
Clinical value of echocardiography in evaluating hemodynamics and right ventricular function in patients with chronic thromboembolic pulmonary hypertension after balloon pulmonary angioplasty
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare pulmonary vascular disease caused by chronic and persistent organized thrombi of major pulmonary arteries. CTEPH is associated with progressive right heart failure and poor patient prognosis (1-4). Pulmonary endarterectomy (PEA) improves the symptoms and prognosis of patients with proximal CTEPH (5,6). Notably, the degree of improvement in pulmonary hypertension and tricuspid regurgitation and patient survival after PEA is dependent on the type and location of pulmonary thromboembolic (7). PEA is associated with limitations such as major pulmonary vessel obstruction and small vessel arterial obstruction (8). Pulmonary vascular resistance (PVR) and right heart pressure do not significantly reduce after surgery for patients with distal small vessel arteriopathy or surgically inaccessible thrombus (2,7,9). Balloon pulmonary angioplasty (BPA) is an effective method for patients with inoperable CTEPH or residual or recurrent pulmonary hypertension following PEA (10,11). However, the effects of BPA on pulmonary hemodynamics and right ventricular (RV) function in patients with CTEPH have not been explored. The current gold standard for evaluation of RV hemodynamics is right heart catheterization (RHC). However, RHC is not effective for exploring the RV structure and function and is not suitable for repeated examinations due to its invasiveness and time-consuming (12). Cardiovascular magnetic resonance (CMR) is used to effectively to explore RV function including RV stroke volume, which is a hemodynamic parameter that indicates the prognosis of patients with CTEPH (13). A recent study reported that CMR can be used to determine reversibility of RV remodeling by catheter intervention in the area of CTEPH by evaluating RV functional parameters (14). However, CMR is time-consuming and expensive when repetitive examination is required. Therefore, it is imperative to explore simple and non-invasive tools for evaluation of the therapeutic effect of BPA in outpatient setting. Echocardiography techniques are used for non-invasive evaluation of RV function. In addition, the approaches are inexpensive and less time and labor intensive for repetitive examination. Previous findings on echocardiography indicate that BPA can improve RV structure and function in patients with CTEPH (12,15,16). However, this finding was only observed at a timepoint of 3 months or more after BPA. Studies have not explored whether the RV function and structure is improved within a few hours after BPA. Studies should explore RV structural changes and RV systolic and diastolic function under physiological conditions as well as pathophysiological conditions after BPA.
Therefore, the aim of the present study was to evaluate the short-term effects of BPA on pulmonary hemodynamics, right ventricular function and structure in patients with CTEPH. This article is presented in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1536/rc).
Methods
Study design and participants
A total of 30 consecutive CTEPH adult patients undergoing BPA procedures at The First Affiliated Hospital of Guangzhou Medical University from September 2019 to September 2020 were enrolled in the present single-centered, retrospective, observational study. The criteria were as follows: (I) patients admitted to the hospital with inoperable CTEPH diagnosed based on a detailed medical history, results of pulmonary angiography, RHC, and radionuclide lung ventilation/perfusion scans, (II) patients diagnosed with pulmonary artery thrombosis and thrombosis persisted after three-month anticoagulation treatment, (III) and gave oral or written informed consent. Pregnant and uncollaborative patients were excluded from the study. BPA treatment selection criteria: (I) pulmonary artery stenosis ≥70% confirmed through pulmonary angiography; (II) pulmonary hypertension with intermediate to high-risk stratification or right ventricular insufficiency (10); (III) CTEPH diagnosis and treatment progress; 4) No contraindications related to interventional therapy. A total of 41 CTEPH patients were registered to the study. Out of the 41 patients, 8 patients did not have complete clinical data before BPA, and 3 patients had not undergone echocardiography within 24 hours after BPA. Therefore, 30 patients were finally enrolled in the study.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee at The First Affiliated Hospital of Guangzhou Medical University (Approval No. [2020]108). All patients signed written informed consent prior to participation in the current study.
BPA procedure
BPA was performed as described previously (17). RHC was performed through the right femoral venous using a Swan-Ganz catheter (Edwards) before and after surgery. A 5F pigtail angiography catheter (Cordis) was used to perform digital subtraction angiography (DSA) of the target pulmonary artery area. The guidewire (ASAHI) was passed through the target lesion, and target segment/subsegmental branches were expanded by manual balloon inflations using a semi-compliant balloon until the indentation disappeared or the balloon was fully inflated. The contrast agent was injected into the treated vessel after inflation to evaluate the angiographic effect of the operation. Super-selective and multi-angle pulmonary angiography was performed to determine the next target vessel after completing the balloon expansion of the lung segment, then the balloon was extended to the vessel. Patients were administered with local anesthesia during the procedures.
RHC
RHC was performed using a Swan-Ganz catheter (Edwards Lifesciences) before and after surgery. RHC evaluates hemodynamic parameters, including right atrial pressure (RAP), pulmonary artery pressure (PAP), pulmonary capillary wedge pressure (PCWP), PVR and cardiac output (CO). Mean right atrial pressure (mRAP), mean pulmonary artery pressure (mPAP) and mean PCWP were recorded during continuous ECG monitoring. CO was calculated using the Fick method, whereas the cardiac index (CI) was calculated by dividing the CO by the body surface area.
Echocardiography
A Philips EPIQ 7C (Philips Healthcare) Doppler ultrasound machine equipped with an S5-1 transducer (1–5 MHz) was used for echocardiography. Determination of right heart function parameters was conducted according to the 2015 ASE Adult Echocardiography Cardiac Chamber Quantification Guidelines (18). The apical four-chamber view of the right ventricle was displayed and the RV function parameters including tissue Doppler-derived tricuspid lateral annular systolic velocity (S’), tricuspid annular plane systolic excursion (TAPSE), right ventricular index of myocardial performance (RIMP) and right ventricular fractional area change (RVFAC) were determined. Echocardiography RV structural parameters including inner diameter of main pulmonary artery in end diastolic (DMPA), right ventricular basal diameter (RVD); right atrium diameter (RAD); right ventricular end-diastolic area (RVEDA), right ventricular end-systolic area (RVESA), right atrium end-diastolic area (RAEDA) and right atrium end-systolic area (RAESA) were determined in the present study. In addition, left ventricular stroke volume (LVSV) was evaluated as the left heart function parameter of echocardiography. RIMP was calculated by Pulse Tissue Doppler Imaging [RIMP = (TCO − ET)/ET]. RAD was measured in the 4-chamber apical view in the axis parallel to the interatrial septum from the center of the tricuspid annulus to the superior wall of the right atrium. Pulmonary artery systolic pressure (PASP) was estimated from tricuspid regurgitation pressure difference. Echocardiographic evaluation after BPA was performed at the department of ultrasound in the morning of the day after BPA treatment (within 24 h). Ultrasound images were independently reviewed by two researchers with extensive experience in echocardiography training. Inconsistencies between the assessment results of the two independent researchers were resolved by discussion and consultation with a third researcher. All researchers evaluating echocardiography results were blinded to the clinical details.
Statistical analysis
SPSS software version 26.0 was used for statistical analysis. Data were presented as mean ± SD or number (%) depending on the distribution type. The correlation between variables was evaluated by linear regression analysis. Paired t-test was conducted to compare the changes in hemodynamic and RV functional parameters before and after BPA. A P value <0.05 was considered statistically significant.
Results
Baseline patient characteristics
A total of 30 consecutive patients were enrolled to the current study between September 2019 to September 2020. Participants included 9 males (30.0%) and 21 females (70.0%). The average age of participants was 57.5±12.4 years and they had an average BMI of 23.9±3.1. Demographic and clinical characteristics of patients are presented in Table 1. All 30 patients included in the present study underwent BPA surgeries. Out of the 30 patients, 11 (36.7%) were classified as World Health Organization functional class (WHO-FC) III or IV. mRAP, mPAP and PVR of the patients at baseline were elevated to 6.47±3.21 mmHg, 35.17±10.79 mmHg and 7.34±4.19 Wood units, whereas CI was reduced (2.73±1.11 L/min per m2) (Table 1).
Table 1
| Characteristic | Patients, N=30 |
|---|---|
| Age, years | 57.5±12.4 |
| Male, n (%) | 9 (30.0) |
| BMI, kg/m2 | 23.9±3.1 |
| WHO-FC, n (%) | |
| Class I | 2 (6.7) |
| Class II | 17 (56.7) |
| Class III | 9 (30.0) |
| Class IV | 2 (6.7) |
| pro-BNP, pg/mL | 644.21±888.67 |
| Hemodynamic data before procedure | |
| Mean RAP, mmHg | 6.47±3.21 |
| Mean PAP, mmHg | 35.17±10.79 |
| CI, L/min per m2 | 2.73±1.11 |
| PVR, Wood units | 7.34±4.19 |
Data are presented as mean ± SD or n (%). BMI, body mass index; WHO-FC, World Health Organization functional class; pro-BNP, pro-brain natriuretic peptide; RAP, right atrial pressure; PAP, pulmonary artery pressure; CI, cardiac index; PVR, pulmonary vascular resistance.
Hemodynamic parameters assessed by RHC before and after BPA
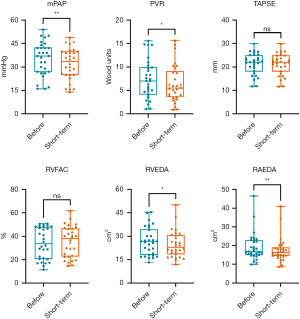
All BPA procedures in the present study were successfully performed without any deaths or major complications. Analysis showed that the vessel was dilated successfully by angiography after BPA (Figure 1). RHC data before vs. after BPA are presented in Table 2. Notably, some RHC hemodynamic parameters including PASP (60.97±21.13 vs. 54.43±18.65 mmHg, P<0.001), pulmonary artery diastolic pressure (PADP) (20.17±6.91 vs. 17.20±6.50 mmHg, P=0.006), mPAP (35.17±10.79 vs. 32.10±10.02 mmHg, P=0.009) and PVR (7.34±4.19 vs. 6.41±3.96 Wood units, P=0.014) were significantly improved after BPA (Figure 2). However, mean PCWP, CI and mean RAP did not show significant changes after BPA.
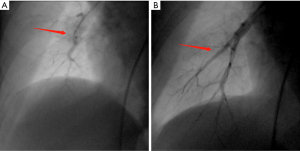
Table 2
| Variables | Before | After | Difference (95% CI) | P value |
|---|---|---|---|---|
| PASP, mmHg | 60.97±21.13 | 54.43±18.65 | −6.53 (−9.79 to −3.27) | <0.001 |
| PADP, mmHg | 20.17±6.91 | 17.20±6.50 | −2.97 (−5.03 to −0.91) | 0.006 |
| Mean PAP, mmHg | 35.17±10.79 | 32.10±10.02 | −3.07 (−5.31 to −0.82) | 0.009 |
| Mean PCWP, mmHg | 8.95±2.95 | 8.57±2.84 | −0.38 (−0.98 to 0.21) | 0.196 |
| CI, L/min per m2 | 2.73±1.11 | 2.97±1.19 | 0.24 (−0.20 to 0.67) | 0.276 |
| PVR, Wood units | 7.34±4.19 | 6.41±3.96 | −0.93 (−1.66 to −0.20) | 0.014 |
| Mean RAP, mmHg | 6.47±3.21 | 6.23±2.84 | −0.23 (−1.11 to 0.64) | 0.590 |
Data are presented as mean ± SD. P values were calculated using paired t-test for continuous variables. Before: before BPA; after: within 24 hours after BPA. RHC, right heart catheterization; CI, cardiac index; PASP, pulmonary artery systolic pressure; PADP, pulmonary artery diastolic pressure; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; BPA, balloon pulmonary angioplasty.
Comparison of echocardiography parameters before vs. after BPA
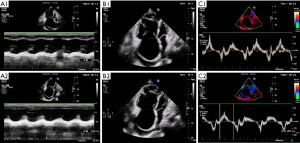
The results from blind evaluation of echocardiography parameters were highly consistent with the results obtained for RHC parameters (Figure 3). All examinations were performed within 24 h. PASP at baseline was 62.90±27.70 mmHg as determined by echocardiography, as opposed to 60.97±21.13 mmHg as determined by RHC. RHC and echocardiography results were significantly correlated (r=0.807; P<0.0001). Echocardiography function parameters, echocardiography structural parameters and echocardiography PASP of the 30 patients before vs. after BPA are presented in Table 3. Notably, some echocardiography function parameters were significantly improved after BPA (Figure 2). In addition, echocardiography structural parameters were significantly improved after BPA. Right atrium parameters including RAD (54.62±9.86 vs. 51.61±9.97, P<0.001), RAEDA (19.31±7.43 vs. 17.61±6.52, P=0.002), RAESA (13.90±7.45 vs. 12.66±6.88, P<0.001) were significantly less after BPA relative to the values before BPA. In addition, right ventricular parameters including RVEDA (26.72±8.99 vs. 24.82±8.52, P=0.022), RVESA (18.19±8.35 vs. 16.51±7.93, P=0.013) decreased after BPA. Moreover, echocardiography PASP after BPA was significantly lower compared with that before BPA (62.90±27.70 vs. 57.02±24.99, P=0.047). However, the results showed no significant changes in S’, TAPSE, RIMP, RVFAC, and LVSV after BPA, however, the mean values of these parameters were slightly improved (P>0.05; Table 3). A representative echocardiography images of one CTEPH patient before and after BPA treatment is presented in Figure 4.
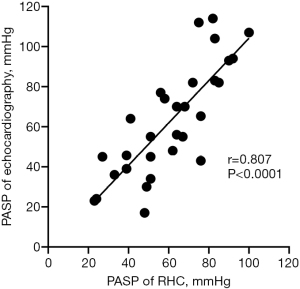
Table 3
| Variables | Before | After | Difference (95% CI) | P value |
|---|---|---|---|---|
| Echocardiography function parameters | ||||
| S’, cm/s | 12.66±2.15 | 12.95±2.28 | 0.29 (−0.51 to 1.09) | 0.469 |
| TAPSE, mm | 20.10±5.07 | 21.33±4.56 | 1.24 (−0.07 to 2.54) | 0.063 |
| RIMP | 0.60±0.23 | 0.60±0.21 | 0.00 (−0.07 to 0.07) | 0.992 |
| RVFAC, % | 33.92±13.45 | 35.46±13.30 | 1.54 (−1.65 to 4.72) | 0.331 |
| LVSV, mL | 57.89±14.92 | 62.21±15.21 | 4.32 (−0.68 to 9.31) | 0.088 |
| Echocardiography structural parameters | ||||
| DMPA, mm | 27.60±5.89 | 27.35±5.76 | −0.25 (−1.72 to 1.22) | 0.734 |
| RVD, mm | 41.00±8.44 | 40.22±8.62 | −0.77 (−2.08 to 0.53) | 0.235 |
| RAD, mm | 54.62±9.86 | 51.61±9.97 | −3.01 (−4.27 to −1.75) | <0.001 |
| RVEDA, cm2 | 26.72±8.99 | 24.82±8.52 | −1.90 (−3.50 to −0.29) | 0.022 |
| RVESA, cm2 | 18.19±8.35 | 16.51±7.93 | −1.68 (−2.97 to −0.38) | 0.013 |
| RAEDA, cm2 | 19.31±7.43 | 17.61±6.52 | −1.69 (−2.73 to −0.66) | 0.002 |
| RAESA, cm2 | 13.90±7.45 | 12.66±6.88 | −1.24 (−1.76 to −0.72) | <0.001 |
| Echocardiography PASP, mmHg | 62.90±27.70 | 57.02±24.99 | −5.88 (−11.66 to −0.09) | 0.047 |
Data are presented as mean ± SD. P values were calculated using paired t-test for continuous variables. Before: before BPA; after: within 24 hours after BPA. CI, confidence interval; S’, tissue Doppler-derived tricuspid lateral annular systolic velocity; TAPSE, tricuspid annular plane systolic excursion; RIMP, right ventricular index of myocardial performance; RVFAC, right ventricular fractional area change; LVSV, left ventricular stroke volume; DMPA, inner diameter of main pulmonary artery; RVD, right ventricular basal diameter; RAD, right atrium diameter; RVEDA, right ventricular end-diastolic area; RVESA, right ventricular end-systolic area; RAEDA, right atrium end-diastolic area; RAESA, right atrium end- systolic area; PASP, pulmonary artery systolic pressure.
Discussion
The major findings of the present study were as follows: (I) BPA reduces pulmonary artery pressure and RV volume in inoperable CTEPH patients; (II) the right heart volume load and pulmonary artery pressure load of CTEPH patients were rapidly decreased within 24 hours after BPA treatment; (III) RV structure improved within 24 hours after BPA, but the RV function did not improve, indicating that the right ventricle has not undergone significant reverse remodeling.
Chronic pressure overload causes RV morphological changes such as myocardial hypertrophy and RV dilatation in CTEPH patients. BPA significantly improves pulmonary blood flow distribution, increases pulmonary vascular volume and decreases RV overload. A previous study on the use of BPA as an alternative interventional strategy for CTEPH patients without surgical potential reported that BPA significantly reduced pulmonary hypertension in CTEPH patients, but is associated with severe complications such as reperfusion pulmonary edema (19). A refined BPA procedure was recently reported which significantly improves the clinical status and hemodynamics of inoperable CTEPH patients, and is associated with a lower mortality rate (20). Echocardiography evaluation results from clinical studies indicate that BPA reduces pulmonary artery pressure and improves RV function in CTEPH patients (12,16,21). A previous study showed that CMR can be used to indicate a decrease in RV volume and mass after BPA (14). Although CMR provides excellent image clarity and volumetric accuracy, it is costly. In addition, CMR has limited clinical value in evaluation of hemodynamics. The high consistency between echocardiography findings and RHC results indicates that echocardiography is a reliable tool for evaluating severity and prognosis of CTEPH patients after BPA. Moreover, echocardiography techniques can be used to non-invasively assess RV function, making it inexpensive and convenient for repetitive examination. Moreover, echocardiography is more effective for follow-up after BPA compared with traditional methods.
Evaluation of RV is challenging owing to its complex geometry. The ASE guidelines indicate that assessment of RV systolic function should involve at least one of the following methods or a combination of multiple methods: RVFAC, S’ and TAPSE (18). S’ and TAPSE are used to evaluate the longitudinal motor function of RV, and they have a significant clinical predictive value. Notably, determination of S’ and TAPSE is angularly dependent and cannot fully represent the overall RV function. S’ and TAPSE analysis can underestimate or overestimate the RV systolic function, if the Doppler signal is not parallel to the direction of the annulus motion. RVFAC is a parameter for quantitatively evaluating the overall systolic function of the right ventricle, including longitudinal and radial directions. RVFAC is more comprehensive in evaluating RV function compared with S’ and TAPSE. RIMP is used to determine the overall systolic and diastolic function of the right ventricle, and is less affected by heart rate. However, RIMP can give false low results in conditions associated with elevated RA pressures (18).
The right heart volume load and pulmonary artery pressure load of CTEPH patients were significantly decreased within 24 hours after BPA treatment. However, RV function did not improve, indicating that the right ventricle had not undergone significant reverse remodeling within 24 hours after BPA. A study by Broch (16) reported that RV undergoes significant reverse remodeling after 3 months or more after BPA, as indicated by reduction of RV volume load and improvement of RV function. Although the parameters of RV function did not improve significantly in the current study, the trend of RVFAC indicates that RV function gradually improved. The pulmonary blood flow is rapidly restored and RV afterload is reduced by dilation of the narrowed lesions or recanalizing the occluded pulmonary vessels immediately after BPA, thereby improving pulmonary hemodynamics and RV volume. The function of the RV is gradually improved, and a significant reverse remodeling of the RV occurs. BPA surgery is associated with improvements in pulmonary hemodynamics, lung perfusion, exercise endurance, WHO-FC, and 6-minute walking distance in the short term (22). However, RV function does not improve immediately after BPA treatment, and positive effects of this procedure may only be observed after several months (15,16,21). This phenomenon can be attributed to the unique myocardial structure of RV. Longitudinal cardiomyocytes are mainly located in the subendocardium of RV, and are more susceptible to high pressure and insufficient coronary perfusion caused by RV dilation (23). Therefore, the function of damaged myocardial fibers may take longer to be restored after BPA reduces the load on the RV.
The 2015 ESC/ERS pulmonary hypertension guidelines state that BPA is a Class IIb indication for patients with inoperable CTEPH (10). The role of BPA was further reported at the Sixth World Symposium on Pulmonary Hypertension (24). Further studies should explore the role of BPA in treatment of patients with CTEPH. Right heart failure is the leading cause of death in patients with CTEPH and can be reversed by BPA (25). Echocardiography techniques are used to non-invasively assess RV function parameters. Notably, the right atrium volume parameter, right ventricular volume parameter and PASP, which are sensitive indicators for evaluating the effect of BPA, were significantly improved in the present study.
The present study had some limitations. First, this study was conducted retrospectively at a single center with a limited number of patients. Second, only treatment group was enrolled in the present study, therefore, there was no control group for comparison of the results. Third, the follow-up period of this study was relatively short. Fourth, the study did explore the effects of new echocardiographic techniques, such as strain, 3-dimensional transthoracic echocardiography (3D-TTE). Fifth, only single echocardiographic parameters were determined and no multivariate echocardiographic indicators were evaluated to estimate right ventricular function, which were more consistent with invasive measurements compared with the univariate analysis results (26). Sixth, the influence of BPA complications, such as post-reperfusion pulmonary edema, on the obtained measurement results was not factored during analysis. The findings from the present study should be further verified through a multicenter study with a large cohort.
Conclusions
The results of the present study show that a single BPA procedure improves the prognosis of CTEPH patients by reducing the RV volume load in the short-term. However, RV systolic function is not significantly improved within 24 hours. The findings show that echocardiography is a useful modality for evaluation of RV function in patients with inoperable CTEPH after BPA.
Acknowledgments
We thank Dr. Jerry, from HOME for Researchers (https://www.home-for-researchers.com), for editing the English text of a draft of this manuscript.
Funding: The study was funded by the open project of the State Key Laboratory of Respiratory Diseases (No. SKLRD-OP-202112), Zhongnanshan Medical Foundation of Guangdong Province (No. ZNSA-2020013) and the First Affiliated Hospital of Guangzhou Medical University Achievements and Clinical Transformation Nursery Project (No. ZH202104).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1536/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1536/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1536/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee at The First Affiliated Hospital of Guangzhou Medical University (Approval No. [2020]108). All patients signed written informed consent prior to participation in the current study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lang IM, Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation 2014;130:508-18. [Crossref] [PubMed]
- Piazza G, Goldhaber SZ. Chronic thromboembolic pulmonary hypertension. N Engl J Med 2011;364:351-60. [Crossref] [PubMed]
- Riedel M, Stanek V, Widimsky J, et al. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982;81:151-8. [Crossref] [PubMed]
- Hoeper MM, Mayer E, Simonneau G, et al. Chronic thromboembolic pulmonary hypertension. Circulation 2006;113:2011-20. [Crossref] [PubMed]
- Cannon JE, Su L, Kiely DG, et al. Dynamic Risk Stratification of Patient Long-Term Outcome After Pulmonary Endarterectomy: Results From the United Kingdom National Cohort. Circulation 2016;133:1761-71. [Crossref] [PubMed]
- Jenkins D, Madani M, Fadel E, et al. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017;26:160111. [Crossref] [PubMed]
- Thistlethwaite PA, Madani M, Jamieson SW. Outcomes of pulmonary endarterectomy surgery. Semin Thorac Cardiovasc Surg 2006;18:257-64. [Crossref] [PubMed]
- Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest 1993;103:685-92. [Crossref] [PubMed]
- Simonneau G, Torbicki A, Dorfmüller P, et al. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017;26:160112. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903-75. [Crossref] [PubMed]
- Fukuda K, Date H, Doi S, et al. Guidelines for the Treatment of Pulmonary Hypertension (JCS 2017/JPCPHS 2017). Circ J 2019;83:842-945. [Crossref] [PubMed]
- Tsugu T, Murata M, Kawakami T, et al. Significance of echocardiographic assessment for right ventricular function after balloon pulmonary angioplasty in patients with chronic thromboembolic induced pulmonary hypertension. Am J Cardiol 2015;115:256-61. [Crossref] [PubMed]
- van Wolferen SA, Marcus JT, Boonstra A, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 2007;28:1250-7. [Crossref] [PubMed]
- Fukui S, Ogo T, Morita Y, et al. Right ventricular reverse remodelling after balloon pulmonary angioplasty. Eur Respir J 2014;43:1394-402. [Crossref] [PubMed]
- Zhang X, Guo D, Wang J, et al. Speckle tracking for predicting outcomes of balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Echocardiography 2020;37:841-9. [Crossref] [PubMed]
- Broch K, Murbraech K, Ragnarsson A, et al. Echocardiographic evidence of right ventricular functional improvement after balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2016;35:80-6. [Crossref] [PubMed]
- Lin JL, Chen HM, Lin FC, et al. Application of DynaCT angiographic reconstruction in balloon pulmonary angioplasty. Eur Radiol 2020;30:6950-7. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-70. [Crossref] [PubMed]
- Feinstein JA, Goldhaber SZ, Lock JE, et al. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001;103:10-3. [Crossref] [PubMed]
- Mizoguchi H, Ogawa A, Munemasa M, et al. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012;5:748-55. [Crossref] [PubMed]
- Kanar BG, Mutlu B, Atas H, et al. Improvements of right ventricular function and hemodynamics after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Echocardiography 2019;36:2050-6. [Crossref] [PubMed]
- Fukui S, Ogo T, Goto Y, et al. Exercise intolerance and ventilatory inefficiency improve early after balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Int J Cardiol 2015;180:66-8. [Crossref] [PubMed]
- Maschke SK, Schoenfeld CO, Kaireit TF, et al. MRI-derived Regional Biventricular Function in Patients with Chronic Thromboembolic Pulmonary Hypertension Before and After Pulmonary Endarterectomy. Acad Radiol 2018;25:1540-7. [Crossref] [PubMed]
- Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019;53:1801915. [Crossref] [PubMed]
- Delcroix M, Lang I, Pepke-Zaba J, et al. Long-Term Outcome of Patients With Chronic Thromboembolic Pulmonary Hypertension: Results From an International Prospective Registry. Circulation 2016;133:859-71. [Crossref] [PubMed]
- Mańczak R, Kurzyna M, Piłka M, et al. Prediction of Prognostic Hemodynamic Indices in Pulmonary Hypertension Using Non-Invasive Parameters. Diagnostics (Basel) 2020;10:644. [Crossref] [PubMed]


