Update on clinical trials in home mechanical ventilation
Introduction
Mechanical ventilation in the home is not a new idea. Over half a century ago, individuals with polio led the challenge of maintaining mechanical ventilation outside of institutions. Limited data are available on the number of patients with chronic respiratory failure treated outside of hospital. A European survey in 2005 reported that almost 22,000 patients with chronic respiratory failure were receiving home mechanical ventilation (HMV) with either non-invasive ventilation (NIV) or invasive ventilation (1). In this study, the estimated prevalence of HMV in Europe was 6.6 per 100,000 people. An accurate count of the number of patients receiving HMV in the US is unknown (2).
Differing drivers have fuelled demand for HMV: rising costs of hospital care, the advent of commercially available non-invasive masks and positive-pressure ventilators, the rise in obesity and the desire of individuals to maintain a quality of life (QOL) at home (3). Questions remain regarding patient benefit in a number of groups and this review describes some of the recent completed and ongoing studies that have sought to shed light on this growing field.
Chronic obstructive pulmonary disease (COPD)
The empirical appeal of treating chronic respiratory failure in patients with COPD and physiological data from small single centre studies (4,5) has led to continued enthusiasm for HMV despite disappointing data from large clinical trials (6,7). The reasons for the failure to translate physiological data from small studies performed in highly specialist centres, to improved clinical outcomes in large multicenter trials, has largely been attribute to study design, rather than failure of the intervention. Further impetus for definitive clinical trials has been provided by increasing data suggesting that high intensity ventilation, targeting CO2 clearance, has beneficial effects over the standard low pressure ventilation (8,9).
There have been two recent high impact, well designed studies, examining the role of HMV in COPD (10,11). These two studies have each examined a different patient population, one stable chronic hypercapnic COPD and the other post-acute exacerbation, and are as such complementary to each other.
COPD—stable hypercapnic
Köhnlein et al. (11) investigated the effect of long-term NIV, targeted to markedly reduce hypercapnia, on survival in patients with clinically stable (4-week run-in period) advanced COPD (Global Initiative for Chronic Obstructive Lung Disease, GOLD stage 4). The study was an open label, multicenter, randomized controlled trial (RCT), in patients with a PaCO2 of ≥7 kPa and pH >7.35, originally powered for 300 patients. NIV was targeted to reduce baseline PaCO2 by at least 20% or to achieve PaCO2 values <6.5 kPa. Over a 7-year period [2004–2011] across 36 respiratory units in Germany and Austria, 195 patients were randomly assigned to NIV (n=102) or control (n=93). The primary outcome was 1-year all-cause mortality. Settings (data available in 83%) included mean inspiratory positive airway pressure (IPAP) 22±5 cmH2O and expiratory positive airway pressure (EPAP) 5±2 cmH2O with adherence (data available in 47%) 5.9±3.1 hours/day.
The trial was terminated early as the mortality reduction in the intervention arm was greater than expected and a change in national guidelines occurred, which recommended NIV in this patient group thus rendering further recruitment unfeasible. One-year mortality was 12% in the intervention vs. 33% in the control group; hazard ratio (HR) 0.24, 95% confidence interval (CI) 0.11–0.49; P=0.0004; number needed to treat to prevent one death: 5. QOL (assessed by the St George’s Respiratory Questionnaire summary score) improved more in the intervention group (6.2 points, 95% CI 0.7–11.8, P=0.0289). Notwithstanding the problems of recruitment to such clinical trials, the protracted time to recruitment and lack of information on numbers screened raises the issue of selection bias (12). Although reported as having a low incidence of admissions over the year in both groups, the 2.2±10.2 admissions per patient in the NIV and 3.1±5.4 admissions per patient in the control group would classify them as frequent exacerbators (13). Interestingly, only three patients in the control arm received NIV during the year (indication was PaCO2 >10 kPa, irrespective of pH). Furthermore, the cohort enrolled into the study had preserved exercise capacity despite their very severe COPD, with a 6-minute walk test (6MWT) of 227±121 m in the NIV group and 250±145 m in the control. This is in stark contrast to those patients enrolled into the RESCUE trial, in whom the incremental shuttle walk test results were not reported, as the patients were so frequently unable to perform it (10). The data from this study suggests that when NIV is applied to effectively reduce PaCO2 in stable COPD patients with a preserved exercise capacity and moderate hypercapnia, a significant survival benefit can be obtained.
COPD—post-acute exacerbation
In acute hospital admissions of COPD complicated by hypercapnic respiratory failure, NIV reduces hospital deaths and complications associated with invasive ventilation and length of hospital stay (14). However, patients who have required an admission with decompensated respiratory failure have a poor prognosis over the following 12 months (15). The RESCUE trial (10), an RCT from the Netherlands, was designed to assess if the addition of HMV improved patient outcomes in this high risk group. The trial primary outcome was respiratory admission-free survival. In total 201 patients were enrolled following an acute exacerbation of COPD, complicated by respiratory acidosis requiring treatment with NIV, to receive domiciliary NIV or standard care. Patients were GOLD stage 3/4, with persistent hypercapnia 48 hours after cessation of acute NIV. Therapy was established across four expert home ventilation centres, using high pressure ventilation: IPAP of 19.2±3.4 cmH2O and EPAP of 4.8±1.0 cmH2O, with a moderate back-up rate of 15±3 breaths per minute.
The intervention reduced mean nocturnal partial pressure of transcutaneous CO2 (tcCO2) in the NIV arm compared to standard treatment [mean difference tcCO2 −0.8 kPa (−0.4 to −1.3); P<0.001]. There was also a treatment effect on daytime pCO2 favouring the NIV arm at 1-year [mean difference pCO2 −0.5 kPa (−0.04 to −0.9); P<0.05]. However, there was also an improvement in daytime pCO2 in the standard treatment arm, and the between-group effect was lost when the pCO2 data were standardised to the condition state in which the measurement was taken, such as the addition of supplementary oxygen, at baseline and 1-year follow-up.
Patient characteristics, ventilator settings and adherence were similar to the study of Köhnlein et al. (11). However, no effect was noted on survival. Mortality at 1-year in both the control and intervention groups was similar to that in the control arm of the study of Köhnlein et al. (11). The reasons for this discordant result may be attributed to spontaneous resolution of respiratory failure that occurs with recovery from an acute exacerbation. As such, recruited patients may not have had significant chronic hypercapnic respiratory failure, that could be expected to be improved with NIV. Furthermore, the adverse influence on survival of the index exacerbation may have been dominant over any effect of NIV (16).
Two recent small single centre RCTs have provided further but conflicting data to the RESCUE study in the post-acute exacerbation setting. The two trials operated different designs with Cheung et al. (17) in Hong Kong, using a sham RCT. The study randomised 47 COPD patients (age 75.9±5.8 years) to receive NIV (n=23) or continuous positive airway pressure (CPAP) (n=24), following acute admissions requiring NIV (having screened 235). Patients were enrolled 48 h after being weaned from NIV and although hypercapnia was not an entry requirement, had moderate hypercapnic respiratory failure (PaCO2 7.7±1.0 kPa in the NIV and 7.3±1.0 kPa in the CPAP groups). The primary end point was respiratory deterioration due to hypercapnic exacerbation, defined as the requirement for NIV in the sham CPAP arm, or escalation of NIV to greater than 12 h/day in the NIV arm. Relatively low ventilator pressures were used with a mean IPAP of 14.8±1.1 cmH2O, EPAP 5 cmH2O but with high levels of adherence to NIV therapy (7–9 h/night). This trial showed a significant benefit of NIV with 38.5% of the intervention group vs. 60.2% of the control group requiring admission and ventilatory support at 1 year [log-rank test, P=0.039; HR = 0.39 (0.16–0.98), P=0.047]. However, it must be noted that the trial did not achieve its planned sample size and did not find a difference in survival, all cause admissions, arterial blood gasses and adverse events, between groups.
Funk et al. (18) utilised a withdrawal open labelled RCT and randomized 26 patients to continuation (n=13) or withdrawal (n=13) of NIV 6 months after an acute hypercapnic exacerbation following which NIV was established. A large number of patients were screened in order to complete the trial (n=998). The primary outcome was respiratory deterioration requiring either re-initiation of NIV, extended NIV use, or invasive ventilation, depending on group allocation. Re-initiation of NIV could be due to deteriorating objective (respiratory failure) or subjective (patient or clinician determined clinical worsening). There was a significant benefit of NIV in terms of the primary outcome, although this was principally due to subjective rather than objective criteria for re-initiation of NIV and as such, subject to potential bias. Furthermore, there was no benefit in terms of all-cause re-admission or exacerbation frequency.
The reason for conflicting results from these two small studies compared to the much larger RESCUE study is largely attributable to study design, with the choice of primary outcome in the work by Funk et al. and Cheung et al. favouring a benefit from NIV. The more applicable primary outcome in the RESCUE study, that of respiratory admission free survival, and its large sample size, makes the data more generalizable. Currently, data therefore does not support the routine use of NIV in patients post-acute exacerbation of COPD. Further data is needed in light of the study by Köhnlein and co-workers to establish if those patients with moderate hypercapnia (PaCO2 >7 kPa), following achieving a degree of clinical stability, will benefit. This group is being examined in a prospective randomised controlled study in the UK that has completed recruitment and is in the follow-up phase (HOT HMV UK; UKCRN 8059).
Synthesis of existing clinical trials suggests domiciliary NIV is unlikely to be beneficial if PaCO2 is <7 kPa during the stable state and patients should therefore be reassessed following an acute exacerbation for the persistence of hypercapnia after a period of recovery. Effective NIV should be confirmed by overnight monitoring of tcCO2 and ventilator settings adjusted, to achieve a greater than 20% reduction in PaCO2 during spontaneous breathing during the initiation phase. The four largest RCTs investigating the effects of HMV in hypercapnic COPD are summarised in Table 1 (6,7,10,11).
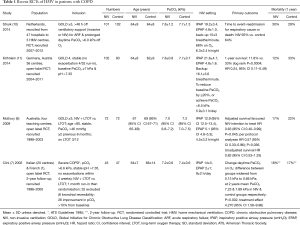
Full table
Novel modes of ventilation
Novel NIV modes have been introduced previously, but have not frequently made the transition to be incorporated into clinical practice (19,20). More recently hybrid pressure support (PS) volume targeted modes have been introduced, with some evidence of enhanced overnight control of ventilation, but at the expense of detriment to sleep quality (21,22). Subsequent trials have failed to demonstrate clinically meaningful differences between these hybrid modes and standard fixed bi-level ventilation when titration methods have been standardized and have suggested no significant impact on objective sleep quality (23,24). Further developments in ventilator technology have incorporated control of back-up rate and automatic titration of EPAP to optimize upper airway patency and maintain minute ventilation.
Intelligent volume-assured pressure support (iVAPS) is a hybrid mode of NIV, providing continual automatic adjustment of PS to achieve a set target volume, with the addition of a monitored back-up rate design to maximise patient triggered breaths. In a randomised non-inferiority trial, Kelly and colleagues (25) investigated iVAPS as an alternative mode to standard PS, in 18 patients with chronic obstructive or restrictive lung disease being established on HMV. iVAPS achieved similar control of sleep disordered breathing, with no significant difference in objective sleep parameters. Interestingly, the iVAPS mode delivered a lower median PS [8 (inter-quartile range 6–10) vs. 10 (inter-quartile range 9–11) cmH2O; P=0.001] with an associated increase in adherence [median 5:40 (4:42–6:49) vs. 4:20 (2:27–6:17) h:min/night; P=0.004]. In addition to enhanced adherence patients had a preference for treatment with the iVAPS mode, however this is confounded by the open label nature of the trial design. Further work with this mode has demonstrated equivalent control of overnight ventilation compared with high intensity NIV but again with improvements in subjective sleep quality (26).
In addition to the breath-by-breath adaptation of PS to achieve target volumes and manipulation of back-up rates to ensure minimum ventilation, a novel NIV mode has been introduced that assesses upper airway patency, in order to ensure overnight titration of EPAP termed average-volume assured pressure support-auto-titrating EPAP (AVAPS-AE). This mode has been evaluated in 10 patients established on domiciliary NIV for COPD-obstructive sleep apnoea (OSA) overlap syndrome in a non-randomized, open label study (27). Similar to other work described above, the novel mode showed clinical equivalence compared to standard fixed level PS in terms of control of sleep disordered breathing and objective sleep quality. As with other novel modes AVAPS-AE was associated with lower mean delivered PS (AVAPS-AE 15±3 cmH2O, fixed level PS 18±7 cmH2O, P=0.155), which translated to improved subjective sleep quality [sleep comfort visual analogue scale mean change 12 mm (95% CI 3–21 mm); P=0.013] and enhanced ventilator adherence (AVAPS-AE 8:27±1:31 h:min vs. fixed level PS 6:21±2:02 h:min; P=0.035). Table 2 summarizes four of these novel mode studies.
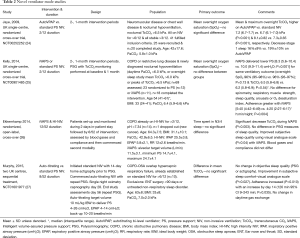
Full table
Pulmonary rehabilitation (PR) & home mechanical ventilation (HMV)
The significant benefits of PR in patients with COPD are well established (28,29), however the role of NIV and PR remains unclear (30). Márquez-Martín et al. (31) compared the combined use of exercise training and NIV, with the two interventions separately. Forty-five patients with severe COPD (GOLD 4) and hypercapnic respiratory failure (PaO2 <60 mmHg, PaCO2 >45 mmHg), clinically stable for three months, were recruited over a 4-year period and randomized into three groups for an intervention of 12 weeks. Forty-three completed the study and 27 were on long-term oxygen therapy (LTOT). Median IPAP was 16 cmH2O and EPAP was 4 cmH2O. Exercise capacity improved in the rehabilitation and the combined group, but not in the ventilation alone group. In the 6MWT, the group receiving both NIV and training had a median improvement of 83 m vs. 40 m in the group receiving ventilation alone and 42 m in the group that underwent training alone. Though the differences were not statistically significant difference, potentially due to sample size, they do concur with two previous studies suggesting an additive effect of NIV and PR (32,33).
Patient-ventilator asynchrony (PVA)
Despite clinical benefits, a significant proportion of patients are unable to adhere to their HMV prescription (34). PVA describes the poor interaction between patient and ventilator. Ramsay and colleagues (35) using parasternal electromyography during HMV set-up, found PVA is frequent and, in contrast to other studies (36,37), not associated with an adverse impact on nocturnal gas exchange. The significance of this high observed level of PVA is unclear, with further data pending on the relationship between ventilator comfort and adherence (http://www.clinicaltrials.gov, NCT01371149).
Obesity hypoventilation syndrome (OHS)
OHS is defined as the presence of obesity [body mass index (BMI) >30 kg/m2] and unexplained arterial hypercapnia, in the presence of sleep disordered breathing, usually OSA (38). There are a wide range of clinical phenotypes that meet the definition of OHS which can be categorized by the type of sleep disordered breathing present (39). Unfortunately, much of the data currently available groups all of these phenotypes together and therefore fail to assess whether response to treatment differs (summary of recent trials provided in Table 3). There are uncontrolled and small RCT data to support the use of CPAP (43), fixed level NIV (41,44) and volume assured NIV (23), in the treatment of OHS, with the only direct comparison on these therapies showing equivalence (40).
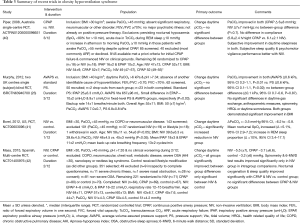
Full table
Masa et al. (42) performed a three-limb multi-centre RCT in which 221 patients with OHS and severe OSA were randomised to lifestyle modification alone, or PAP therapy (CPAP or NIV) combined with lifestyle modification, to assess clinical efficacy, as measured by improvement in daytime hypercapnia following 2 months of treatment. CPAP was titrated using overnight polysomnography and NIV initiated with volume targeting hybrid PS mode, with a moderate backup rate. EPAP was titrated using overnight polysomnography. All treatment arms showed improvement in hypercapnia, with the magnitude of improvement greatest for patients randomized to NIV. The magnitude of improvement in PaCO2 in the NIV arm was significantly better than for lifestyle modification alone. However, NIV was not significantly superior to CPAP. The study was not powered to detect small differences in change in PaCO2 and the patients were only modestly hypercapnic (PaCO2 50–51 mmHg) at baseline. Both PAP arms produced improvements in subjective sleep quality and health related QOL that was not found with lifestyle advice alone. NIV therapy was, in addition, associated with statistically significant improvements in lung function and exercise capacity not found with either CPAP or life style modification [ratio of forced expiratory volume in one second (FEV1) improved 4.8%±13%; P<0.01 and 6MWT increased by 32±58 m; P<0.001]. The study represents the largest RCT performed in OHS and as such greatly informs the management of patients. Whilst the data must only be applied to those patients with significant OSA, as patients with ‘lone OHS’ were excluded, this represents the phenoytype of the majority of patients encountered (45). Furthermore, the suggestion of superiority of NIV in terms of both weight loss and exercise capacity requires more detailed analysis. Previous data using volume targeted NIV in OHS indicated a reduction in weight following treatment associated with increased daytime physical activity (23) and is in contrast to the weight gain associated with CPAP therapy in eucapnic OSA (46). Whilst the data does not yet indicate a clear physiological or clinical superiority, multi-dimensional therapy, targeting weight loss, as well as the control of sleep disordered breathing, are essential (47). This study is the first phase of the Pickwick project, a larger study of 36 months duration that has hospitalization as the primary outcome with the original control group being re-randomized to NIV or CPAP at the end of the initial 3 months phase. The future outcomes of this trial will be greatly anticipated.
Amyotrophic lateral sclerosis (ALS) [motor neuron disease (MND)]
The landmark trial demonstrating benefit of NIV in ALS has established the therapy as gold standard in patients who develop respiratory insufficiency in the absence of significant bulbar involvement (48). Due to the poor tolerance of NIV in some patients, leading to treatment failure, there has been interest in alternative methods of managing respiratory failure in ALS. Diaphragm pacing has shown potential benefit in pilot work, necessitating further investigation (49).
The Diaphragm pacing in ALS (DiPALS) study assessed the potential benefit of diaphragm pacing in patients with ALS. Patients were randomized to standard care (NIV alone) or the addition of diaphragm pacing at the point of clinically significant respiratory insufficiency (50). The trial was powered to recruit 108 patients (randomized 1:1) assuming a 25% absolute survival benefit at 12 months in favour of diaphragm pacing. The trial was terminated early at the recommendation of the independent Data Monitoring and Ethics Committee (DMEC) with 37 patients randomised in each group. At study completion in December 2014 median survival in the pacing group was 11.0 vs. 22.5 months in the NIV only arm; adjusted HR 2.27 (1.22–4.25); P=0.009. Twenty-eight (76%) patients died in the pacing group and 19 (51%) patients died in the NIV alone group, with 162 adverse events (5.9 events per person-year) in the pacing group, of which 46 events were serious, compared with 81 events (2.5 events per person-year) in the NIV alone group, of which 31 events were serious. The pacing group were older (60±10 vs. 54±12) but otherwise baseline differences were similar and minimization was employed to balance for differences in age, sex, forced vital capacity (FVC), and bulbar function. It is not yet clear whether the excess deaths related to the effects of the surgery or the pacing itself, however, the trial illustrated the necessity for good quality evidence prior to the introduction of novel medical devices.
Two further studies, one in the US and one from France investigating application of diaphragm pacing are still pending (ClinicalTrials.gov, numbers NCT01938495 and NCT01583088), though the later study has suspended recruitment and in light of the DiPALS results careful consideration will be needed prior to any future work in this area.
Heart failure (HF)
SERVE-HF an international, multicentre RCT (51) investigated the impact on mortality and morbidity of treating predominantly central sleep apnoea (CSA) in a symptomatic HF population [New York Heart Association classification (NYHA) ≥II] with impaired left ventricular ejection fraction (LVEF ≤45%) with adaptive servo-ventilation (ASV, n=666) or control (n=659). The incidence of the primary end point (time to all-cause mortality or unplanned hospitalization for worsening HF) did not differ significantly between the ASV group and the control group [54.1% and 50.8%, respectively; HR 1.13 (0.97–1.31); P=0.10]. However, all-cause mortality and cardiovascular mortality were significantly higher in the ASV group [HR for death from any cause 1.28 (1.06–1.55); P=0.01; and HR for cardiovascular death 1.34 (1.09–1.65); P=0.006]. The company (Resmed) currently advises that ASV is contraindicated in patients with symptomatic, chronic HF (NYHA ≥II, with LVEF ≤45%) and moderate to severe predominant CSA.
The cause(s) of the increase in cardiovascular mortality in the ASV group is of great interest. It has previously been argued that CSA with Cheyne-Stokes pattern of respiration (CSA-CSR) is a compensatory response to severe HF, and in itself may not be injurious (52). Indeed, potential beneficial effects of CSA-CSR include augmentation of stroke volume, increased lung volume with intrinsic positive end-expiratory pressure (PEEP) and cyclic respiratory muscle rest (52). Abolishment of this adaptation could thus be deleterious. A second explanation is that reduction in stroke volume, that occurs in HF patients with low pulmonary-capillary wedge pressures from application of NIV or CPAP (53-55), has significant consequences in this high risk group. In contrast, deployment of CPAP in patients with acute HF +/− high wedge pressures does not appear to impair cardiac performance (54-56).
ADaptive-servo VENTilation for treatment of OSA and CSA in Heart Failure (ADVENT-HF) (NCT01128816), a multi-centre, multi-national RCT aiming to randomize 860 patients with CSA and symptomatic systolic HF (American Heart Association B–D, LVEF <45%) had recruited 301 patients by March 2015. A DMEC review instigated following the publication of the SERVE-HF data did not recommend trial cessation and recruitment is ongoing.
In Japan a multicentre, open-label blinded-endpoint RCT—Study of the Effects of Adaptive Servo-ventilation Therapy on Cardiac Function and Remodelling in Patients with Chronic HF (SAVIOR-C)—recruited 213 outpatients with mild to severe HF (LVEF <40% and NYHA ≥II) assigned to ASV and medical therapy or medical therapy alone for 24 weeks (57). The primary outcome of LVEF did not differ between the two groups (both groups improved significantly), though NYHA class and Activities of daily living (ADL) improved significantly in the ASV group compared to the control arm.
Interstitial lung disease (ILD)
There have been few studies of ILD and HMV. PR in ILD is safe and improves functional exercise capacity, dyspnoea and QOL (58). Dreher and colleagues investigated the effects of PR in hypercapnic ILD patients (59). Those with hypercapnia received NIV (n=29); the remaining ILD patients served as a comparison group (n=319). PR improved the 6MWT distance achieved by 64.4±67.1 m vs. baseline (P<0.0001) in NIV patients and by 43.2±55.1 m (P<0.0001) in the control group [difference 21.1 (0.5–41.8) m; P=0.045]. PR improved the SF-36 mental component score vs. baseline in both groups. The results must be viewed in the context of the study design that compared hypercapnic ILD patients treated with NIV prior to PR, to eucapnic patients receiving PR alone. However, hypercapnia is acknowledged to be a poor prognostic feature in ILD and thus the significant physiological and clinical improvements would lead to a recommendation for initiating NIV in this group prior to attempting PR in order to maximize benefits.
The elderly
Three retrospective studies of patients commenced on HMV aged ≥75 have previously reported encouraging results; subsequent admissions to hospital were reduced in two studies (60,61) and the other study found no statistically significant differences between those aged ≥75 and the younger age groups in blood gas parameters, adherence and adverse events (62). In support of these findings Comer et al. (63) reported their experience of 256 patients set-up with HMV, including 103 aged ≥75. They found HMV in the elderly group was well tolerated and indeed found gas exchange to be improved compared to the younger groups.
Health care costs
The cost of maintaining ventilator dependent patients in an institutional setting is substantial, with cost benefits as well as patient preference for the home care setting (64). Care must be taken when evaluating HMV patients during acute admission as early tracheostomy and transfer to long term acute care facilities is incentivised in certain health care systems but will lead to high numbers of tracheostomy ventilated patients with the associated high long term morbidity and cost to the health care system (65). Thought should be given to the aggressive management of such patients with 24 h NIV and mechanical insufflation-exsufflation, which has excellent outcomes in maintaining neuromuscular patients during acute exacerbations, without the need for tracheostomy (66).
In addition to long term costs of care, a focus has been made on cost saving by utilizing out-patient setup of NIV in stable patients. This has been driven not just by cost pressures, but also the benefit of maintaining dependent patients within their established care environments. A recent RCT from Hazenberg et al. (67) investigated initial set-up of HMV at home (n=38) or in hospital (n=39), in patients diagnosed with chronic respiratory failure due to a neuromuscular or thoracic cage disease. Primary outcome was PaCO2, while QOL and costs were secondary outcomes. At 6 months there was no significant difference between the two groups in improvements in PaCO2 or QOL. However, cost savings in the home group were €3000 per patient, suggesting equivalent physiological and clinical efficacy, but with enhanced cost-effectiveness. It must be remembered when extrapolating these data that the study was conducted in established ventilation centres, with significant experience in HMV in neuromuscular disease (NMD). Further data is pending on whether such benefits are experienced in other less dependent patients with chronic respiratory failure who are able to be setup in a daycase outpatient setting with the assistance of novel automated ventilator technology (http://www.isrctn.com, 51420481) (68).
Conclusions and future directions
Although many benefits of HMV have been established by either randomized controlled data or consensus opinion for certain disorders, such as NMD and obesity, it remains unclear what the optimum ventilator setup strategy should be, whether polysomnography is required, or if outpatient setup is clinically safe and efficacious. Furthermore, it is unclear what if any importance should be given to patient ventilator asynchrony and novel modes of ventilation, or what measures are most appropriate to assess efficacy of HMV. In other diagnostic groups, such as COPD the data is more equivocal and large-scale studies are still need to delineate the phenotypes of patients who may benefit from HMV.
Acknowledgements
None.
Footnote
Conflicts of Interest: The Clinical Respiratory Physiology Research Centre has received unrestricted research grants from ResMed, Abingdon, Oxfordshire, UK; Philips-Respironics, Murrysville, PA, USA; Fisher & Paykel Healthcare, Auckland, New Zealand and B&D ElectroMedical, Stratford-upon-Avon, Warwickshire, UK. PB Murphy has received hospitality for conferences and lecturing from Philips-Respironics; lecturing from Fisher & Paykel; hospitality for conferences from ResMed.
References
- Lloyd-Owen SJ, Donaldson GC, Ambrosino N, et al. Patterns of home mechanical ventilation use in Europe: results from the Eurovent survey. Eur Respir J 2005;25:1025-31. [PubMed]
- King AC. Long-term home mechanical ventilation in the United States. Respir Care 2012;57:921-30; discussion 930-2. [PubMed]
- McKim DA, Road J, Avendano M, et al. Home mechanical ventilation: a Canadian Thoracic Society clinical practice guideline. Can Respir J 2011;18:197-215. [PubMed]
- Elliott MW, Mulvey DA, Moxham J, et al. Domiciliary nocturnal nasal intermittent positive pressure ventilation in COPD: mechanisms underlying changes in arterial blood gas tensions. Eur Respir J 1991;4:1044-52. [PubMed]
- Díaz O, Bégin P, Andresen M, et al. Physiological and clinical effects of diurnal noninvasive ventilation in hypercapnic COPD. Eur Respir J 2005;26:1016-23. [PubMed]
- McEvoy RD, Pierce RJ, Hillman D, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 2009;64:561-6. [PubMed]
- Clini E, Sturani C, Rossi A, et al. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J 2002;20:529-38. [PubMed]
- Windisch W, Vogel M, Sorichter S, et al. Normocapnia during nIPPV in chronic hypercapnic COPD reduces subsequent spontaneous PaCO2. Respir Med 2002;96:572-9. [PubMed]
- Windisch W, Haenel M, Storre JH, et al. High-intensity non-invasive positive pressure ventilation for stable hypercapnic COPD. Int J Med Sci 2009;6:72-6. [PubMed]
- Struik FM, Sprooten RT, Kerstjens HA, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax 2014;69:826-34. [PubMed]
- Köhnlein T, Windisch W, Köhler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med 2014;2:698-705. [PubMed]
- Elliott M. Domiciliary NIV for COPD: where are we now? Lancet Respir Med 2014;2:672-3. [PubMed]
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128-38. [PubMed]
- Ram FS, Picot J, Lightowler J, et al. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2004.CD004104. [PubMed]
- Murray I, Paterson E, Thain G, et al. Outcomes following non-invasive ventilation for hypercapnic exacerbations of chronic obstructive pulmonary disease. Thorax 2011;66:825-6. [PubMed]
- Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847-52. [PubMed]
- Cheung AP, Chan VL, Liong JT, et al. A pilot trial of non-invasive home ventilation after acidotic respiratory failure in chronic obstructive pulmonary disease. Int J Tuberc Lung Dis 2010;14:642-9. [PubMed]
- Funk GC, Breyer MK, Burghuber OC, et al. Long-term non-invasive ventilation in COPD after acute-on-chronic respiratory failure. Respir Med 2011;105:427-34. [PubMed]
- Hart N, Hunt A, Polkey MI, et al. Comparison of proportional assist ventilation and pressure support ventilation in chronic respiratory failure due to neuromuscular and chest wall deformity. Thorax 2002;57:979-81. [PubMed]
- Piquilloud L, Tassaux D, Bialais E, et al. Neurally adjusted ventilatory assist (NAVA) improves patient-ventilator interaction during non-invasive ventilation delivered by face mask. Intensive Care Med 2012;38:1624-31. [PubMed]
- Storre JH, Seuthe B, Fiechter R, et al. Average volume-assured pressure support in obesity hypoventilation: A randomized crossover trial. Chest 2006;130:815-21. [PubMed]
- Janssens JP, Metzger M, Sforza E. Impact of volume targeting on efficacy of bi-level non-invasive ventilation and sleep in obesity-hypoventilation. Respir Med 2009;103:165-72. [PubMed]
- Murphy PB, Davidson C, Hind MD, et al. Volume targeted versus pressure support non-invasive ventilation in patients with super obesity and chronic respiratory failure: a randomised controlled trial. Thorax 2012;67:727-34. [PubMed]
- Jaye J, Chatwin M, Dayer M, et al. Autotitrating versus standard noninvasive ventilation: a randomised crossover trial. Eur Respir J 2009;33:566-71. [PubMed]
- Kelly JL, Jaye J, Pickersgill RE, et al. Randomized trial of 'intelligent' autotitrating ventilation versus standard pressure support non-invasive ventilation: impact on adherence and physiological outcomes. Respirology 2014;19:596-603. [PubMed]
- Ekkernkamp E, Storre JH, Windisch W, et al. Impact of intelligent volume-assured pressure support on sleep quality in stable hypercapnic chronic obstructive pulmonary disease patients: a randomized, crossover study. Respiration 2014;88:270-6. [PubMed]
- Murphy PB, Arbane G, Ramsay M, et al. Safety and efficacy of auto-titrating noninvasive ventilation in COPD and obstructive sleep apnoea overlap syndrome. Eur Respir J 2015;46:548-51. [PubMed]
- Puhan MA, Gimeno-Santos E, Scharplatz M, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011.CD005305. [PubMed]
- McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015;2:CD003793. [PubMed]
- Menadue C, Piper AJ, van 't Hul AJ, et al. Non-invasive ventilation during exercise training for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014;5:CD007714. [PubMed]
- Márquez-Martín E, Ruiz FO, Ramos PC, et al. Randomized trial of non-invasive ventilation combined with exercise training in patients with chronic hypercapnic failure due to chronic obstructive pulmonary disease. Respir Med 2014;108:1741-51. [PubMed]
- Duiverman ML, Wempe JB, Bladder G, et al. Two-year home-based nocturnal noninvasive ventilation added to rehabilitation in chronic obstructive pulmonary disease patients: a randomized controlled trial. Respir Res 2011;12:112. [PubMed]
- Köhnlein T, Schönheit-Kenn U, Winterkamp S, et al. Noninvasive ventilation in pulmonary rehabilitation of COPD patients. Respir Med 2009;103:1329-36. [PubMed]
- Criner GJ, Brennan K, Travaline JM, et al. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest 1999;116:667-75. [PubMed]
- Ramsay M, Mandal S, Suh ES, et al. Parasternal electromyography to determine the relationship between patient-ventilator asynchrony and nocturnal gas exchange during home mechanical ventilation set-up. Thorax 2015;70:946-52. [PubMed]
- Fanfulla F, Delmastro M, Berardinelli A, et al. Effects of different ventilator settings on sleep and inspiratory effort in patients with neuromuscular disease. Am J Respir Crit Care Med 2005;172:619-24. [PubMed]
- Fanfulla F, Taurino AE, Lupo ND, et al. Effect of sleep on patient/ventilator asynchrony in patients undergoing chronic non-invasive mechanical ventilation. Respir Med 2007;101:1702-7. [PubMed]
- Piper AJ, Grunstein RR. Obesity hypoventilation syndrome: mechanisms and management. Am J Respir Crit Care Med 2011;183:292-8. [PubMed]
- Hart N, Mandal S, Manuel A, et al. Rebuttal: 'Obesity hypoventilation syndrome (OHS): does the current definition need revisiting?'. Thorax 2014;69:955. [PubMed]
- Piper AJ, Wang D, Yee BJ, et al. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax 2008;63:395-401. [PubMed]
- Borel JC, Tamisier R, Gonzalez-Bermejo J, et al. Noninvasive ventilation in mild obesity hypoventilation syndrome: a randomized controlled trial. Chest 2012;141:692-702. [PubMed]
- Masa JF, Corral J, Alonso ML, et al. Efficacy of Different Treatment Alternatives for Obesity Hypoventilation Syndrome. Pickwick Study. Am J Respir Crit Care Med 2015;192:86-95. [PubMed]
- Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis 1983;128:177-81. [PubMed]
- Piper AJ, Sullivan CE. Effects of short-term NIPPV in the treatment of patients with severe obstructive sleep apnea and hypercapnia. Chest 1994;105:434-40. [PubMed]
- Kessler R, Chaouat A, Schinkewitch P, et al. The obesity-hypoventilation syndrome revisited: a prospective study of 34 consecutive cases. Chest 2001;120:369-76. [PubMed]
- Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax 2015;70:258-64. [PubMed]
- Murphy PB, Polkey MI, Hart N. Obesity hypoventilation syndrome: the need for a multifaceted approach to treatment. Chest 2012;142:540-1; author reply 541-2. [PubMed]
- Bourke SC, Tomlinson M, Williams TL, et al. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol 2006;5:140-7. [PubMed]
- Scherer K, Bedlack RS. Diaphragm pacing in amyotrophic lateral sclerosis: a literature review. Muscle Nerve 2012;46:1-8. [PubMed]
- DiPALS Writing Committee; DiPALS Study Group Collaborators. Safety and efficacy of diaphragm pacing in patients with respiratory insufficiency due to amyotrophic lateral sclerosis (DiPALS): a multicentre, open-label, randomised controlled trial. Lancet Neurol 2015;14:883-92. [PubMed]
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N Engl J Med 2015;373:1095-105. [PubMed]
- Naughton MT. Cheyne-Stokes respiration: friend or foe? Thorax 2012;67:357-60. [PubMed]
- Philip-Joët FF, Paganelli FF, Dutau HL, et al. Hemodynamic effects of bilevel nasal positive airway pressure ventilation in patients with heart failure. Respiration 1999;66:136-43. [PubMed]
- Bradley TD, Holloway RM, McLaughlin PR, et al. Cardiac output response to continuous positive airway pressure in congestive heart failure. Am Rev Respir Dis 1992;145:377-82. [PubMed]
- De Hoyos A, Liu PP, Benard DC, et al. Haemodynamic effects of continuous positive airway pressure in humans with normal and impaired left ventricular function. Clin Sci (Lond) 1995;88:173-8. [PubMed]
- Lenique F, Habis M, Lofaso F, et al. Ventilatory and hemodynamic effects of continuous positive airway pressure in left heart failure. Am J Respir Crit Care Med 1997;155:500-5. [PubMed]
- Momomura S, Seino Y, Kihara Y, et al. Adaptive servo-ventilation therapy for patients with chronic heart failure in a confirmatory, multicenter, randomized, controlled study. Circ J 2015;79:981-90. [PubMed]
- Dowman L, Hill CJ, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev 2014;10:CD006322. [PubMed]
- Dreher M, Ekkernkamp E, Schmoor C, et al. Pulmonary rehabilitation and noninvasive ventilation in patients with hypercapnic interstitial lung disease. Respiration 2015;89:208-13. [PubMed]
- Farrero E, Prats E, Manresa F, et al. Outcome of non-invasive domiciliary ventilation in elderly patients. Respir Med 2007;101:1068-73. [PubMed]
- Janssens JP, Cicotti E, Fitting JW, et al. Non-invasive home ventilation in patients over 75 years of age: tolerance, compliance, and impact on quality of life. Respir Med 1998;92:1311-20. [PubMed]
- Crespo A, Muñoz X, Torres F, et al. Noninvasive home mechanical ventilation in elderly patients. Gerontology 2010;56:150-6. [PubMed]
- Comer DM, Oakes A, Mukherjee R. Domiciliary non-invasive ventilation in the elderly. Effective, tolerated and justified. Ulster Med J 2015;84:22-5. [PubMed]
- Bach JR, Intintola P, Alba AS, et al. The ventilator-assisted individual. Cost analysis of institutionalization vs rehabilitation and in-home management. Chest 1992;101:26-30. [PubMed]
- Bach JR, Tran J, Durante S. Cost and physician effort analysis of invasive vs. noninvasive respiratory management of Duchenne muscular dystrophy. Am J Phys Med Rehabil 2015;94:474-82. [PubMed]
- Bach JR, Sinquee DM, Saporito LR, et al. Efficacy of mechanical insufflation-exsufflation in extubating unweanable subjects with restrictive pulmonary disorders. Respir Care 2015;60:477-83. [PubMed]
- Hazenberg A, Kerstjens HA, Prins SC, et al. Initiation of home mechanical ventilation at home: a randomised controlled trial of efficacy, feasibility and costs. Respir Med 2014;108:1387-95. [PubMed]
- Mandal S, Arbane G, Murphy P, et al. Medium-term cost-effectiveness of an automated non-invasive ventilation outpatient set-up versus a standard fixed level non-invasive ventilation inpatient set-up in obese patients with chronic respiratory failure: a protocol description. BMJ Open 2015;5:e007082. [PubMed]


