
Prognostic impact of surgical treatment for high-grade neuroendocrine carcinoma of the lung: a multi-institutional retrospective study
Introduction
Small cell lung cancer (SCLC) and large cell neuroendocrine carcinoma (LCNEC) are high-grade neuroendocrine carcinomas (HGNEC) of the lung that behave aggressively and have a poor prognosis. Although the vast majority of HGNEC patients have metastatic disease at diagnosis, cure can be expected in patients with early-stage or locally advanced disease who undergo surgical treatment and adjuvant chemotherapy (1). Lobectomy with mediastinal lymph node dissection followed by adjuvant chemotherapy with platinum-doublet agents is standard treatment (ST) for resectable HGNEC. Limited surgery and omission of postoperative chemotherapy are associated with worse survival (2-5). In real-world clinical practice, not all HGNEC patients can receive multimodality treatment because of heavy smoking, respiratory comorbidities, or reduced organ function. Despite the many studies of surgical treatment in HGNEC patients, the differences in rates of recurrence and survival between ST and non-standard treatment (NST) remain unclear. We conducted a multi-institutional retrospective cohort study using a prospectively maintained clinical database to compare clinicopathological characteristics, postoperative recurrence, and survival outcomes between surgically treated HGNEC patients who received ST and those who received NST. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1938/rc).
Methods
Patient selection
The database records of 104 patients diagnosed with primary HGNEC of the lung (including combined SCLC and LCNEC) who underwent surgical treatment with curative intent at Tottori University Hospital and its four affiliated hospitals (Tottori Prefectural Central Hospital, Tottori Prefectural Kousei Hospital, Yonago Medical Center, and Matsue Medical Center) between January 2005 and December 2020 were retrospectively reviewed and analyzed. Patients who underwent induction therapy, incomplete resection were excluded from this study. Patients who received surgical biopsy of extensive disease small-cell lung cancer for histological diagnosis were also excluded. Surgical resection was categorized as sublobar resection (wedge resection or segmentectomy), lobectomy, or greater than lobectomy (bilobectomy or pneumonectomy). The following clinicopathological factors were examined: age, sex, smoking status, body mass index, serum pro-gastrin releasing peptide (pro-GRP) concentration, cardiovascular and respiratory comorbidities, percent vital capacity (%VC), forced expiratory volume in one second (FEV1.0%), laterality and lobe of primary tumor, surgical procedure, mediastinal lymph node dissection, adjuvant chemotherapy, pathological stage, and histology.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the institutional review board of Tottori University Hospital (No. 20A002) in May 2020. The requirement for written informed consent was waived because of the study’s retrospective design.
Histological evaluation
Surgical specimens were fixed with 10% formalin and embedded in paraffin. Serial sections were stained using hematoxylin-eosin (HE) and histopathologically examined. Additional immunohistochemical analysis of neuroendocrine markers (Synaptophysin, Chromogranin A, and CD56) was performed if HGNEC was highly suspected. Histological specimens were reviewed by a qualified pathologist (Y.U.) and certified thoracic surgeons (S.M. and Y.O.) at the time of diagnosis. Combined SCLC (C-SCLC) and combined LCNEC (C-LCNEC) were defined according to the World Health Organization classification as SCLC or LCNEC combined with additional components consisting of other histological non-small cell lung carcinoma (NSCLC) types (usually adenocarcinoma, squamous cell carcinoma, or less common histological types) (6). Tumors in the early study period were initially staged according to the 6th and 7th editions of the TNM classification; these were later restaged according to the 8th edition prior to analysis.
Group classification and definition of each recurrence
Patients were grouped according to treatment (ST or NST). ST was defined as lobectomy, bilobectomy, or pneumonectomy with mediastinal lymph node dissection followed by adjuvant platinum-doublet chemotherapy with more than two cycles. NST was defined as limited resection, surgery without mediastinal lymph node dissection, or less than two cycles of adjuvant chemotherapy. Locoregional recurrence was defined as recurrence in the (I) bronchial stump or staple line of the lung parenchyma, (II) ipsilateral pleura and/or chest wall, or (III) ipsilateral hilar and/or mediastinal lymph nodes, as described in our previous study (7). Recurrence was defined as distant if it was located in a different lobe of the ipsilateral lung, contralateral thorax, lymph nodes other than ipsilateral hilar and/or mediastinal, or an organ such as the liver, adrenal gland, brain, bone, or other sites. Median follow-up for censored cases was 30 months (range, 1–129 months), and minimum follow-up of living patients was 3 months.
Statistical analysis
Statistical analyses were performed using SPSS software version 22 (IBM Corp., Armonk, NY, USA), BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan), and GraphPad prism 8.4.1 for Windows (GraphPad Software, La Jolla, CA, USA). Group comparisons were performed using Fisher’s exact probability test, Pearson’s chi-square test, or the Mann-Whitney U test as appropriate. Recurrence free survival (RFS) and overall survival (OS) were estimated using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate Cox proportional hazards regression was used to identify clinicopathological factors associated with survival outcomes. Variables with P<0.2 in the univariate analyses were included in the multivariate model. P<0.05 (two-sided) was considered significant.
Results
Clinicopathological characteristics
Patient demographics and clinicopathological characteristics are shown in Table 1. Patients in the ST group were significantly younger than those in the NST group (P=0.01) and had significantly fewer respiratory complications (P=0.03). Pulmonary function (%VC and FEV1.0%) did not significantly differ between the groups. Among the patients in the NST group, 47% underwent limited resection, mediastinal lymph node dissection was omitted in 67%, and 75% did not receive adjuvant chemotherapy. Pathological stage and histology were not significantly different between the groups.
Table 1
| Standard therapy (n=31) | Non-standard therapy (n=73) | P value | ||||
|---|---|---|---|---|---|---|
| N or Median [IQR] | % or range | N or Median [IQR] | % or range | |||
| Age | 69 [10] | 50–80 | 72 [10] | 54–90 | 0.010 | |
| Sex | 1.000 | |||||
| Men | 28 | 90 | 65 | 89 | ||
| Women | 3 | 10 | 8 | 11 | ||
| Smoking status | 0.087 | |||||
| Ever | 29 | 94 | 73 | 100 | ||
| Never | 2 | 6 | 0 | 0 | ||
| BMI | 22.4 [3.4] | 16.0- 27.7 | 21.6 [4.2] | 16.4–31.0 | 0.599 | |
| Pro-GRP | 63.9 [64.5] | 17.1–908.0 | 56.5 [52.0] | 19.0–854.0 | 0.515 | |
| Cardiovascular comorbidities | 0.823 | |||||
| Yes | 10 | 32 | 27 | 36 | ||
| No | 21 | 68 | 46 | 64 | ||
| Respiratory comorbidities | 0.030 | |||||
| Yes | 8 | 26 | 37 | 51 | ||
| No | 23 | 74 | 36 | 49 | ||
| %VC | 96.0 [22.5] | 68.3–137.2 | 95.6 [23.5] | 31.3–137.3 | 0.201 | |
| FEV1.0% | 73.7 [9.9] | 44.6–91.3 | 69.6 [14.6] | 35.2–100.0 | 0.133 | |
| Primary tumor laterality | 0.080 | |||||
| Right | 14 | 45 | 48 | 66 | ||
| Left | 17 | 55 | 25 | 34 | ||
| Primary tumor lobe | 0.664 | |||||
| Upper & Middle lobe | 20 | 65 | 43 | 59 | ||
| Lower lobe | 11 | 35 | 30 | 41 | ||
| Surgical procedure | <0.001 | |||||
| Limited resection | 0 | 0 | 34 | 47 | ||
| Lobectomy or more | 31 | 100 | 39 | 53 | ||
| Mediastinal lymph node dissection | <0.001 | |||||
| Yes | 31 | 100 | 27 | 37 | ||
| No | 0 | 0 | 46 | 63 | ||
| Adjuvant chemotherapy | <0.001 | |||||
| Yes | 31 | 100 | 18 | 25 | ||
| No | 0 | 0 | 55 | 75 | ||
| Pathological stage | 0.183 | |||||
| Stage IA | 11 | 35 | 26 | 36 | ||
| Stage IB | 7 | 23 | 28 | 38 | ||
| Stage II or more | 13 | 42 | 19 | 26 | ||
| Histology | 0.133 | |||||
| SCLC | 15 | 48 | 31 | 42 | ||
| LCNEC | 5 | 16 | 23 | 32 | ||
| Combined SCLC | 2 | 7 | 9 | 12 | ||
| Combined LCNEC | 9 | 29 | 10 | 14 | ||
IQR, interquartile range; BMI, body mass index; Pro-GRP, pro-gastrin releasing peptide; VC, vital capacity; FEV, forced expiratory volume; SCLC, small cell lung cancer; LCNEC, large cell neuroendocrine carcinoma.
Incidence of recurrence and distribution of initial recurrence sites
Table 2 shows the incidence of postoperative recurrence and distribution of initial recurrence sites in both groups. During follow-up, 50 patients (48.1%) developed recurrence. The proportion of patients who developed recurrence did not significantly differ between the ST and NST groups (35% vs. 53%; P=0.133). No patient developed local recurrence at the bronchial stump or lung staple line in either group. Ipsilateral lymph node recurrence occurred in 1 ST group patient (3%) and 15 NST group patients (21%). Distant recurrence occurred in 9 ST group patients (29%) and 31 NST group patients (42%). A significantly higher proportion of patients in the NST group developed ipsilateral lymph node recurrence (21% vs. 3%; P=0.035) and ipsilateral or contralateral lung recurrence (15% vs. 0%; P=0.031).
Table 2
| Standard therapy | Non-standard therapy | P value | ||||
|---|---|---|---|---|---|---|
| N=31 | % | N=73 | % | |||
| Postoperative recurrence | ||||||
| No | 20 | 65 | 34 | 47 | 0.133 | |
| Yes | 11 | 35 | 39 | 53 | ||
| Locoregional recurrence | 2 | 6 | 8 | 11 | 0.282 | |
| Locoregional and distant recurrences | 0 | 0 | 8 | 11 | ||
| Distant recurrence | 9 | 29 | 23 | 31 | ||
| Sites of initial recurrence | ||||||
| Ipsilateral pleura | 1 | 3 | 5 | 7 | 0.667 | |
| Ipsilateral lymph nodes | 1 | 3 | 15 | 21 | 0.035 | |
| Other lymph nodes | 0 | 0 | 3 | 4 | 0.553 | |
| Lung | 0 | 0 | 11 | 15 | 0.031 | |
| Liver | 2 | 6 | 8 | 11 | 1.000 | |
| Brain | 4 | 13 | 7 | 10 | 0.729 | |
| Bone | 2 | 6 | 5 | 7 | 1.000 | |
| Adrenal gland | 2 | 6 | 2 | 3 | 0.581 | |
| Other sites | 2 | 6 | 5 | 7 | 1.000 | |
Survival analysis
Figure 1 shows Kaplan-Meier survival curves of the ST and NST groups. Five-year RFS was 58.2% in the ST group and 29.3% in the NST group (P=0.013). The 5-year OS rates were 64.2% and 38.3%, respectively (P=0.038). In ST group subanalysis, 5-year RFS and OS were higher in patients with pathological stage I disease than those with higher stage disease (P=0.046 and 0.053, respectively; Figure S1). Multivariate Cox proportional hazards regression showed that NST was independently associated with worse RFS (hazard ratio, 1.965; 95% confidence interval, 1.038–3.719; P=0.038) and OS (hazard ratio, 2.044; 95% confidence interval, 1.016–4.113; P=0.045; Table 3).
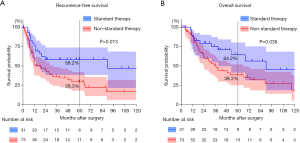
Table 3
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | (95% confidence interval) | P value | Hazard ratio | (95% confidence interval) | P value | |||
| Univariate and multivariate analysis for RFS | ||||||||
| Age >70 y.o. | 1.375 | (0.812–2.329) | 0.236 | |||||
| Male | 0.856 | (0.388–1.892) | 0.701 | |||||
| Cardiovascular complications | 0.901 | (0.516–1.574) | 0.715 | |||||
| Respiratory complications | 1.486 | (0.885–2.494) | 0.134 | 1.110 | (0.641–1.921) | 0.710 | ||
| Left side | 1.066 | (0.629–1.805) | 0.813 | |||||
| Lower lobe | 1.806 | (1.076–3.030) | 0.025 | 1.659 | (0.970–2.838) | 0.065 | ||
| Pathological stage II or more | 1.325 | (0.768–2.284) | 0.312 | |||||
| SCLC/C-SCLC histology | 1.299 | (0.767–2.201) | 0.331 | |||||
| Non-standard therapy | 2.127 | (1.143–3.959) | 0.017 | 1.965 | (1.038–3.719) | 0.038 | ||
| Univariate and multivariate analysis for OS | ||||||||
| Age >70 y.o. | 1.818 | (1.005–3.289) | 0.048 | 2.015 | (1.090–3.723) | 0.025 | ||
| Male | 0.660 | (0.294–1.483) | 0.314 | |||||
| Cardiovascular complications | 1.263 | (0.687–2.322) | 0.452 | |||||
| Respiratory complications | 1.373 | (0.770–2.447) | 0.283 | |||||
| Left side | 1.461 | (0.814–2.622) | 0.204 | |||||
| Lower lobe | 1.631 | (0.918–2.899) | 0.095 | 2.142 | (1.144–4.012) | 0.017 | ||
| Pathological stage II or more | 1.830 | (1.016–3.294) | 0.044 | 2.883 | (1.496–5.559) | 0.002 | ||
| SCLC/C-SCLC histology | 1.091 | (0.610–1.951) | 0.769 | |||||
| Non-standard therapy | 2.010 | (1.021–3.958) | 0.043 | 2.044 | (1.016–4.113) | 0.045 | ||
RFS, recurrence free survival; OS, overall survival; y.o., years old; SCLC, small cell lung cancer; C-SCLC, combined SCLC.
Discussion
This study evaluated clinicopathological features and survival outcomes in patients with HGNEC of the lung who underwent surgical resection using data obtained from a prospectively maintained regional multi-institutional database. Our findings show that lobectomy and mediastinal lymph node dissection followed by at least two cycles of adjuvant chemotherapy with platinum-doublet agents can provide a better survival outcome than other more limited treatments.
SCLC is more malignant than other types of lung cancer because of its rapid growth and metastatic spread early in the disease course. For early-stage SCLC, treatment with surgery and adjuvant chemotherapy are generally recommended. A recent large-scale cohort study showed that lobectomy with adjuvant chemotherapy in stage I/II SCLC patients was associated with significantly longer median OS than concurrent chemoradiation therapy (48.6 vs. 28.7 months; P<0.0001) (8). LCNEC is a rare neoplasm that was classified with high-grade neuroendocrine tumors of the lung in 2015 (9) and behaves more aggressively than other types of lung cancer. Five-year OS is approximately 50% even in patients who receive curative-intent surgery (10,11). Previous retrospective and prospective studies of adjuvant chemotherapy for LCNEC have shown better survival in patients who receive a SCLC regimen such as cisplatin and etoposide (12,13). Although the differences between LCNEC and SCLC in terms of pathogenesis and detailed molecular and pathological features remain controversial, they are commonly addressed together in clinical practice as HGNEC.
Lobectomy is a standard surgical procedure for primary lung cancer and is generally performed in patients with early to locally advanced HGNEC. Several studies have reported that lobectomy for HGNEC achieves better long-term outcomes than limited surgery such as wedge resection or segmentectomy (2,3,14). In our study, 34 of 104 patients (33%) received limited resection rather than standard resection because of advanced age, respiratory and/or other organ dysfunction, or presence of multiple lung tumors. In addition, most limited resection procedures did not include hilar or mediastinal lymph node sampling or dissection. Considering that 21% of the patients in our study experienced local recurrence in the ipsilateral lymph nodes after NST, we hypothesize that omitting lymph node dissection can contribute to poor outcome. Regardless of the type of lung resection, sampling or dissection of the hilar and mediastinal lymph nodes should be performed when possible to allow proper pathological staging and avoid regional lymph node recurrence when treating HGNEC.
Postoperative systemic chemotherapy is important when treating HGNEC even in early-stage disease because of its high degree of biological malignancy. Yang et al. reported that adjuvant chemotherapy was associated with significantly improved survival in patients with pT1-2N0M0 SCLC (4). Raman et al. reported that adjuvant chemotherapy appeared to confer an additional OS advantage in patients with completely resected stage IB LCNEC (5). Kenmotsu et al. conducted a large randomized phase III study in patients with completely resected stage I–IIIA HGNEC of the lung to compare postoperative adjuvant chemotherapy regimens (JCOG1205/1206) (15). They demonstrated that irinotecan plus cisplatin was not superior to etoposide plus cisplatin for improving RFS. Therefore, etoposide plus cisplatin remains the standard. In theory, all HGNEC patients should be given etoposide and cisplatin as adjuvant chemotherapy after complete resection; however, this is difficult to implement in real-world clinical practice. In our study population, approximately half of the patients could not receive adjuvant chemotherapy because of advanced age, decreased activities of daily living after surgery, and concurrent respiratory or other systemic comorbidities. The use of immune-checkpoint inhibitors or molecular-targeted therapeutic agents for NSCLC adjuvant therapy has been investigated in recent years (16,17). Moreover, recent advances in molecular pathology have led to the discovery of new potential HGNEC therapeutic targets (18,19). Hopefully, effective and well-tolerated adjuvant therapies with few side effects will be developed for treating HGNEC in the future.
Other additional treatments for surgically resected HGNEC remain controversial. Wakeam et al. investigated the effect of postoperative radiation therapy (PORT) on survival outcomes in SCLC patients and concluded that it was associated with longer survival in node-positive patients and those undergoing sublobar resection (20). Zhou et al. conducted a large multi-institutional cohort study to examine survival and recurrence patterns in patients with limited-stage SCLC who underwent surgical resection followed by various adjuvant therapies and reported that mediastinal PORT did not improve OS or reduce locoregional recurrence (21). Survival benefit of prophylactic cranial irradiation (PCI) after surgery in patients with HGNEC is also unclear. Although Zhou et al. reported that PCI did not reduce the incidence of brain metastasis or improve OS (21), Yang et al. suggested that PCI might be associated with a favorable survival advantage and lower risk of brain metastasis in their meta-analysis of PCI in patients with resected SCLC (22). In our study, only three patients received PORT and/or PCI because these treatments are not commonly utilized in HGNEC patients in Japan. Large-scale prospective studies are needed to determine their benefit in patients with surgically resected HGNEC.
There are several limitations of this study. Its retrospective nature and differences in surgeons, hospital equipment, and treatment decisions among the participating institutions might have introduced bias. Another limitation is the time-duration of the data collection from 2005–2020. During this time period much has changed in the treatment of lung cancer in terms of diagnosis, staging, surgical, and radiation techniques. Furthermore, its sample size was small because of the relative rarity of HGNEC patients undergoing resection; therefore, our findings cannot be considered definitive.
Conclusions
In conclusion, lobectomy and mediastinal lymph node dissection followed by at least two cycles of adjuvant chemotherapy with platinum-doublet agents provided better outcomes than more limited treatment in patients with HGNEC of the lung undergoing resection. Patients who received more limited treatment experienced worse RFS, worse OS, and more frequent recurrence in the regional lymph nodes and lungs. Lymph node dissection should be performed when possible to avoid regional lymph node recurrence. Patients who receive more limited treatment upfront may require additional treatment.
Acknowledgments
We thank the Edanz Group (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding: This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Number JP 19K18215).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1938/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1938/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1938/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Welter S, Aigner C, Roesel C. The role of surgery in high grade neuroendocrine tumours of the lung. J Thorac Dis 2017;9:S1474-83. [Crossref] [PubMed]
- Takei H, Kondo H, Miyaoka E, et al. Surgery for small cell lung cancer: a retrospective analysis of 243 patients from Japanese Lung Cancer Registry in 2004. J Thorac Oncol 2014;9:1140-5. [Crossref] [PubMed]
- Raman V, Jawitz OK, Yang CJ, et al. Outcomes for Surgery in Large Cell Lung Neuroendocrine Cancer. J Thorac Oncol 2019;14:2143-51. [Crossref] [PubMed]
- Yang CF, Chan DY, Speicher PJ, et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:1057-64. [Crossref] [PubMed]
- Raman V, Jawitz OK, Yang CJ, et al. Adjuvant Therapy for Patients With Early Large Cell Lung Neuroendocrine Cancer: A National Analysis. Ann Thorac Surg 2019;108:377-83. [Crossref] [PubMed]
- Travis WD. The 2015 WHO classification of lung tumors. Pathologe 2014;35:188. [Crossref] [PubMed]
- Haruki T, Miwa K, Araki K, et al. Distribution and Prevalence of Locoregional Recurrence after Video-Assisted Thoracoscopic Surgery for Primary Lung Cancer. Thorac Cardiovasc Surg 2016;64:526-32. [PubMed]
- Wakeam E, Acuna SA, Leighl NB, et al. Surgery Versus Chemotherapy and Radiotherapy For Early and Locally Advanced Small Cell Lung Cancer: A Propensity-Matched Analysis of Survival. Lung Cancer 2017;109:78-88. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Moriya Y, et al. Prognostic impact of large cell neuroendocrine histology in patients with pathologic stage Ia pulmonary non-small cell carcinoma. J Thorac Cardiovasc Surg 2006;132:312-5. [Crossref] [PubMed]
- Kim KW, Kim HK, Kim J, et al. Outcomes of Curative-Intent Surgery and Adjuvant Treatment for Pulmonary Large Cell Neuroendocrine Carcinoma. World J Surg 2017;41:1820-7. [Crossref] [PubMed]
- Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol 2005;23:8774-85. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg 2006;82:1802-7. [Crossref] [PubMed]
- Mochizuki E, Matsuura S, Oishi K, et al. Surgical resection for clinical stage I high-grade neuroendocrine carcinoma of the lung. World J Surg Oncol 2018;16:33. [Crossref] [PubMed]
- Kenmotsu H, Niho S, Tsuboi M, et al. Randomized Phase III Study of Irinotecan Plus Cisplatin Versus Etoposide Plus Cisplatin for Completely Resected High-Grade Neuroendocrine Carcinoma of the Lung: JCOG1205/1206. J Clin Oncol 2020;38:4292-301. [Crossref] [PubMed]
- Yotsukura M, Nakagawa K, Suzuki K, et al. Recent advances and future perspectives in adjuvant and neoadjuvant immunotherapies for lung cancer. Jpn J Clin Oncol 2021;51:28-36. [Crossref] [PubMed]
- Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. [Crossref] [PubMed]
- Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer 2017;17:725-37. [Crossref] [PubMed]
- Lantuejoul S, Fernandez-Cuesta L, Damiola F, et al. New molecular classification of large cell neuroendocrine carcinoma and small cell lung carcinoma with potential therapeutic impacts. Transl Lung Cancer Res 2020;9:2233-44. [Crossref] [PubMed]
- Wakeam E, Giuliani M, Leighl NB, et al. Indications for Adjuvant Mediastinal Radiotherapy in Surgically Resected Small Cell Lung Cancer. Ann Thorac Surg 2017;103:1647-53. [Crossref] [PubMed]
- Zhou N, Bott M, Park BJ, et al. Predictors of survival following surgical resection of limited-stage small cell lung cancer. J Thorac Cardiovasc Surg 2021;161:760-771.e2. [Crossref] [PubMed]
- Yang Y, Zhang D, Zhou X, et al. Prophylactic cranial irradiation in resected small cell lung cancer: A systematic review with meta-analysis. J Cancer 2018;9:433-9. [Crossref] [PubMed]


