
Reference equations of the impulse oscillatory in healthy Thai adults
Introduction
Impulse oscillometry (IOS) is a simple, non-invasive method that requires only tidal breathing for measuring both airway resistance and airway reactance (1,2). This technique has been developed by Michaelson et al. (3) since 1975. Previous study suggested that it is more sensitive than spirometry for detecting small airway dysfunction (4). Nowadays, IOS has been used for assessing various respiratory diseases especially for the diagnosis of chronic obstructive pulmonary disease (COPD) (5-11) and assessment of asthma control (12-16). The most common IOS parameters reported are resistance at 5 Hz (R5), resistance at 20 Hz (R20), difference of resistance (R5–R20), resonant frequency (Fres), reactance at 5 Hz (X5), and area under reactance curve between 5 Hz and resonant frequency (AX) (2,17).
Following other lung function tests including spirometry, diffusing lung capacity for carbon monoxide (DLCO), and total lung capacity (TLC), choosing optimal reference values is crucial for the interpretation of IOS results. Previous studies have demonstrated that IOS indices are associated with sex, age, height, and bodyweight (18-22). Previous study also suggested that there are differences in lung volumes between ethnic groups (23). The IOS prediction equations should be separately provided for men and women (24). Moreover, the predictive values of IOS can be different depending on the types of device that is used (25). This may have an impact on the predictive values and normal ranges of IOS parameters. The lack of appropriate reference equations and normal ranges of IOS have hindered the application of IOS in clinical practice. The previous study suggested that ethnicity is significantly under-reported and this may limit the applicability of current equations to heterogeneous patient populations (24). Establishing predictive equations for the IOS in the Thai adult population is still required. Therefore, this study aimed to establish reference equations for the IOS in the Thai adult population. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1989/rc).
Methods
Study procedures
This retrospective cross-sectional study of IOS parameters in healthy Thai adults aged greater than 20 years old in the previous studies were included (11,16). The data of healthy controls in the Coronavirus Disease 2019 (COVID-19) study and wildland firefighter study were also included. All subjects were recruited from the same area in Chiang Mai, Thailand. This study was conducted at the Lung health center, Division of Pulmonary, Critical Care, and Allergy, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Research Ethics Committee, Faculty of Medicine, Chiang Mai University (study code: MED-2564-08533, date of approval: 12 November 2021) and filed under Clinical Trials Registry (study ID: TCTR20211014002, date of approval: 14 October 2021). Individual consent for this retrospective analysis was waived.
Subjects
One hundred and fifty-four subjects were screened for eligibility for enrolment in the study. Subjects unable to perform spirometry and IOS were excluded. IOS or spirometry not met standard recommended by King et al. (26) according to the European Respiratory Society (ERS) standard and ERS/American Thoracic Society (ATS) 2019 standard (27), respectively were also excluded. Subjects had a history of cigarette smoking greater than 5 pack-years, had a history of asthma, other chronic lung disease or current acute respiratory tract infection, and obesity [body mass index (BMI) >30 kg/m2] were also excluded. The healthy control subjects were subjects with normal spirometry [forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and the ratio of FEV1/FVC greater than the statistically defined fifth percentile of normal (lower limit of normal; LLN)] (27), and who had no known other chronic systemic diseases (e.g., cardiovascular diseases, kidney diseases, liver diseases, neurological diseases, musculoskeletal diseases, autoimmune diseases, and malignancy). Baseline characteristics including age, height, bodyweight, BMI, underlying disease, and history of smoking were recorded. Pulmonary function test data including IOS and spirometry results were also recorded.
IOS
Pre-bronchodilator (BD) IOS was performed in all subjects before spirometry for ensuring that there was no effect of forced expiration on the respiratory tract smooth muscle (28). The IOS was measured using IOS (Master Screen IOS, Viasys GmbH, Hoechberg, Germany). The parameters in IOS that represent the resistance of the airways include the R5, R20, and R5–R20 values. The parameters in IOS that represent the reactance of the airways include the X5, Fres, and AX. All subjects were asked to perform tidal breathing for 30–45 seconds via a mouthpiece that was connected to a loudspeaker which generates pressure oscillations composed of multiple frequencies. Subjects were asked to sit on a chair in an upright position, wore a nose clip, and were directed to firmly support their cheeks with both hands. A minimum of three trials was performed following the standard recommended by ERS standard (26). The average values from three IOS measurements were recorded. The coherence of each measurement was ≥0.8 at 5 Hz and ≥0.9 at 20 Hz. Unacceptable data were excluded from the analysis.
Spirometry
Spirometry was assessed in all subjects using a spirometer (Vmax Encore 22, Care Fusion, Hoechberg, Germany). Pre-BD spirometry was performed according to the standards of ATS/ERS 2019 (27). Spirometry parameters including FVC, FEV1, ratio of FEV1/FVC, and forced expiratory flow at 25–75% of FVC (FEF25–75%) were recorded. The predicted values and z-score of FVC, FEV1, ratio of FEV1/FVC, and FEF25–75% were calculated from the Global Lung Initiative (GLI) 2012 (Southeast Asian sub-group) reference equations (29).
Study size calculation
The study size of the study was calculated based on data from the previous study (19) using G*Power Version 3.1.9.2 which included two factors and R2 of R5–R20 was 0.2249. Therefore, at least 74 subjects (37 for each gender) needed to be included in this study (power =0.8 with statistical significance <0.05).
Statistical analysis
Results for continuous data were expressed as mean ± standard deviation (SD) or median, interquartile range (IQR) as appropriate. Results for categorical data were expressed as frequencies and percentages. Independent sample t-tests and the Mann-Whitney U test were used to compare differences between the sex groups for parametric and non-parametric data, respectively. Fisher’s exact test was used to compare the categorical data between groups. Reference equations were calculated separately for men and women using multivariable linear regression analysis. Scatter plots were drawn to observe the linear relationship between IOS indices and predictor variables [Figure S1 (male), Figure S2 (female)]. Normal P-P plots and residual plots were drawn to examine the normality and equal variance of the residuals. As R5–R20 and AX were non-normal distribution, thus R5–R20 and AX were calculated as log10 transformation (logR5–R20 and logAX) in the equations. Predictor variables including age, height, and bodyweight were selected using the stepwise method, in which predictors would enter the model if the P value <0.10. The fitness of the model was assessed by the coefficient of determination (R2). The upper limit of normal (ULN) and LLN of IOS parameters was calculated as followed = predictive value + 1.645 × root mean square error (RMSE) and predictive value − 1.645 × RMSE, respectively. All statistical analyses were performed using STATA version 16 (StataCorp, College Station, TX, USA).
Results
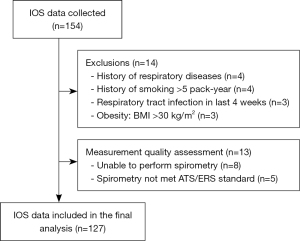
A total of 154 subjects were recruited. However, fourteen subjects were excluded due to various reasons including having a history of chronic respiratory diseases (n=4), having a history of smoking >5 pack-years (n=4), having respiratory tract infection in the last four weeks (n=3), and obesity (n=3). Eight subjects were unable to perform spirometry. Therefore, they were excluded. After the measurement of the quality of spirometry and IOS, five subjects were excluded due to the spirometry not meeting ATS/ERS 2019 standard. More data are shown in Figure 1. The distribution of age, height, and bodyweight according to sex is shown in Figure 2.

The baseline characteristics of the study population are presented in the Table 1. The mean age of men and women was 46.7±18.2 and 52.6±12.9 years, respectively. All of the females were non-smokers and 72.4% of male subjects were non-smokers. More data are shown in Table 1.
Table 1
| Clinical characteristics | Men (n=87) | Women (n=40) |
|---|---|---|
| Age, years (range) | 46.7±18.2 (22–92) | 52.6±12.9 (25–74) |
| Height, cm (range) | 165.6±7.6 (148–184) | 152.9±4.1 (145–162) |
| Bodyweight, kg (range) | 68.4±11.7 (43.0–99.0) | 53.5±7.5 (39.0–70.0) |
| BMI, kg/m2 (range) | 24.8±2.9 (19.1–30.0) | 22.9±2.9 (17.1–29.5) |
| Smoking status | ||
| Non-smoker | 63 (72.4) | 40 (100.0) |
| Current-smoker | 4 (4.6) | 0 (0.0) |
| Ex-smoker | 20 (23.0) | 0 (0.0) |
| Smoking pack-year | 1.3±1.8 | 0.0±0.0 |
Data are mean ± SD unless otherwise stated. BMI, body mass index; SD, standard deviation.
The spirometric and IOS data of subjects are shown in Table 2. There was no significant difference between the percent predicted and z-score of FVC, FEV1, the ratio of FEV1/FVC, and FEF25–75% between men and women. The R5, R20, and AX were significantly higher in women compared to men. The R5–R20 tends to be higher in women compared to men but not a significant difference. The X5 was more negative in women compared to men but not a significant difference. However, there was no significant difference between the Fres between men and women. More data are shown in Table 2.
Table 2
| Parameters | Men (n=87) | Women (n=40) | P value |
|---|---|---|---|
| Spirometry | |||
| % predicted FVC | 100.0±14.1 | 102.6±10.1 | 0.299 |
| z-score of FVC | 0.107 (−0.595, 0.805) | 0.148 (−0.251, 0.639) | 0.575 |
| % predicted FEV1 | 99.0±12.8 | 101.7±12.4 | 0.264 |
| z-score of FEV1 | 0.081 (−0.716, 0.616) | 0.142 (−0.517, 0.634) | 0.400 |
| FEV1/FVC, % | 82.3±5.8 | 82.9±5.0 | 0.589 |
| z-score of FEV1/FVC | −0.208 (−0.684, 0.370) | −0.094 (−0.553, 0.372) | 0.684 |
| % predicted FEF25–75% | 103.0±24.1 | 108.9±32.7 | 0.264 |
| z-score of FEF25–75% | 0.065 (−0.609, 0.568) | 0.335 (−0.827, 0.842) | 0.447 |
| IOS | |||
| R5, cmH2O/L/s | 3.29±0.99 | 4.17±1.11 | <0.001 |
| R20, cmH2O/L/s | 2.79±0.79 | 3.57±0.87 | <0.001 |
| R5–R20, cmH2O/L/s, median (IQR) | 0.38 (0.20, 0.71) | 0.52 (0.33, 0.78) | 0.108 |
| X5, cmH2O/L/s | −0.90±0.44 | −1.08±0.57 | 0.061 |
| Fres, Hz | 12.38±3.71 | 12.37±3.82 | 0.997 |
| AX, cmH2O/L, median (IQR) | 2.15 (1.30, 4.08) | 3.98 (2.03, 5.41) | 0.024 |
Data are mean ± SD unless otherwise stated. IOS, impulse oscillometry; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; FEF25–75%, forced expiratory flow at 25–75% of FVC; R5, resistance at 5 Hz; R20, resistance at 20 Hz; R5–R20, heterogeneity of resistance between R5 and R20; IQR, interquartile range; X5, reactance at 5 Hz; Fres, resonant frequency; AX, the area under reactance curve between 5 Hz and resonant frequency; SD, standard deviation.
The spirometric and IOS data of smokers and non-smokers in male subjects are shown in Table 3. The percent predicted and z-score of FVC, FEV1, the ratio of FEV1/FVC, and FEF25–75% between smokers and non-smokers were not significantly different. All IOS indices were also comparable between smokers and non-smokers. More data are shown in Table 3.
Table 3
| Parameters | Smokers (n=24) | Non-smokers (n=63) | P value |
|---|---|---|---|
| Spirometry | |||
| % predicted FVC | 96.1±11.3 | 101.6±14.8 | 0.104 |
| z-score of FVC | −0.020 (−0.883, 0.259) | 0.135 (−0.521, 0.884) | 0.171 |
| % predicted FEV1 | 97.4±10.0 | 99.6±13.7 | 0.476 |
| z-score of FEV1 | −0.095 (0.779, 0.486) | 0.081 (−0.716, 0.748) | 0.314 |
| FEV1/FVC, % | 83.4±5.1 | 81.9±6.0 | 0.280 |
| z-score of FEV1/FVC | 0.030 (−0.612, 0.694) | −0.208 (−0.708, 0.175) | 0.301 |
| % predicted FEF25–75% | 105.2±25.4 | 102.2±23.7 | 0.616 |
| z-score of FEF25–75% | −0.109 (−0.565, 0.672) | 0.088 (−0.649, 0.559) | 0.613 |
| IOS | |||
| R5, cmH2O/L/s | 3.47±1.04 | 3.22±0.97 | 0.287 |
| R20, cmH2O/L/s | 2.85±0.92 | 2.76±0.75 | 0.624 |
| R5–R20, cmH2O/L/s, median (IQR) | 0.43 (0.30, 0.89) | 0.34 (0.17, 0.70) | 0.145 |
| X5, cmH2O/L/s | −0.99±0.48 | −0.87±0.42 | 0.259 |
| Fres, Hz | 12.74±3.58 | 12.23±3.77 | 0.575 |
| AX, cmH2O/L, median (IQR) | 2.15 (1.41, 6.16) | 2.12 (1.22, 3.87) | 0.314 |
Data are mean ± SD unless otherwise stated. IOS, impulse oscillometry; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; FEF25–75%, forced expiratory flow at 25–75% of FVC; R5, resistance at 5 Hz; R20, resistance at 20 Hz; R5–R20, heterogeneity of resistance between R5 and R20; X5, reactance at 5 Hz; Fres, resonant frequency; AX, the area under reactance curve between 5 Hz and resonant frequency; SD, standard deviation.
The reference equations for the IOS parameters for men and women are presented in Table 4. Age, height, and bodyweight were shown to be the influential predictor as they contributed to the most of IOS indices except for the R5–R20 in men equations. Bodyweight was shown to be the influential predictor as it contributed to the most of IOS indices except for the X5 in women’s equations. Age was shown to be the influential predictor for IOS indices in men but not for women except for the Fres equation. More data are shown in Table 4.
Table 4
| Parameters | Equations | RMSE | Adjusted R2 |
|---|---|---|---|
| Men | |||
| R5, cmH2O/L/s | 18.7428 − 0.0163 (age) − 0.1106 (height) + 0.0531 (weight) | 0.8194 | 0.3431 |
| R20, cmH2O/L/s | 13.6060 − 0.0169 (age) − 0.0743 (height) + 0.0332 (weight) | 0.6818 | 0.2933 |
| logR5–R20, cmH2O/L/s | 1.0414 − 0.0087 (height) | 0.4131 | 0.0249 |
| X5, cmH2O/L/s | −6.5532 + 0.0045 (age) + 0.0349 (height) − 0.0051 (weight) | 0.3940 | 0.2182 |
| Fres, Hz | 49.1797 + 0.0296 (age) − 0.2560 (height) + 0.0615 (weight) | 3.3240 | 0.2233 |
| logAX, cmH2O/L | 4.3201 − 0.0255 (height) + 0.0039 (weight) | 0.3413 | 0.1676 |
| Women | |||
| R5, cmH2O/L/s | 12.6580 − 0.0838 (height) + 0.0809 (weight) | 0.9868 | 0.2489 |
| R20, cmH2O/L/s | 15.7253 − 0.0977 (height) + 0.0519 (weight) | 0.7834 | 0.2333 |
| logR5–R20, cmH2O/L/s | −4.2198 + 0.0052 (age) + 0.0166 (height) + 0.0202 (weight) | 0.3170 | 0.2046 |
| X5, cmH2O/L/s | −6.1651 + 0.0333 (height) | 0.5648 | 0.0560 |
| Fres, Hz | −4.2714 + 0.1079 (age) + 0.2048 (weight) | 3.4200 | 0.2415 |
| logAX, cmH2O/L | −0.7026 + 0.0064 (age) + 0.0162 (weight) | 0.3573 | 0.1296 |
Models were built using multivariable linear regression analysis taking age (in years), weight (in kg), and height (in centimeters) as main explanatory variables. IOS, impulse oscillometry; RMSE, root mean square error; R5, resistance at 5 Hz; R20, resistance at 20 Hz; R5–R20, heterogeneity of resistance between R5 and R20; X5, reactance at 5 Hz; Fres, resonant frequency; AX, the area under reactance curve between 5 Hz and resonant frequency.
Discussion
Our study provided the reference equations for the IOS parameters in Thai healthy adults for both men and women. IOS indices including R5, R20, and AX were significantly higher in women compared to men. Compared to the previously published IOS predictive values in the Chinese and Australian populations that used the same IOS device (19,20), we found that the R5 and R20 in Thai adults were higher. We also found that the X5 was more negative in Thai adults compared to the previous findings (19,20).
Sex-specific IOS reference equations of the commonly used IOS indices are created for Thai adults. Our results showed that age, height, and bodyweight were shown to be the influential predictors as they contributed to the most of IOS indices in men equations which were supported by the previous studies indicating that anthropometric parameters including height and weight are influential predictors for contributing to the IOS reference equation in men (21,22). For women, our study showed that height and bodyweight were shown to be the influential predictors for the contribution of the IOS predictive value which was supported by the previous studies indicating that height and weight were predictors that affect the IOS reference equation in women (21,22,30). We found a negative correlation between height and airway resistance indices including R5 and R20 which were supported by the previous studies (18,19,21,22,30). The systematic review suggested that the relation between airway resistance and height may be due to the relation between height and lung volume (24). We also found a positive correlation between bodyweight and airway resistance and reactance indices which were supported by the previous studies (19,21,22,30). The previous finding also suggested that the increase in airway resistance and decrease in X5 with increasing weight supported that weight plays a role in the determination of impedance (24). Pellegrino et al. (31) hypothesized that these findings may reflect obesity-related pulmonary inhomogeneity, possibly as a consequence of mechanical airway compression and microatelectasis.
Our study showed a significant increase in airway resistance including R5 and R20 in men compared to women which were comparable to those of previous studies, which showed that the R5 and R20 were significantly higher in men (20,30). Previous studies postulated that higher airway resistance in women in comparison to men likely results from smaller average lung volumes or possibly smaller airway diameters in women (32,33). We also found a higher airway reactance (more negative X5) in females compared to males which was similar to the previous studies (20,21). Previous studies explained that one plausible explanation for this finding could be a higher BMI in women compared to men (21). However, the BMI of women in our study was lower compared to men. Therefore, the more negative X5 in females may be due to the shorter height which was suggested in the previous findings (19).
A previous study suggested that ethnicity is significantly under-reported and this may limit the applicability of current equations to heterogeneous patient populations (24). Despite the increasing use of IOS in clinical practice, population-specific reference equations are lacking (21). Some IOS indices in our Thai prediction value were higher when compared with the Chinese and Australian predictive values (19,20). For example, the R5 were higher about 22.5–32.1% and 11.9–15.4% for men and women, respectively when compared to previous findings (19,20). The R20 were also higher about 11.9–15.4% and 19.5–20.6% for men and women, respectively when compared to the Chinese and Australian predictive values (19,20). Moreover, the X5 in our prediction value were more negative when compared with the Chinese and Australian predictive values (ranging from −6.2% to −6.9% and −5.6% to −14.1% for men and women, respectively) (19,20). The differences in airway resistance and reactance between our study and the previous studies may be due to the average height of our study were shorter compared to the previous studies (165.6 and 152.9 cm vs. 170.0 to 175.9 cm and 159.0 to 164.6 cm for men and women, respectively (19,20). Moreover, the differences in airway impedance between our study and the previous one may be due to the average BMI of our study were lower compared to the previous study in Australian population (24.8 and 22.9 kg/m2vs. 27.2 and 28.5 kg/m2 for men and women, respectively (20). Additionally, the diversity of ethnic origins may have resulted in differences in airway resistance and reactance (24). The utility of IOS is fast gaining acceptability for accurate clinical diagnosis, and its use has increased significantly among Thai clinicians in recent years because of the ease to perform compared to spirometry. Thus, developing IOS reference equations for the Thai population is important. To the best of our knowledge, our study is the first study to present the IOS reference equation in the Thai adult population aged 22–92 years.
Strength and limitation of this study
The strength of our study is its value as the first study that provides the IOS reference equation in the Thai adult population. However, this study has some limitations. Firstly, there was no data from childhood and adolescent subjects aged less than 22 years due to limitations of available data. Thus, the Thai IOS reference equation from childhood and adolescence should be presented in future studies. Secondly, this is a single-center study that small samples in Northern Thailand were included. Thus, the multicenter study that included more sample size in all parts of Thailand is needed to confirm these findings. However, we had calculated sample size and could recruit subjects more than calculated one. Thirdly, the data about air pollutants and biofuel exposure were not mentioned in our study. This could have an impact on IOS parameters. However, annual report of the particulate matter with a diameter of 2.5 micrometres or less (PM2.5) in our city was 30.6±4.2 microgram/m3 (ranged from 24.0 to 36.0 microgram/m3) over the past decade (34) whereas the gaseous pollutants were within acceptable level by the World Health Organization (WHO). Thus, the detail about air pollutants and biofuel exposure should be focused in future studies. Fourthly, 24 (27.6%) of male subjects with smoking history of was 1.3±1.8 pack-year were included in this study. However, all included subjects were classified as non-smoker according to the U.S. Centers for Disease Control which defines a never-smoker as someone who has smoked <100 cigarettes or 5 pack-year per lifetime (35). Although, the smoking history of these light smokers was classified as non-smokers, IOS possibly might detect very early airway changes in them. Therefore, only absolute non-smokers should be recruited in future studies.
Conclusions
Our study provided the reference equations for the IOS parameters in Thai adults for both men and women. IOS indices including R5, R20, and AX were significantly higher in women compared to men.
Acknowledgments
The authors would like to thank all subjects who participated in this study. The authors also wish to acknowledge the contribution of the staff of the Division of Pulmonary, Critical Care and Allergy, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University to this trial. The authors would like to thank Ruth Leatherman, Research Administration Section, Faculty of Medicine, Chiang Mai University for native English proofreading.
Funding: This study was funded by the Faculty of Medicine, Chiang Mai University Research Fund under grant No. 037/2563.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1989/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1989/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1989/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Research Ethics Committee, Faculty of Medicine, Chiang Mai University (study code: MED-2564-08533, date of approval: 12 November 2021) and filed under Clinical Trials Registry (study ID: TCTR20211014002, date of approval: 14 October 2021). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oostveen E, MacLeod D, Lorino H, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J 2003;22:1026-41. [Crossref] [PubMed]
- Bickel S, Popler J, Lesnick B, et al. Impulse oscillometry: interpretation and practical applications. Chest 2014;146:841-7. [Crossref] [PubMed]
- Michaelson ED, Grassman ED, Peters WR. Pulmonary mechanics by spectral analysis of forced random noise. J Clin Invest 1975;56:1210-30. [Crossref] [PubMed]
- Saadeh C, Saadeh C, Cross B, et al. Advantage of impulse oscillometry over spirometry to diagnose chronic obstructive pulmonary disease and monitor pulmonary responses to bronchodilators: An observational study. SAGE Open Med 2015;3:2050312115578957. [Crossref] [PubMed]
- Li Y, Chen Y, Wang P. Application of impulse oscillometry and bronchial dilation test for analysis in patients with asthma and chronic obstructive pulmonary disease. Int J Clin Exp Med 2015;8:1271-5. [PubMed]
- Gong SG, Yang WL, Liu JM, et al. Change in pulmonary function in chronic obstructive pulmonary disease stage 0 patients. Int J Clin Exp Med 2015;8:21400-6. [PubMed]
- Liu Z, Lin L, Liu X. Clinical application value of impulse oscillometry in geriatric patients with COPD. Int J Chron Obstruct Pulmon Dis 2017;12:897-905. [Crossref] [PubMed]
- Aarli BB, Calverley PM, Jensen RL, et al. Variability of within-breath reactance in COPD patients and its association with dyspnoea. Eur Respir J 2015;45:625-34. [Crossref] [PubMed]
- Al-Mutairi SS, Sharma PN, Al-Alawi A, et al. Impulse oscillometry: an alternative modality to the conventional pulmonary function test to categorise obstructive pulmonary disorders. Clin Exp Med 2007;7:56-64. [Crossref] [PubMed]
- Wei X, Shi Z, Cui Y, et al. Impulse oscillometry system as an alternative diagnostic method for chronic obstructive pulmonary disease. Medicine (Baltimore) 2017;96:e8543. [Crossref] [PubMed]
- Chaiwong W, Namwongprom S, Liwsrisakun C, et al. Diagnostic Ability of Impulse Oscillometry in Diagnosis of Chronic Obstructive Pulmonary Disease. COPD 2020;17:635-46. [Crossref] [PubMed]
- Schulze J, Biedebach S, Christmann M, et al. Impulse Oscillometry as a Predictor of Asthma Exacerbations in Young Children. Respiration 2016;91:107-14. [Crossref] [PubMed]
- Shi Y, Aledia AS, Galant SP, et al. Peripheral airway impairment measured by oscillometry predicts loss of asthma control in children. J Allergy Clin Immunol 2013;131:718-23. [Crossref] [PubMed]
- Shi Y, Aledia AS, Tatavoosian AV, et al. Relating small airways to asthma control by using impulse oscillometry in children. J Allergy Clin Immunol 2012;129:671-8. [Crossref] [PubMed]
- Takeda T, Oga T, Niimi A, et al. Relationship between small airway function and health status, dyspnea and disease control in asthma. Respiration 2010;80:120-6. [Crossref] [PubMed]
- Chaiwong W, Namwongprom S, Liwsrisakun C, et al. The roles of impulse oscillometry in detection of poorly controlled asthma in adults with normal spirometry. J Asthma 2022;59:561-71. [Crossref] [PubMed]
- Brashier B, Salvi S. Measuring lung function using sound waves: role of the forced oscillation technique and impulse oscillometry system. Breathe (Sheff) 2015;11:57-65. [Crossref] [PubMed]
- Shiota S, Katoh M, Fujii M, et al. Predictive equations and the reliability of the impulse oscillatory system in Japanese adult subjects. Respirology 2005;10:310-5. [Crossref] [PubMed]
- Liang XL, Gao Y, Guan WJ, et al. Reference values of respiratory impedance with impulse oscillometry in healthy Chinese adults. J Thorac Dis 2021;13:3680-91. [Crossref] [PubMed]
- Newbury W, Crockett A, Newbury J. A pilot study to evaluate Australian predictive equations for the impulse oscillometry system. Respirology 2008;13:1070-5. [Crossref] [PubMed]
- Moitra S, Moitra S, Ghosh AK, et al. Reference values of impulse oscillometry (IOS) for healthy Indian adults. Int J Tuberc Lung Dis 2020;24:536-9. [Crossref] [PubMed]
- Schulz H, Flexeder C, Behr J, et al. Reference values of impulse oscillometric lung function indices in adults of advanced age. PLoS One 2013;8:e63366. [Crossref] [PubMed]
- Clausen JL. Prediction of normal values in pulmonary function testing. Clin Chest Med 1989;10:135-43. [Crossref] [PubMed]
- Kalchiem-Dekel O, Hines SE. Forty years of reference values for respiratory system impedance in adults: 1977-2017. Respir Med 2018;136:37-47. [Crossref] [PubMed]
- Porojan-Suppini N, Fira-Mladinescu O, Marc M, et al. Lung Function Assessment by Impulse Oscillometry in Adults. Ther Clin Risk Manag 2020;16:1139-50. [Crossref] [PubMed]
- King GG, Bates J, Berger KI, et al. Technical standards for respiratory oscillometry. Eur Respir J 2020;55:1900753. [Crossref] [PubMed]
- Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019;200:e70-88. [Crossref] [PubMed]
- Vink GR, Arets HG, van der Laag J, et al. Impulse oscillometry: a measure for airway obstruction. Pediatr Pulmonol 2003;35:214-9. [Crossref] [PubMed]
- Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324-43. [Crossref] [PubMed]
- Aarli BB, Eagan TM, Ellingsen I, et al. Reference values for within-breath pulmonary impedance parameters in asymptomatic elderly. Clin Respir J 2013;7:245-52. [Crossref] [PubMed]
- Pellegrino R, Gobbi A, Antonelli A, et al. Ventilation heterogeneity in obesity. J Appl Physiol (1985) 2014;116:1175-81. [Crossref] [PubMed]
- Obol BJ. Tests of ventilatory function not requiring maximal subject effort. II. The measurement of total respiratory impedance. Am Rev Respir Dis 1968;97:868-79. [PubMed]
- Martin TR, Castile RG, Fredberg JJ, et al. Airway size is related to sex but not lung size in normal adults. J Appl Physiol (1985) 1987;63:2042-7. [Crossref] [PubMed]
- Pollution Control Department, Thailand. Reports on Smog Situation in the North Home page (in Thai). Available online: http://www.pcd.go.th
- Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults--United States, 2000. MMWR Morb Mortal Wkly Rep 2002;51:642-5. [PubMed]


