
Effect of prior cancer history on survival of patients with esophageal carcinoma: a propensity score matching, population-based study
Introduction
Esophageal cancer is the ninth most common type of tumor in both sexes worldwide (1). Squamous cell carcinoma and adenocarcinoma are two typical histological subtypes. While squamous cell carcinoma accounts for approximately 87% of cases worldwide, a recent study has found that the incidence rate of esophageal adenocarcinoma is rapidly increasing or even exceeding that of squamous cell carcinoma in some developed countries (2). With a high mortality, which ranks seventh among all cancer types (3), esophageal carcinoma carries a relatively poor prognosis, considering that the 5-year survival rate is approximately 20% in Europe and the United States and less than 5% in low- and middle-income countries (4).
Given that prior malignancy is commonly believed to affect the prognosis of cancer patients, prior cancer history is regularly regarded as a common exclusion criterion in many clinical trials (5-7). A previous study employing pan-cancer analysis has suggested that prior cancer has varying impact on overall survival depending on the type of previous cancer (8). For instance, several studies on nasopharyngeal carcinoma, advanced breast cancer, and lung cancer have shown no significant impact of prior malignancy on survival (9-11). However, for laryngeal cancer and ovarian cancer, prior cancer history adversely interferes with prognosis (12,13). Except for one small-scale study that has merely shown no adverse effect for patients with stage IV esophageal cancer (14), there is no other study to confirm the influence of prior cancer diagnosis on survival across all stages of esophageal carcinoma. Therefore, the common understanding may lead to excessive exclusion criteria, eventually weakening the efficacy of clinical trials. To address this issue, this study used the Surveillance, Epidemiology, and End Results (SEER) database to precisely evaluate the impact of prior cancer on survival of patients with esophageal cancer across all stages and guide the formulation of eligibility criteria for clinical trials. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1707/rc).
Methods
Study design and patients
Clinical data of patients with esophageal carcinoma were obtained from the SEER database using the SEER*Stat software version 8.3.9 (https://seer.cancer.gov/, accession number 12569-Nov2020) (15). The SEER database sponsored by the National Cancer Institute provides population-based cancer incidence data that cover approximately 34.6% of the U. S. population. Individuals with a histologically confirmed esophageal carcinoma between 2011 and 2016 were identified to ensure at least a 5-year follow-up period in this study. The exclusion criteria were as follows: (I) age at diagnosis <18 years; (II) incomplete survival data and follow-up survival information; (III) diagnosis made at autopsy or via death certificates only. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Demographic and clinicopathological data, including age at diagnosis, sex, race, marital status, primary site, grade, histology recode-broad groupings, American Joint Committee on Cancer (AJCC) Stage Group (6th), surgical information, radiotherapy and chemotherapy records, cause of death, and follow-up information, were extracted from the SEER database. Age at diagnosis (a continuous variable) was converted to a categorical variable (<65 and ≥65 years). The race was categorized into White, Black, and others (American Indian/AK Native, Asian/Pacific Islander). Marital status was classified as married, single, separated/widowed/divorced (sep/wid/div), and unknown. The histological type was described as adenoma and adenocarcinoma, squamous cell carcinoma, and others.
Prior cancer history was determined from the SEER sequence number, as described in a previous study (8). The sequence number represents the order of all malignancies diagnosed over the lifetime. The interval time between the diagnosis of esophageal carcinoma and the most recent prior cancer was calculated by SEER*Stat Program and was subsequently divided into 6–12 months, 1–5 years, 5–10 years, and >10 years for further analysis.
Study outcomes
The primary outcome was all-cause survival, which referred to the interval time from the date of diagnosis to the date of death caused by all reasons, including esophageal cancer and other diseases, or last follow-up calculated by the SEER*Stat Program. The secondary outcome was esophageal cancer-specific survival, and patients were censored if they died from causes other than esophageal cancer. We evaluated the impact of prior cancer on prognosis of patients with esophageal carcinoma by analyzing all-cause survival and esophageal cancer-specific survival.
Statistical analysis
According to prior cancer history, patients in this study were divided into two groups, including the group with prior cancer and the group without prior cancer. The Pearson chi-square test was applied to analyze the differences between the two groups. We employed the propensity score matching (PSM) method to balance the confounding bias caused by covariates, including age, sex, race, marital status, primary site, grade, histological type, stage, surgical information, and radiotherapy and chemotherapy records (16). A one-to-one nearest PSM between patients with prior cancer and without prior cancer was performed with a caliper of 0.2. An adjusted cohort was built for subsequent analysis. The Kaplan-Meier analysis was utilized to compare survival function via log-rank tests in all-cause and esophageal cancer-specific survival. Multivariate propensity score-adjusted Cox proportional hazards models were also constructed incorporating covariates such as age, sex, race, marital status, primary site, grade, histological type, AJCC stage, surgical information, radiotherapy, and chemotherapy records to identify independent predictors of survival. Furthermore, subgroup analysis stratified by age, sex, race, grade, histological type, and AJCC stage of esophageal carcinoma, as well as timing, type, and stage of prior cancer was conducted to investigate the impact of prior malignancy on prognosis more deeply. Two-tailed P values <0.05 were considered statistically significant. Statistical analysis was performed using R software (version 4.0.1).
Results
Patients characteristics
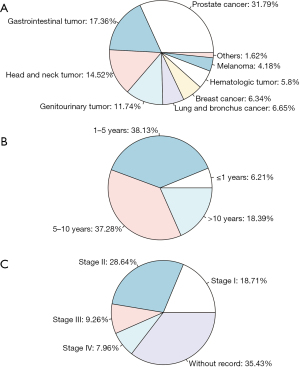
In this study, 17,123 eligible patients with esophageal carcinoma were identified from the SEER database, including 2,224 (13%) patients with prior cancer and 14,899 (87%) patients without prior cancer. Esophageal carcinoma most often occurred in male White individuals aged ≥65 years. The most frequent primary site at diagnosis was the lower third of the esophagus (54.5% for the group with prior cancer; 61.5% for the group without prior cancer). In terms of histological types, adenoma and adenocarcinoma had a slightly higher incidence rate than squamous cell carcinoma (53.7% vs. 37.8%, respectively, for the group with prior cancer; 61.9% vs. 29.6%, respectively, for the group without prior cancer). Compared with cases without prior malignancy, patients with prior cancer were more often older than 65 years (77.9% vs. 55.1%, P<0.001), White (86.1% vs. 84.6%, P=0.003), and married (56.9% vs. 52.7%, P<0.001), and they were also more likely to have squamous cell carcinoma (37.8% vs. 29.6%, P<0.001). Patients with prior cancer less often received surgical treatments (21.5% vs. 25.0%, P<0.001), radiotherapy (51.2% vs. 56.6%, P<0.001), and chemotherapy (54.2% vs. 63.2%, P<0.001). After adjustment for the propensity scores, all variables were well-balanced between patients with and without prior cancer (P>0.05). Table 1 summarizes the baseline characteristics of patients with esophageal carcinoma who died of all causes and esophageal carcinoma grouped by prior cancer history both before PSM and after PSM. Figure 1 depicts the types, diagnostic time, and stage of prior cancer. Prostate cancer (31.79%), gastrointestinal tumor (17.36%), head and neck tumor (14.52%), and genitourinary tumor (11.74%) were the most common types of prior cancer. The median time between the diagnostic time of the recent prior cancer and the esophageal cancer was 69 months. As shown in Figure 1B, 1–5 years occupied most of the timing of prior cancer. Almost 35.43% esophageal cancer patients with prior cancer did not have a record of prior cancer stage (Figure 1C).
Table 1
| Characteristics | Death from all-cause | Death from esophageal cancer | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before PSM | After PSM | Before PSM | After PSM | ||||||||||||
| No prior cancer, N=14,899 (87.0%) | With prior cancer, N=2,224 (13.0%) | P | No prior cancer, N=2,224 (50%) | With prior cancer, N=2,224 (50%) | P | No prior cancer, N=12,804 (88.1%) | With prior cancer, N=1,728 (11.9%) | P | No prior cancer, N=1,728 (50.0%) | With prior cancer, N=1,728 (50.0%) | P | ||||
| Age (years) | <0.001 | 0.402 | <0.001 | 1.000 | |||||||||||
| <65 | 6,685 (44.9) | 491 (22.1) | 467 (21.0) | 491 (22.1) | 5,865 (45.8) | 385 (22.3) | 384 (22.2) | 385 (22.3) | |||||||
| ≥65 | 8,214 (55.1) | 1,733 (77.9) | 1,757 (79.0) | 1,733 (77.9) | 6,939 (54.2) | 1,343 (77.7) | 1,344 (77.8) | 1,343 (77.7) | |||||||
| Gender | 0.184 | 0.080 | 0.356 | 0.546 | |||||||||||
| Female | 3,052 (20.5) | 428 (19.2) | 382 (17.2) | 428 (19.2) | 2,646 (20.7) | 340 (19.7) | 325 (18.8) | 340 (19.7) | |||||||
| Male | 11,847 (79.5) | 1,796 (80.8) | 1,842 (82.8) | 1,796 (80.8) | 10,158 (79.3) | 1,388 (80.3) | 1,403 (81.2) | 1,388 (80.3) | |||||||
| Race | 0.003 | 0.293 | 0.017 | 0.282 | |||||||||||
| Black | 1,444 (9.7) | 221 (9.9) | 194 (8.7) | 221 (9.9) | 1,199 (9.4) | 166 (9.6) | 153 (8.9) | 166 (9.6) | |||||||
| White | 12,600 (84.6) | 1,915 (86.1) | 1,950 (87.7) | 1,915 (86.1) | 10,856 (84.8) | 1,490 (86.2) | 1,518 (87.8) | 1,490 (86.2) | |||||||
| Others/unknown | 855 (5.7) | 88 (4.0) | 80 (3.6) | 88 (4.0) | 749 (5.8) | 72 (4.2) | 57 (3.3) | 72 (4.2) | |||||||
| Marital status | <0.001 | 0.779 | <0.001 | 0.916 | |||||||||||
| Married | 7,845 (52.7) | 1,266 (56.9) | 1,265 (56.9) | 1,266 (56.9) | 6,802 (53.1) | 985 (57.0) | 1,005 (58.2) | 985 (57.0) | |||||||
| Single | 2,755 (18.5) | 239 (10.7) | 243 (10.9) | 239 (10.7) | 2,352 (18.4) | 186 (10.8) | 178 (10.3) | 186 (10.8) | |||||||
| Sep/wid/div | 3,357 (22.5) | 550 (24.7) | 531 (23.9) | 550 (24.7) | 2,840 (22.2) | 426 (24.7) | 416 (24.1) | 426 (24.7) | |||||||
| Unknown | 942 (6.3) | 169 (7.6) | 185 (8.3) | 169 (7.6) | 810 (6.3) | 131 (7.6) | 129 (7.5) | 131 (7.6) | |||||||
| Primary site | <0.001 | 0.895 | <0.001 | 0.803 | |||||||||||
| Cervical esophagus | 239 (1.6) | 78 (3.5) | 69 (3.1) | 78 (3.5) | 201 (1.6) | 55 (3.2) | 46 (2.7) | 55 (3.2) | |||||||
| Thoracic esophagus | 528 (3.5) | 91 (4.1) | 92 (4.1) | 91 (4.1) | 463 (3.6) | 70 (4.1) | 55 (3.2) | 70 (4.1) | |||||||
| Abdominal esophagus | 84 (0.6) | 13 (0.6) | 9 (0.4) | 13 (0.6) | 68 (0.5) | 10 (0.6) | 9 (0.5) | 10 (0.6) | |||||||
| Upper third of esophagus | 704 (4.7) | 161 (7.2) | 158 (7.1) | 161 (7.2) | 602 (4.7) | 114 (6.6) | 103 (6.0) | 114 (6.6) | |||||||
| Middle third of esophagus | 2,135 (14.3) | 383 (17.2) | 375 (16.9) | 383 (17.2) | 1,863 (14.6) | 293 (17.0) | 301 (17.4) | 293 (17.0) | |||||||
| Lower third of esophagus | 9,165 (61.5) | 1,211 (54.5) | 1,244 (55.9) | 1,211 (54.5) | 7,848 (61.3) | 972 (56.2) | 988 (57.2) | 972 (56.2) | |||||||
| Overlapping lesion of esophagus | 680 (4.6) | 75 (3.4) | 63 (2.8) | 75 (3.4) | 603 (4.7) | 59 (3.4) | 61 (3.5) | 59 (3.4) | |||||||
| Esophagus, NOS | 1,364 (9.2) | 212 (9.5) | 214 (9.6) | 212 (9.5) | 1,156 (9.0) | 155 (9.0) | 165 (9.5) | 155 (9.0) | |||||||
| Grade | 0.230 | 0.775 | 0.470 | 0.892 | |||||||||||
| Well differentiated | 732 (4.9) | 99 (4.5) | 91 (4.1) | 99 (4.5) | 639 (5.0) | 74 (4.3) | 74 (4.3) | 74 (4.3) | |||||||
| Moderately differentiated | 4,956 (33.3) | 738 (33.2) | 712 (32.0) | 738 (33.2) | 4,248 (33.2) | 584 (33.8) | 561 (32.5) | 584 (33.8) | |||||||
| Poorly differentiated | 6,122 (41.1) | 882 (39.7) | 888 (39.9) | 882 (39.7) | 5,282 (41.3) | 694 (40.2) | 699 (40.5) | 694 (40.2) | |||||||
| Undifferentiated | 180 (1.2) | 27 (1.2) | 32 (1.4) | 27 (1.2) | 155 (1.2) | 25 (1.4) | 23 (1.3) | 25 (1.4) | |||||||
| Unknown | 2,909 (19.5) | 478 (21.5) | 501 (22.5) | 478 (21.5) | 2,480 (19.4) | 351 (20.3) | 371 (21.5) | 351 (20.3) | |||||||
| Histology type | <0.001 | 0.683 | <0.001 | 0.793 | |||||||||||
| Squamous cell carcinoma | 4,416 (29.6) | 841 (37.8) | 817 (36.7) | 841 (37.8) | 3,831 (29.9) | 646 (37.4) | 656 (38.0) | 646 (37.4) | |||||||
| Adenoma and adenocarcinoma | 9,222 (61.9) | 1,194 (53.7) | 1,223 (55.0) | 1,194 (53.7) | 7,916 (61.8) | 948 (54.9) | 948 (54.9) | 948 (54.9) | |||||||
| Other types | 1,261 (8.5) | 189 (8.5) | 184 (8.3) | 189 (8.5) | 1,057 (8.3) | 134 (7.8) | 124 (7.2) | 134 (7.8) | |||||||
| AJCC stage (6th) | <0.001 | 0.562 | <0.001 | 0.626 | |||||||||||
| I | 1,992 (13.4) | 420 (18.9) | 383 (17.2) | 420 (18.9) | 1,691 (13.2) | 303 (17.5) | 287 (16.6) | 303 (17.5) | |||||||
| II | 2,685 (18.0) | 447 (20.1) | 447 (20.1) | 447 (20.1) | 2,332 (18.2) | 360 (20.8) | 332 (19.2) | 360 (20.8) | |||||||
| III | 3,138 (21.1) | 363 (16.3) | 391 (17.6) | 363 (16.3) | 2,755 (21.5) | 311 (18.0) | 326 (18.9) | 311 (18.0) | |||||||
| IV | 5,265 (35.3) | 606 (27.2) | 621 (27.9) | 606 (27.2) | 4,516 (35.3) | 497 (28.8) | 509 (29.5) | 497 (28.8) | |||||||
| Unknown | 1,819 (12.2) | 388 (17.4) | 382 (17.2) | 388 (17.4) | 1,510 (11.8) | 257 (14.9) | 274 (15.9) | 257 (14.9) | |||||||
| Surgery | <0.001 | 0.826 | 0.006 | 0.416 | |||||||||||
| No/unknown | 11,167 (75.0) | 1,745 (78.5) | 1,752 (78.8) | 1,745 (78.5) | 9,431 (73.7) | 1,327 (76.8) | 1,348 (78.0) | 1,327 (76.8) | |||||||
| Yes | 3,732 (25.0) | 479 (21.5) | 472 (21.2) | 479 (21.5) | 3,373 (26.3) | 401 (23.2) | 380 (22.0) | 401 (23.2) | |||||||
| Radiotherapy | <0.001 | 0.118 | 0.010 | 0.811 | |||||||||||
| No/unknown | 6,472 (43.4) | 1,086 (48.8) | 1,033 (46.4) | 1,086 (48.8) | 5,460 (42.6) | 794 (45.9) | 786 (45.5) | 794 (45.9) | |||||||
| Yes | 8,427 (56.6) | 1,138 (51.2) | 1,191 (53.6) | 1,138 (51.2) | 7,344 (57.4) | 934 (54.1) | 942 (54.5) | 934 (54.1) | |||||||
| Chemotherapy | <0.001 | 0.416 | <0.001 | 0.428 | |||||||||||
| No/unknown | 5,481 (36.8) | 1,018 (45.8) | 990 (44.5) | 1,018 (45.8) | 4,575 (35.7) | 742 (42.9) | 718 (41.6) | 742 (42.9) | |||||||
| Yes | 9,418 (63.2) | 1,206 (54.2) | 1,234 (55.5) | 1,206 (54.2) | 8,229 (64.3) | 986 (57.1) | 1,010 (58.4) | 986 (57.1) | |||||||
PSM, propensity score matching; Sep/wid/div, separated/widowed/divorced; NOS, not otherwise specified; AJCC, American Joint Committee on Cancer.
Effect of prior cancer on all-cause and esophageal cancer-specific survival
To evaluate the impact of prior malignancy on survival, Kaplan-Meier survival curves before PSM did not demonstrate significant difference between patients with prior cancer and those without prior cancer in terms of all-cause survival (HR =1.047, 95% CI: 0.995–1.102, P=0.077) (Figure 2A) and esophageal cancer-specific survival (HR =0.986, 95% CI: 0.928–1.048, P=0.65) (Figure 2B), which implied that prior cancer probably had no adverse effect on prognosis of patients with esophageal cancer. For patients with prior cancer history, the 1-, 3-, and 5-year all-cause survival rates were 45.2%, 22.2%, and 15.6%, respectively. For patients with no prior malignancy, the 1-, 3-, and 5-year all-cause survival rates were 46.5%, 24.3%, and 17.5%, respectively.
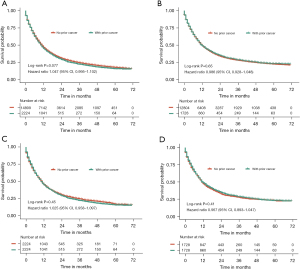
After PSM, the survival curves also showed no significant difference between the two groups in terms of all-cause (HR =1.025, 95% CI: 0.958–1.097, P=0.45) (Figure 2C) and esophageal cancer-specific survival (HR =0.967, 95% CI: 0.893–1.047, P=0.41) (Figure 2D). Multivariate covariate-adjusted Cox regression models revealed that prior malignancy history was not significantly associated with inferior all-cause (HR =1.002, 95% CI: 0.936–1.072, P=0.965) and esophageal cancer-specific survival (HR =0.964, 95% CI: 0.890–1.045, P=0.374) (Table 2).
Table 2
| Variables | All-cause adjusted HR (95% CI) | P value | Esophageal cancer-specific adjusted HR (95% CI) | P value |
|---|---|---|---|---|
| Prior cancer | ||||
| No prior cancer | Reference | Reference | ||
| With prior cancer | 1.002 (0.936, 1.072) | 0.965 | 0.964 (0.890, 1.045) | 0.374 |
| Age (years) | ||||
| <65 | Reference | Reference | ||
| ≥65 | 1.121 (1.028, 1.222) | 0.010 | 1.122 (1.0133, 1.242) | 0.027 |
| Gender | ||||
| Female | Reference | Reference | ||
| Male | 1.151 (1.049, 1.262) | 0.003 | 1.159 (1.041, 1.291) | 0.007 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 1.022 (0.906, 1.154) | 0.721 | 0.943 (0.817, 1.088) | 0.421 |
| Others/unknown | 0.858 (0.695, 1.060) | 0.157 | 1.009 (0.793, 1.284) | 0.943 |
| Marital status | ||||
| Married | Reference | |||
| Single | 1.062 (0.948, 1.191) | 0.300 | 1.161 (1.015, 1.329) | 0.030 |
| Sep/wid/div | 1.206 (1.109, 1.312) | <0.001 | 1.193 (1.080, 1.318) | <0.001 |
| Unknown | 0.894 (0.782, 1.021) | 0.099 | 0.869 (0.739, 1.022) | 0.090 |
| Primary site | ||||
| Abdominal esophagus | Reference | Reference | ||
| Cervical esophagus | 0.542 (0.334, 0.878) | 0.013 | 0.781 (0.452, 1.349) | 0.376 |
| Thoracic esophagus | 0.634 (0.395, 1.018) | 0.059 | 0.856 (0.501, 1.464) | 0.570 |
| Upper third of esophagus | 0.594 (0.374, 0.944) | 0.027 | 0.724 (0.430, 1.219) | 0.224 |
| Middle third of esophagus | 0.590 (0.376, 0.927) | 0.022 | 0.842 (0.509, 1.393) | 0.504 |
| Lower third of esophagus | 0.602 (0.386, 0.938) | 0.025 | 0.857 (0.522, 1.406) | 0.541 |
| Overlapping lesion of esophagus | 0.704 (0.437, 1.136) | 0.150 | 0.943 (0.553, 1.608) | 0.829 |
| Esophagus, NOS | 0.591 (0.375, 0.934) | 0.024 | 0.831 (0.499, 1.383) | 0.475 |
| Grade | ||||
| Well differentiated | Reference | Reference | ||
| Moderately differentiated | 1.648 (1.349, 2.013) | <0.001 | 1.606 (1.245, 2.072) | <0.001 |
| Poorly differentiated | 2.017 (1.654, 2.461) | <0.001 | 1.968 (1.529, 2.534) | <0.001 |
| Undifferentiated | 2.593 (1.838, 3.658) | <0.001 | 2.657 (1.789, 3.948) | <0.001 |
| Unknown | 1.401 (1.141, 1.719) | 0.001 | 1.421 (1.095, 1.845) | 0.008 |
| Histology type | ||||
| Adenoma and adenocarcinoma | Reference | Reference | ||
| Squamous cell carcinoma | 1.113 (1.012, 1.224) | 0.028 | 1.087 (0.971, 1.218) | 0.903 |
| Other types | 1.046 (0.920, 1.190) | 0.492 | 0.990 (0.846, 1.159) | 0.149 |
| AJCC stage (6th) | ||||
| I | Reference | Reference | ||
| II | 1.506 (1.317, 1.723) | <0.001 | 1.616 (1.366, 1.911) | <0.001 |
| III | 2.112 (1.835, 2.430) | <0.001 | 2.498 (2.109, 2.958) | <0.001 |
| IV | 3.229 (2.844, 3.665) | <0.001 | 3.752 (3.209, 4.387) | <0.001 |
| Unknown | 1.649 (1.448, 1.877) | <0.001 | 1.862 (1.581, 2.194) | <0.001 |
| Surgery | ||||
| No/unknown | Reference | Reference | ||
| Yes | 0.310 (0.276, 0.348) | <0.001 | 0.273 (0.237, 0.315) | <0.001 |
| Radiotherapy | ||||
| No/unknown | Reference | Reference | ||
| Yes | 0.803 (0.741, 0.871) | <0.001 | 0.832 (0.756, 0.915) | <0.001 |
| Chemotherapy | ||||
| No/unknown | Reference | Reference | ||
| Yes | 0.412 (0.378, 0.449) | <0.001 | 0.385 (0.348, 0.426) | <0.001 |
HR, hazard ratio; CI, confidence interval; Sep/wid/div, separated/widowed/divorced; NOS, not otherwise specified; AJCC, American Joint Committee on Cancer.
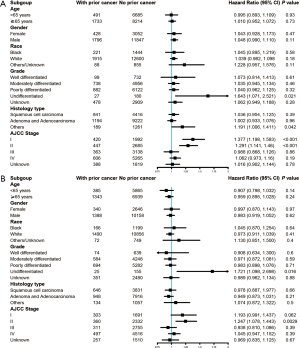
Figure 3A shows the results of subgroup analysis stratified by age, sex, race, grade, histological type, and AJCC stage of esophageal cancer. Except for subgroups of undifferentiated pathological grade, stage I, and stage II, other groups indicated that prior cancer history was not significantly related to the overall survival. For esophageal cancer-specific survival, subgroup analysis stratified by age, sex, race, grade, histological type, and stage of the index cancer showed similar tendency to that of all-cause survival (Figure 3B).
Effect of the diagnostic time, type, and stage of prior cancer on overall survival
Figure 4 illustrates the subgroup analysis stratified by the latency period between prior cancer and esophageal cancer diagnosis, which further investigated the effect of prior cancer on survival. In subgroups of 6–12 months, 1–5 years, and 5–10 years, prior cancer displayed no significant effect on prognosis (P>0.05). For the subgroup of >10 years, patients without prior cancer showed a slightly better survival than patients with previous malignancy (P=0.0096). Multivariate covariate-adjusted Cox regression models for the subgroup analyses stratified by latency period are shown in Table 3, and they confirmed that prior cancer history was not an independent risk factor for overall survival in any of the subgroups.
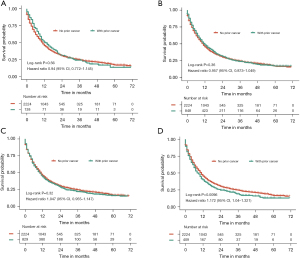
Table 3
| Variables | 6–12 months | 1–5 years | 5–10 years | >10 years | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P values | HR (95% CI) | P values | HR (95% CI) | P values | ||||
| Prior cancer | |||||||||||
| No prior cancer | Reference | Reference | Reference | Reference | |||||||
| With prior cancer | 1.064 (0.871, 1.299) | 0.546 | 0.968 (0.883, 1.062) | 0.494 | 0.985 (0.898, 1.080) | 0.741 | 1.106 (0.979, 1.249) | 0.105 | |||
| Age (years) | |||||||||||
| <65 | Reference | Reference | Reference | Reference | |||||||
| ≥65 | 1.109 (0.983, 1.251) | 0.093 | 1.118 (1.008, 1.240) | 0.035 | 1.122 (1.010, 1.247) | 0.031 | 1.112 (0.989, 1.249) | 0.075 | |||
| Gender | |||||||||||
| Female | Reference | Reference | Reference | Reference | |||||||
| Male | 1.196 (1.049, 1.364) | 0.008 | 1.138 (1.015, 1.276) | 0.026 | 1.143 (1.018, 1.282) | 0.023 | 1.175 (1.041, 1.326) | 0.009 | |||
| Race | |||||||||||
| Black | Reference | Reference | Reference | Reference | |||||||
| White | 1.081 (0.911, 1.284) | 0.372 | 1.000 (0.859, 1.163) | 0.996 | 0.987 (0.849, 1.147) | 0.862 | 1.092 (0.930, 1.281) | 0.283 | |||
| Others/unknown | 0.714 (0.525, 0.970) | 0.031 | 0.707 (0.543, 0.920) | 0.010 | 0.776 (0.598, 1.007) | 0.056 | 0.752 (0.560, 1.009) | 0.058 | |||
| Marital status | |||||||||||
| Married | Reference | Reference | Reference | Reference | |||||||
| Single | 1.180 (1.007, 1.381) | 0.040 | 1.101 (0.958, 1.264) | 0.176 | 1.114 (0.971, 1.279) | 0.122 | 1.073 (0.923, 1.248) | 0.360 | |||
| Sep/wid/div | 1.203 (1.070, 1.353) | 0.002 | 1.174 (1.059, 1.302) | 0.002 | 1.203 (1.087, 1.332) | <0.001 | 1.166 (1.044, 1.302) | 0.006 | |||
| Unknown | 0.988 (0.823, 1.185) | 0.893 | 0.892 (0.762, 1.044) | 0.153 | 1.007 (0.854, 1.188) | 0.932 | 0.902 (0.756, 1.076) | 0.253 | |||
| Primary site | |||||||||||
| Abdominal esophagus | Reference | Reference | Reference | Reference | |||||||
| Cervical esophagus | 0.546 (0.266, 1.119) | 0.098 | 0.554 (0.298, 1.031) | 0.062 | 0.626 (0.330, 1.187) | 0.151 | 0.579 (0.309, 1.085) | 0.088 | |||
| Thoracic esophagus | 0.585 (0.289, 1.184) | 0.136 | 0.627 (0.341, 1.152) | 0.133 | 0.640 (0.340, 1.205) | 0.167 | 0.744 (0.403, 1.374) | 0.344 | |||
| Upper third of esophagus | 0.584 (0.293, 1.166) | 0.127 | 0.584 (0.322, 1.059) | 0.077 | 0.650 (0.349, 1.208) | 0.173 | 0.645 (0.353, 1.178) | 0.154 | |||
| Middle third of esophagus | 0.575 (0.292, 1.130) | 0.108 | 0.626 (0.350, 1.120) | 0.115 | 0.610 (0.332, 1.121) | 0.111 | 0.640 (0.356, 1.151) | 0.136 | |||
| Lower third of esophagus | 0.587 (0.301, 1.144) | 0.118 | 0.655 (0.369, 1.162) | 0.148 | 0.632 (0.347, 1.154) | 0.135 | 0.665 (0.374, 1.183) | 0.165 | |||
| Overlapping lesion of esophagus | 0.780 (0.381, 1.598) | 0.497 | 0.929 (0.503, 1.716) | 0.813 | 0.695 (0.367, 1.316) | 0.263 | 0.808 (0.432, 1.511) | 0.504 | |||
| Esophagus, NOS | 0.606 (0.306, 1.201) | 0.152 | 0.645 (0.358, 1.162) | 0.144 | 0.668 (0.361, 1.234) | 0.197 | 0.640 (0.354, 1.158) | 0.141 | |||
| Grade | |||||||||||
| Well differentiated | Reference | Reference | Reference | Reference | |||||||
| Moderately differentiated | 1.631 (1.228, 2.166) | <0.001 | 1.790 (1.378, 2.326) | <0.001 | 1.670 (1.313, 2.124) | <0.001 | 1.624 (1.251, 2.109) | <0.001 | |||
| Poorly differentiated | 1.995 (1.505, 2.645) | <0.001 | 2.186 (1.685, 2.835) | <0.001 | 2.019 (1.590, 2.563) | <0.001 | 1.957 (1.510, 2.537) | <0.001 | |||
| Undifferentiated | 2.149 (1.358, 3.401) | 0.001 | 2.458 (1.587, 3.806) | <0.001 | 2.448 (1.601, 3.742) | <0.001 | 2.256 (1.418, 3.589) | <0.001 | |||
| Unknown | 1.317 (0.986, 1.760) | 0.062 | 1.478 (1.132, 1.930) | 0.004 | 1.361 (1.065, 1.741) | 0.014 | 1.355 (1.037, 1.771) | 0.026 | |||
| Histology type | |||||||||||
| Adenoma and adenocarcinoma | Reference | Reference | Reference | Reference | |||||||
| Squamous cell carcinoma | 1.095 (0.960, 1.249) | 0.176 | 1.100 (0.978, 1.236) | 0.111 | 1.072 (0.955, 1.203) | 0.241 | 1.101 (0.972, 1.246) | 0.131 | |||
| Other types | 0.990 (0.826, 1.186) | 0.909 | 1.016 (0.866, 1.191) | 0.850 | 0.945 (0.807, 1.107) | 0.483 | 0.965 (0.813, 1.146) | 0.686 | |||
| AJCC stage (6th) | |||||||||||
| I | Reference | Reference | Reference | Reference | |||||||
| II | 1.658 (1.365, 2.013) | <0.001 | 1.663 (1.405, 1.969) | <0.001 | 1.552 (1.312, 1.836) | <0.001 | 1.566 (1.308, 1.875) | <0.001 | |||
| III | 2.360 (1.934, 2.882) | <0.001 | 2.449 (2.058, 2.914) | <0.001 | 2.125 (1.784, 2.533) | <0.001 | 2.386 (1.982, 2.872) | <0.001 | |||
| IV | 3.571 (2.976, 4.285) | <0.001 | 3.473 (2.964, 4.068) | <0.001 | 3.242 (2.767, 3.797) | <0.001 | 3.531 (2.983, 4.179) | <0.001 | |||
| Unknown | 1.851 (1.537, 2.230) | <0.001 | 1.862 (1.583, 2.190) | <0.001 | 1.722 (1.465, 2.024) | <0.001 | 1.788 (1.503, 2.127) | <0.001 | |||
| Surgery | |||||||||||
| No/unknown | Reference | Reference | Reference | Reference | |||||||
| Yes | 0.300 (0.255, 0.352) | <0.001 | 0.305 (0.265, 0.351) | <0.001 | 0.299 (0.259, 0.345) | <0.001 | 0.292 (0.249, 0.341) | <0.001 | |||
| Radiotherapy | |||||||||||
| No/unknown | Reference | Reference | Reference | Reference | |||||||
| Yes | 0.847 (0.757, 0.949) | 0.004 | 0.814 (0.738, 0.898) | <0.001 | 0.833 (0.755, 0.919) | <0.001 | 0.797 (0.717, 0.887) | <0.001 | |||
| Chemotherapy | |||||||||||
| No/unknown | Reference | Reference | Reference | Reference | |||||||
| Yes | 0.398 (0.353, 0.449) | <0.001 | 0.393 (0.354, 0.437) | <0.001 | 0.402 (0.362, 0.446) | <0.001 | 0.405 (0.362, 0.453) | <0.001 | |||
HR, hazard ratio; CI, confidence interval; Sep/wid/div, separated/widowed/divorced; NOS, not otherwise specified; AJCC, American Joint Committee on Cancer.
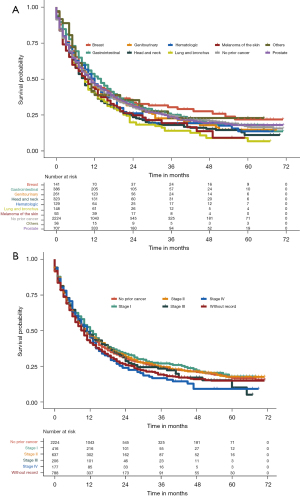
Figure 5 shows overall survival stratified according to the type and stage of prior cancer. Among all of the recorded types of prior cancer, patients with lung and bronchus cancer (P=0.013) or head and neck cancer (P=0.012) had significantly inferior survival compared with patients without prior cancer, while patients with other types of prior cancer showed similar overall survival to those without prior cancer. In subgroup analysis stratified by stage of prior cancer, the survival function of patients with stage I, II, III, and IV prior cancer did not display significant difference compared with patients without prior cancer history.
Discussion
Assuming that prior cancer history may influence the prognosis of cancer patients, prior malignancy is regularly considered an exclusion criterion in cancer clinical trials. However, little evidence has confirmed this hypothesis in different types of cancer. This study focused on the impact of prior cancer on prognosis of patients with esophageal carcinoma. The Kaplan-Meier analysis in this study revealed that for all patients diagnosed with esophageal cancer, prior cancer did not convey any adverse impact on all-cause and esophageal cancer-specific survival before PSM and after PSM. The subgroup analysis stratified by age, sex, race, grade, histological type, and stage of the index cancer confirmed these conclusions. The multivariate Cox regression analysis showed that prior malignancy was not associated with inferior all-cause and esophageal cancer-specific survival. The subgroup analysis stratified by timing of prior cancer showed no significant difference in subgroups of 6–12 months, 1–5 years, and 5–10 years; however, for the subgroup >10 years, prior cancer appeared to be associated with poor prognosis. Long-term malignancy could lead to serious decline in body function and complications, and early diagnosis of indolent prior cancer with long-term healthcare effect might increase the survival. Accordingly, we inferred that prior cancer mainly affected the survival from other causes, including prior cancer, disease progression of prior and index cancer, complications of cancer, and health condition of individuals.
In this study, approximately 13% of patients had a history of prior malignancy, which is a large proportion of the study population. Excessive exclusion criteria would limit trial accrual and low rates of participation would result in prolonged study duration, decreased generalization, and poor accuracy. Therefore, it is essential to analyze the correlation between prior cancer diagnosis and survival outcomes to broaden the inclusion criteria.
To our knowledge, lung cancer, liver cancer, pancreatic adenocarcinoma, breast cancer, and nasopharyngeal cancer have more aggressive tumor biological behavior (17). Previous studies have shown that prior cancer history has no significant effect on the prognosis of the aforementioned types of tumors (10,18-20). However, the malignant behavior of laryngeal cancer is relatively low, and prior cancer history has a significant adverse effect on its prognosis (12). Based on this, we could infer that the degree of the index tumor invasion might be one reason for the different effects of prior cancer history on survival in different types of cancer. This hypothesis was also verified from the subgroup analysis results that prior cancer showed a significant effect on survival for patients with stage I and II esophageal cancer, but not for those in stage III and IV. In this study, the subgroup analysis stratified by prior cancer type revealed that a higher degree of malignancy of a prior cancer, such as lung and bronchus cancer, or head and neck cancer, might have a significant adverse effect on prognosis. In contrast, prior cancer with lower malignancy did not show significant effect on survival of patients with esophageal cancer. Accordingly, we could infer that whether prior cancer history has an impact on survival of esophageal cancer is likely determined by the characteristics of prior cancer and the degree of malignancy of esophageal cancer.
Apart from concerns about the prognostic impact, there are other potential reasons for excluding patients with a prior cancer diagnosis from clinical trials. First, patients with prior malignancy might have received chemotherapy and radiotherapy, which would lower the tolerance for current experimental treatment and interfere with the efficacy of the trial therapy (21). Second, prior cancer could trigger a series of immune responses, damage target organs, or cause complicated diseases, such as immunodeficiency, thereby greatly reducing the effectiveness in experimental patients and the reliability of results. However, alternative strategies could be applied to address this concern. For instance, a number of clinical trials excluded patients with other severe medical comorbidities or organ dysfunction and patients who had previously received prior cancer treatment (22,23).
There are several limitations to our study. First, this study is based on the SEER database, which provides retrospective data; thus, selection bias is inevitable. Although the PSM method was employed to address such bias, other hidden forms of bias caused by unobservable confounders could not be entirely ruled out. Second, detailed information on treatment, such as the types of operation, specific radiotherapy and chemotherapy schemes, genetics, and some lifestyle factors, is not provided by the SEER database. A previous study has confirmed that cigarette smoking and alcohol drinking are two main risk factors for esophageal cancer (24); therefore, we assume that esophageal cancer patients are more likely to be heavy smokers and alcohol abusers. It has been reported that heavy alcohol drinkers and smokers have a worse prognosis in esophageal carcinoma (25). These lifestyle factors should be included in the analysis. Moreover, data on clinical characteristics of prior cancer for many patients in the SEER database were not available and could not be further analyzed, which could have led to the limitations of the findings. Third, the database used in this study only covers approximately 9.4% of the U. S. population; therefore, the generality of our findings has to be further confirmed.
Conclusions
In summary, this study confirmed that prior cancer probably does not exert definite interference with all-cause and esophageal cancer-specific survival. Further research is still essential to explore the appropriateness of such a conclusion. Hence, these findings suggest broader inclusion criteria of clinical trials for patients with esophageal carcinoma in terms of prior malignancy history. This could assist to increase trial enrollment appropriately and reach more generalizable conclusions to guide prospective approaches for esophageal cancer treatment.
Acknowledgments
We would like to thank Editor from Accdon-LetPub for his help in polishing our paper.
Funding: This study was supported by grants from the National Science and Technology Major Project (No. 2020ZX09201021), the Medical Artificial Intelligence Project of Sun Yat-sen Memorial Hospital (No. YXRGZN201902), the National Natural Science Foundation of China (No. 81572596 and 81972471), the Natural Science Foundation of Guangdong Province (No. 2017A030313828), the Guangzhou Science and Technology Major Program (No. 201704020131), the Guangdong Science and Technology Department (No. 2017B030314026), the Sun Yat-sen University Clinical Research 5010 Program (No. 2018007), the Sun Yat-sen Clinical Research Cultivating Program (No. SYS-C-201801), the Guangdong Medical Science and Technology Program (No. A2020558), and Tencent Charity Foundation (Nos. SYSU-81000-20200311-0001 and SYSU-05160-20200506-0001).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1707/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1707/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1707/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381-7. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Murphy G, McCormack V, Abedi-Ardekani B, et al. International cancer seminars: a focus on esophageal squamous cell carcinoma. Ann Oncol 2017;28:2086-93. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- Kato K, Doki Y, Ura T, et al. Long-term efficacy and predictive correlates of response to nivolumab in Japanese patients with esophageal cancer. Cancer Sci 2020;111:1676-84. [Crossref] [PubMed]
- Shoji T, Komiyama S, Kigawa J, et al. An open-label, randomized, phase II trial evaluating the efficacy and safety of standard of care with or without bevacizumab in platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer patients previously treated with bevacizumab for front-line or platinum-sensitive ovarian cancer: rationale, design, and methods of the Japanese Gynecologic Oncology Group study JGOG3023. BMC Cancer 2018;18:771. [Crossref] [PubMed]
- Zhou H, Huang Y, Qiu Z, et al. Impact of prior cancer history on the overall survival of patients newly diagnosed with cancer: A pan-cancer analysis of the SEER database. Int J Cancer 2018;143:1569-77. [Crossref] [PubMed]
- Zhou H, Zhang Y, Liu J, et al. Impact of prior cancer on outcomes in nasopharyngeal carcinoma. Ann Transl Med 2019;7:299. [Crossref] [PubMed]
- Lin C, Wu J, Ding S, et al. Impact of Prior Cancer History on the Clinical Outcomes in Advanced Breast Cancer: A Propensity Score-Adjusted, Population-Based Study. Cancer Res Treat 2020;52:552-62. [Crossref] [PubMed]
- Laccetti AL, Pruitt SL, Xuan L, et al. Prior cancer does not adversely affect survival in locally advanced lung cancer: A national SEER-medicare analysis. Lung Cancer 2016;98:106-13. [Crossref] [PubMed]
- Zhu K, Lin R, Zhang Z, et al. Impact of prior cancer history on the survival of patients with larynx cancer. BMC Cancer 2020;20:1137. [Crossref] [PubMed]
- Bian X, Xia J, Wang K, et al. The effects of a prior malignancy on the survival of patients with ovarian cancer: a population-based study. J Cancer 2020;11:6178-87. [Crossref] [PubMed]
- Saad AM, Al-Husseini MJ, Elgebaly A, et al. Impact of prior malignancy on outcomes of stage IV esophageal carcinoma: SEER based study. Expert Rev Gastroenterol Hepatol 2018;12:417-23. [Crossref] [PubMed]
- Cronin KA, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Cancer 2014;120:3755-7. [Crossref] [PubMed]
- Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399-424. [Crossref] [PubMed]
- Cree IA. Cancer biology. Methods Mol Biol 2011;731:1-11. [Crossref] [PubMed]
- Laccetti AL, Pruitt SL, Xuan L, et al. Effect of prior cancer on outcomes in advanced lung cancer: implications for clinical trial eligibility and accrual. J Natl Cancer Inst 2015;107:djv002. [Crossref] [PubMed]
- He C, Zhang Y, Cai Z, et al. Effect of prior cancer on survival outcomes for patients with pancreatic adenocarcinoma: a propensity score analysis. BMC Cancer 2019;19:509. [Crossref] [PubMed]
- Wang YQ, Lv JW, Tang LL, et al. Effect of prior cancer on trial eligibility and treatment outcomes in nasopharyngeal carcinoma: Implications for clinical trial accrual. Oral Oncol 2019;90:23-9. [Crossref] [PubMed]
- Gerber DE, Laccetti AL, Xuan L, et al. Impact of prior cancer on eligibility for lung cancer clinical trials. J Natl Cancer Inst 2014;106:dju302. [Crossref] [PubMed]
- Wang F, Wei XL, Wang FH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol 2019;30:1479-86. [Crossref] [PubMed]
- Ruhstaller T, Thuss-Patience P, Hayoz S, et al. Neoadjuvant chemotherapy followed by chemoradiation and surgery with and without cetuximab in patients with resectable esophageal cancer: a randomized, open-label, phase III trial (SAKK 75/08). Ann Oncol 2018;29:1386-93. [Crossref] [PubMed]
- Zhao X, Lim F. Lifestyle Risk Factors in Esophageal Cancer: An Integrative Review. Crit Care Nurs Q 2020;43:86-98. [Crossref] [PubMed]
- McMenamin ÚC, McCain S, Kunzmann AT. Do smoking and alcohol behaviours influence GI cancer survival? Best Pract Res Clin Gastroenterol 2017;31:569-77. [Crossref] [PubMed]


