individuals had a shorter leucocyte telomere length (a biomarker of aging) and higher risk of cardiovascular diseases than married individuals (32). In addition, increased level of cortisol was related to poorer cardiovascular outcomes. Unmarried patients had higher cortisol levels than married patients (33), which induced cardiac dysfunction (34) and further increases the risk of cardiovascular disease (35).
What’s more important, the present study is the first to evaluate the associations of different unmarried conditions with cardiovascular death risk in unmarried patients with breast cancer. Unmarried patients with breast cancer had elevated cardiovascular death risk, but the harmful effect was heterogeneous with different unmarried conditions. According to marriage history, unmarried breast cancer patients were divided into two subgroups as follows: those without marriage history (e.g., single) and those with marriage history (e.g., widowed and divorced). The cardiovascular death risk in unmarried breast cancer patients without marriage history (e.g., single) was increased by 60% compared to their married counterparts. Among the unmarried breast cancer patients with marriage history, the risk under widowed circumstances was much worse (increased by 50%) than singled while slightly lower (decreased by 15%) in divorced. Our findings were partially consistent with the Japanese prospective cohort study by Tanno et al. (36) who reported that single, divorced, or widowed hemodialysis patients were all at greater cardiovascular death risk than married patients, and the investigation by Otto et al. who found that single, divorced or widowed patients had higher risk of cardiovascular disease and its related death, compared with married individuals (37). Possible potential explanations may be owed to psychological stress under different unmarried status. Subjects with single, divorced and widowed had a tendency of diagnosis with a chronic stress syndrome (38), but the stress status was manifested with heterogeneity based on different unmarried conditions (30). Chronic exposure to stressors caused endocrine and immune system dysfunction that contributed to sustained low-grade inflammation (39). Growing evidence demonstrated that different unmarried status were linked to varying degrees of low-grade inflammation (40,41). It is well-known that low-grade systemic chronic inflammation acts the essential role in occurrence and development of cardiovascular events. Indeed, the highest cardiovascular death risk was found in widowed breast cancer patients. The loss of spouse could render “broken heart” which leaded to lower heart rate variability and higher pro-inflammatory cytokine levels, further putting patients at high cardiovascular death risk (42). These findings suggested that regular test of high-sensitivity C-reactive protein level could be one of the significant strategies to improve cardiovascular prognosis in breast cancer patients with different unmarried conditions.
Strengths and limitations
The strengths in this study were the large sample size and long follow-up time. It was the first population-based study to show the association between marital status and cardiovascular death risk in patients with breast cancer. Some limitations of this study need to be mentioned. Firstly, marital status was only registered at the time of diagnosis of breast cancer in SEER database, and we were unable to further explore the impact of marriage change on cardiovascular death in breast cancer patients. Secondly, the quality of marriage was also unavailable in SEER database, and we could not further investigate the effect of marriage quality on cardiovascular death risk in breast cancer patients. Thirdly, there is a possibility that married women may be physically, psychologically or emotionally healthier than those who were unmarried, which may increase selection bias. Finally, the SEER registries did not provide information about comorbidities and previous medical history. Though we balanced the variables at baseline levels, there might be unobserved variables that might affect study outcomes, such as comorbidities and previous medical history. Similar to the previous SEER-based cardio-oncology studies (6,7,43,44), our study couldn’t further explore the contribution of marital status on cardiovascular death of breast cancer patients after adjusting comorbidities and previous medical history.
Conclusions
Unmarried breast cancer patients (e.g., single, divorced and widowed) were associated with higher cardiovascular death risk compared with married patients, suggesting that more attention and humanistic care should be offered to unmarried patients in the management of female breast cancer patients, especially to those widowed patients.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (81100235), the Guangzhou Science and Technology Project of China (201804010214) and the Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation. (“Climbing Program” Special Funds.) (pdjh2021b0409).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1261/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1261/coif). All authors report that this study was supported by the National Natural Science Foundation of China (81100235), the Guangzhou Science and Technology Project of China (201804010214) and the Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (“Climbing Program” Special Funds.) (pdjh2021b0409). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Patnaik JL, Byers T, DiGuiseppi C, et al. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res 2011;13:R64. [Crossref] [PubMed]
- Thavendiranathan P, Abdel-Qadir H, Fischer HD, et al. Breast Cancer Therapy-Related Cardiac Dysfunction in Adult Women Treated in Routine Clinical Practice: A Population-Based Cohort Study. J Clin Oncol 2016;34:2239-46. [Crossref] [PubMed]
- Saiki H, Petersen IA, Scott CG, et al. Risk of Heart Failure With Preserved Ejection Fraction in Older Women After Contemporary Radiotherapy for Breast Cancer. Circulation 2017;135:1388-96. [Crossref] [PubMed]
- Dhesy-Thind S, Ellis PM, Mukherjee SD, et al. Longitudinal High-Sensitivity Cardiac Troponin I Measurements in Patients With Breast Cancer Receiving Trastuzumab. Can J Cardiol 2019;35:545.e1-2. [Crossref] [PubMed]
- Zaorsky NG, Zhang Y, Tchelebi LT, et al. Stroke among cancer patients. Nat Commun 2019;10:5172. [Crossref] [PubMed]
- Stoltzfus KC, Zhang Y, Sturgeon K, et al. Fatal heart disease among cancer patients. Nat Commun 2020;11:2011. [Crossref] [PubMed]
- Schultz WM, Hayek SS, Samman Tahhan A, et al. Marital Status and Outcomes in Patients With Cardiovascular Disease. J Am Heart Assoc 2017;6:005890. [Crossref] [PubMed]
- Liu YL, Wang DW, Yang ZC, et al. Marital status is an independent prognostic factor in inflammatory breast cancer patients: an analysis of the surveillance, epidemiology, and end results database. Breast Cancer Res Treat 2019;178:379-88. [Crossref] [PubMed]
- Zhai Z, Zhang F, Zheng Y, et al. Effects of marital status on breast cancer survival by age, race, and hormone receptor status: A population-based Study. Cancer Med 2019;8:4906-17. [Crossref] [PubMed]
- Wong CW, Kwok CS, Narain A, et al. Marital status and risk of cardiovascular diseases: a systematic review and meta-analysis. Heart 2018;104:1937-48. [Crossref] [PubMed]
- Dhindsa DS, Khambhati J, Schultz WM, et al. Marital status and outcomes in patients with cardiovascular disease. Trends Cardiovasc Med 2020;30:215-20. [Crossref] [PubMed]
- Chen Z, Yin K, Zheng D, et al. Marital status independently predicts non-small cell lung cancer survival: a propensity-adjusted SEER database analysis. J Cancer Res Clin Oncol 2020;146:67-74. [Crossref] [PubMed]
- Li MU, Gu S, Mao R, et al. County Median Family Income Is an Independent Prognostic Factor for Stage IV Anaplastic Thyroid Cancer. Anticancer Res 2019;39:949-56. [Crossref] [PubMed]
- Guan T, Zhang H, Yang J, et al. Increased Risk of Cardiovascular Death in Breast Cancer Patients Without Chemotherapy or (and) Radiotherapy: A Large Population-Based Study. Front Oncol 2020;10:619622. [Crossref] [PubMed]
- Guan T, Qiu Z, Su M, et al. Cardiovascular Death Risk in Primary Central Nervous System Lymphoma Patients Treated With Chemotherapy: A Registry-Based Cohort Study. Front Oncol 2021;11:641955. [Crossref] [PubMed]
- Fung C, Fossa SD, Milano MT, et al. Cardiovascular Disease Mortality After Chemotherapy or Surgery for Testicular Nonseminoma: A Population-Based Study. J Clin Oncol 2015;33:3105-15. [Crossref] [PubMed]
- Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation 2016;133:601-9. [Crossref] [PubMed]
- Onwudiwe NC, Kwok Y, Onukwugha E, et al. Cardiovascular event-free survival after adjuvant radiation therapy in breast cancer patients stratified by cardiovascular risk. Cancer Med 2014;3:1342-52. [Crossref] [PubMed]
- Fournier S, Muller O, Ludman AJ, et al. Influence of socioeconomic factors on delays, management and outcome amongst patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Swiss Med Wkly 2013;143:w13817. [Crossref] [PubMed]
- Kilpi F, Konttinen H, Silventoinen K, et al. Living arrangements as determinants of myocardial infarction incidence and survival: A prospective register study of over 300,000 Finnish men and women. Soc Sci Med 2015;133:93-100. [Crossref] [PubMed]
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140:e563-95. [Crossref] [PubMed]
- Surman M, Janik ME. Stress and its molecular consequences in cancer progression. Postepy Hig Med Dosw (Online) 2017;71:485-99. [Crossref] [PubMed]
- Goldzweig G, Andritsch E, Hubert A, et al. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann Oncol 2010;21:877-83. [Crossref] [PubMed]
- Goldzweig G, Andritsch E, Hubert A, et al. How relevant is marital status and gender variables in coping with colorectal cancer? A sample of middle-aged and older cancer survivors. Psychooncology 2009;18:866-74. [Crossref] [PubMed]
- Floud S, Balkwill A, Canoy D, et al. Marital status and ischemic heart disease incidence and mortality in women: a large prospective study. BMC Med 2014;12:42. [Crossref] [PubMed]
- Wu JR, Lennie TA, Chung ML, et al. Medication adherence mediates the relationship between marital status and cardiac event-free survival in patients with heart failure. Heart Lung 2012;41:107-14. [Crossref] [PubMed]
- Fang F, Fall K, Mittleman MA, et al. Suicide and cardiovascular death after a cancer diagnosis. N Engl J Med 2012;366:1310-8. [Crossref] [PubMed]
- Bucciarelli V, Caterino AL, Bianco F, et al. Depression and cardiovascular disease: The deep blue sea of women's heart. Trends Cardiovasc Med 2020;30:170-6. [Crossref] [PubMed]
- Shrout MR, Renna ME, Madison AA, et al. Breast cancer survivors' satisfying marriages predict better psychological and physical health: A longitudinal comparison of satisfied, dissatisfied, and unmarried women. Psychooncology 2021;30:699-707. [Crossref] [PubMed]
- Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A 2004;101:17312-5. [Crossref] [PubMed]
- Chen R, Zhan Y, Pedersen N, et al. Marital status, telomere length and cardiovascular disease risk in a Swedish prospective cohort. Heart 2020;106:267-72. [Crossref] [PubMed]
- Chin B, Murphy MLM, Janicki-Deverts D, et al. Marital status as a predictor of diurnal salivary cortisol levels and slopes in a community sample of healthy adults. Psychoneuroendocrinology 2017;78:68-75. [Crossref] [PubMed]
- Johansen IB, Sandblom E, Skov PV, et al. Bigger is not better: cortisol-induced cardiac growth and dysfunction in salmonids. J Exp Biol 2017;220:2545-53. [Crossref] [PubMed]
- Wester VL, van Rossum EF. Clinical applications of cortisol measurements in hair. Eur J Endocrinol 2015;173:M1-10. [Crossref] [PubMed]
- Tanno K, Ohsawa M, Itai K, et al. Associations of marital status with mortality from all causes and mortality from cardiovascular disease in Japanese haemodialysis patients. Nephrol Dial Transplant 2013;28:1013-20. [Crossref] [PubMed]
- Otto CM. Marital status and cardiovascular disease risk. Heart 2018;104:1893-4. [Crossref] [PubMed]
- Ahola K, Honkonen T, Isometsä E, et al. Burnout in the general population. Results from the Finnish Health 2000 Study. Soc Psychiatry Psychiatr Epidemiol 2006;41:11-7. [Crossref] [PubMed]
- Maydych V. The Interplay Between Stress, Inflammation, and Emotional Attention: Relevance for Depression. Front Neurosci 2019;13:384. [Crossref] [PubMed]
- Hamer M, Chida Y. Associations of very high C-reactive protein concentration with psychosocial and cardiovascular risk factors in an ageing population. Atherosclerosis 2009;206:599-603. [Crossref] [PubMed]
- Engström G, Hedblad B, Rosvall M, et al. Occupation, marital status, and low-grade inflammation: mutual confounding or independent cardiovascular risk factors? Arterioscler Thromb Vasc Biol 2006;26:643-8. [Crossref] [PubMed]
- Fagundes CP, Murdock KW, LeRoy A, et al. Spousal bereavement is associated with more pronounced ex vivo cytokine production and lower heart rate variability: Mechanisms underlying cardiovascular risk? Psychoneuroendocrinology 2018;93:65-71. [Crossref] [PubMed]
- Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J 2019;40:3889-97. [Crossref] [PubMed]
- Gaitanidis A, Spathakis M, Tsalikidis C, et al. Risk factors for cardiovascular mortality in patients with colorectal cancer: a population-based study. Int J Clin Oncol 2019;24:501-7. [Crossref] [PubMed]


