National guideline concordance and outcomes for pathologic N2 disease in non-small cell lung cancer
Introduction
The optimal treatment of patients with non-small cell lung cancer (NSCLC) with pathologic mediastinal lymph node involvement is a rapidly evolving field. While the sequence of therapy and modalities utilized continue to be questioned, for patients who are otherwise fit for surgery and are without N3 disease, some combination of surgical therapy with systemic therapy remains the mainstay of care (1-3). While the National Comprehensive Cancer Network (NCCN) guidelines recommend against upfront surgical resection for these patients (4) (with the specific recommendation being for induction chemotherapy plus or minus radiation therapy in potential surgical candidates), no randomized trials have been performed specifically comparing the sequence of therapy for patients with N2 disease.
Several studies utilizing datasets from the early 2000s have suggested that outcomes are similar for neoadjuvant and adjuvant chemotherapy in patients who underwent complete resection (5-8). If such a belief is increasingly held among thoracic surgeons, it may, at least in part, explain low concordance with guidelines for invasive mediastinal staging. As the primary rationale for invasive mediastinal staging is to identify patients with “occult N2” disease so that they can be treated with induction therapy, if there is no difference between neoadjuvant and adjuvant therapy, the utility of invasive staging is lower. A recent paper from the Society of Thoracic Surgeons General Thoracic Database showed overall low use of invasive mediastinal staging, with only 43% of patients with clinical stage IB or higher undergoing invasive staging (9). This may be particularly relevant for patients with a clinically negative mediastinum.
To help shed further light on these important issues, we utilized the National Cancer Database (NCDB) to better understand the association between both clinical nodal stage and timing of therapy on short and long-term outcomes in patients with pathologic N2 NSCLC who underwent anatomic surgical resection. We hypothesized that patients who were occult N2 (cN0/cN1, pN2) would have better outcomes than patients with clinically evident N2 disease. We also hypothesized that there would not be significant clinical differences between cN2 patients who underwent neoadjuvant chemotherapy, compared with those who underwent adjuvant treatment. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1845/rc).
Methods
Data source
The NCDB a joint project of the American Cancer Society and the American College of Surgeon’s Commission on Cancer (CoC), is a nationwide facility-based oncology dataset that currently captures 70% of all newly diagnosed cancers in the United States annually reported from approximately 1,500 hospitals with CoC-accredited cancer programs. The NCDB only includes patients treated at facilities that are continuously accredited by the CoC. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Patient selection
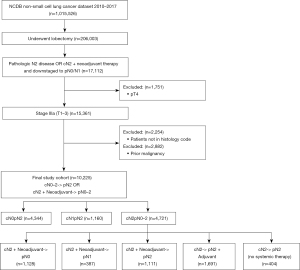
Utilizing the most recent data available from the NCDB, the 2017 participant user file was queried for patients with histologically confirmed N2 disease and an overall stage of IIIA. Per the American Joint Committee on Cancer 7th edition (10), this included T1, T2, and T3 disease. Patients who were clinically staged as IIIA with cN2 disease, received neoadjuvant therapy, and then were downstaged to pN0 or pN1 were also included. Only histology consistent with Adenocarcinoma, Squamous Cell Carcinoma, or Adenosquamous Carcinoma were included based on ICD-0-3 codes (11). Data was analyzed from 2010 onward, as that was when the NCDB started maintaining the most granular information regarding surgical approach. Only patients who underwent lobectomy were evaluated. Patients who had a previous primary lung malignancy, were pT4, or who received non-standard of care treatment including neoadjuvant or adjuvant radiation without chemotherapy were excluded. The patient selection flow chart can be seen in Figure 1.
Variables studied
The NCDB provides patient demographic, clinical, and treatment variables. Trends of treatment guideline concordance based on recommendations from the NCCN were evaluated during this time period (4). The resulting cohort of patients were first categorized by clinical nodal stage with a comparison of demographics and tumor characteristics. A sub-analysis was then performed of only clinical N2 patients separated by utilization and timing of systemic therapy. The primary outcomes were overall survival and guideline concordance. Secondary outcomes included 30-day readmission and 30- and 90-day mortality. This cohort of clinical N2 patients was then further divided based on timing of systemic therapy and nodal response, with overall survival as the primary outcome evaluated.
Statistical analysis
Continuous variables were compared using Student’s t tests, and categorical variables were compared using χ2 and ANOVA tests. Multiple comparisons were made using the Tukey-Kramer method test for ANOVA and log-rank tests, while the Bonferroni correction was used for chi-square tests. Kaplan-Meier methods with log-rank tests were used to analyze overall survival in our cohort. Multivariable survival analysis was performed using Cox Proportional Hazards regression. Statistical analyses were performed using SAS 9.4 statistical software (SAS Institute, Cary, NC, USA). All tests were two-sided, and a P value less than 0.05 was considered statistically significant.
Results
A total of 10,225 Stage IIIA NSCLC patients with pathologic N2 disease and ultimately received surgical lobectomy were included in this study. This included both pT1–3 pN2 patients, and cT1–3 cN2 who received neoadjuvant therapy and were downstaged to pN0 or pN1. Patient demographics and tumor characteristics for each clinical nodal stage cohort are presented in Table 1. Patients with cN2 disease were more likely to have private insurance (cN0 33%, cN1 34%, cN2 41%, P<0.0001) and be treated at an academic center (cN0 34%, cN1 36%, cN2 42%), P<0.0001). Clinical T-Stage correlated with N-Stage, with cN2 having the highest rates of T3–T4 tumors (20% for cN2 vs. 8% for cN0 and 13% for cN1). There was significant understaging of T-stage, regardless of nodal stage. Excluding patients that received neoadjuvant therapy who may have been downstaged, all clinically staged nodal groups had a substantial increase in the T-stage of the tumors after surgery. For this entire cohort, 10% of patients were cT3–4, while after surgery, 17.0% were pT3–4 (Table S1).
Table 1
| Variable | All, N (%) | cN0, N (%) | cN1, N (%) | cN2, N (%) | P value |
|---|---|---|---|---|---|
| Total patients, N | 10,225 | 4,344 | 1,160 | 4,721 | |
| Demographics | |||||
| Age, years [mean ± SD] | 65±10 | 66±10 | 66±10 | 64±10 | <0.0001ab |
| Sex | 0.0016ac | ||||
| Female | 5,450 (53.3) | 2,402 (55.3) | 588 (50.7) | 2,460 (52.1) | |
| Male | 4,775 (46.7) | 1,942 (44.7) | 572 (49.3) | 2,261 (47.9) | |
| Race | 0.184 | ||||
| Caucasian | 8,367 (81.8) | 3,531 (81.3) | 957 (82.5) | 3,879 (82.2) | |
| African American | 985 (9.6) | 410 (9.4) | 102 (8.8) | 473 (10.0) | |
| Hispanic | 345 (3.4) | 169 (3.9) | 42 (3.6) | 134 (2.8) | |
| Asian/Pacific Islander | 396 (3.9) | 180 (4.1) | 44 (3.8) | 172 (3.6) | |
| Other/unknown | 132 (1.3) | 54 (1.2) | 15 (1.3) | 63 (1.3) | |
| Insurance status | <0.0001ab | ||||
| Private | 3,727 (36.4) | 1,416 (32.6) | 392 (33.8) | 1,919 (40.6) | |
| Medicare | 5,388 (52.7) | 2,455 (56.5) | 656 (56.6) | 2,277 (48.2) | |
| Medicaid/other government | 833 (8.1) | 346 (8.0) | 87 (7.5) | 400 (8.5) | |
| None/other | 277 (2.7) | 127 (2.9) | 25 (2.2) | 125 (2.6) | |
| Income | 0.0026a | ||||
| <$38,000 | 1,497 (14.6) | 642 (14.8) | 182 (15.7) | 673 (14.3) | |
| $38,000–$62,999 | 4,631 (45.3) | 1,994 (45.9) | 527 (45.4) | 2,110 (44.7) | |
| ≥$63,000 | 2,977 (29.1) | 1,191 (27.4) | 322 (27.8) | 1,464 (31.0) | |
| Unknown | 1,120 (11.0) | 517 (11.9) | 129 (11.1) | 474 (10) | |
| Location | 0.0134a | ||||
| Metro/Suburban | 8,206 (80.3) | 3,495 (80.5) | 919 (79.2) | 3,792 (80.3) | |
| Urban | 1,463 (14.3) | 640 (14.7) | 169 (14.6) | 654 (13.9) | |
| Rural | 197 (1.9) | 85 (2.0) | 31 (2.7) | 81 (1.7) | |
| Unknown | 359 (3.5) | 124 (2.9) | 41 (3.5) | 194 (4.1) | |
| Facility type | <0.0001ab | ||||
| Non-academic | 6,363 (62.2) | 2,876 (66.2) | 745 (64.2) | 2,742 (58.1) | |
| Academic/research program | 3,862 (37.8) | 1,468 (33.8) | 415 (35.8) | 1,979 (41.9) | |
| Distance to facility, miles, median [Q1–Q3] | 12 [5–28] | 11 [5–27] | 12 [5–28] | 12 [5–28] | 0.8710 |
| Charlson-Deyo comorbidity | <0.0001ab | ||||
| 0 | 5,866 (57.4) | 2,324 (53.5) | 652 (56.2) | 2,890 (61.2) | |
| 1 | 2,974 (29.1) | 1,334 (30.7) | 348 (30.0) | 1,292 (27.4) | |
| ≥2 | 1,385 (13.5) | 686 (15.8) | 160 (13.8) | 539 (11.4) | |
| Tumor characteristics | |||||
| Clinical T stage | <0.0001abc | ||||
| 1 | 4,349 (42.5) | 2,296 (52.9) | 472 (40.7) | 1581 (33.5) | |
| 2 | 4,430 (43.3) | 1,698 (39.1) | 541 (46.6) | 2,191 (46.4) | |
| 3–4 | 1,446 (14.1) | 350 (8.1) | 147 (12.7) | 949 (20.1) | |
| Pathologic T stage | <0.0001abc | ||||
| 1 | 3,551 (34.7) | 1,332 (30.7) | 304 (26.2) | 1,915 (40.6) | |
| 2 | 4,954 (48.4) | 2,313 (53.2) | 603 (52.0) | 2,038 (43.2) | |
| 3 | 1,720 (16.8) | 699 (16.1) | 253 (21.8) | 768 (16.3) | |
| Tumor size, cm, median [Q1–Q3] | 3.2 [2.3–4.8] | 3.0 [2.2–4.4] | 3.4 [2.4–5.1] | 3.5 [2.4–5.0] | <0.0001ac |
| Tumor size (cm) | <0.0001abc | ||||
| <2.0 | 1,684 (16.5) | 790 (18.2) | 143 (12.3) | 751 (15.9) | |
| 2.0–3.9 | 4,672 (45.7) | 2,167 (49.9) | 542 (46.7) | 1,963 (41.6) | |
| 4.0–5.9 | 2,259 (22.1) | 878 (20.2) | 260 (22.4) | 1,121 (23.7) | |
| ≥6.0 | 1,529 (15) | 497 (11.4) | 213 (18.4) | 819 (17.3) | |
| Unknown | 81 (0.8) | 12 (0.3) | 2 (0.2) | 67 (1.4) | |
| Histology | <0.0001ac | ||||
| Adenocarcinoma | 7,528 (73.6) | 3,371 (77.6) | 836 (72.1) | 3,321 (70.3) | |
| Squamous cell carcinoma | 2,365 (23.1) | 809 (18.6) | 289 (24.9) | 1,267 (26.8) | |
| Adenosquamous carcinoma | 332 (3.2) | 164 (3.8) | 35 (3.0) | 133 (2.8) | |
| Treatment | |||||
| Chemotherapy | <0.0001abc | ||||
| None | 1,485 (14.5) | 872 (20.1) | 209 (18) 0 | 404 (8.6) | |
| Neoadjuvant | 2,831 (27.7) | 115 (2.6) | 90 (7.8) | 2,626 (55.6) | |
| Adjuvant | 5,909 (57.8) | 3,357 (77.3) | 861 (74.2) | 1,691 (35.8) | |
| Radiation | 5,339 (52.2) | 1,796 (41.3) | 527 (45.4) | 3,016 (63.9) | <0.0001abc |
| Diagnosis to first treatment, days, median [Q1–Q3] | 32 [16–52] | 34 [15–55] | 34 [20–52] | 30 [15–49] | <0.0001ab |
| Diagnosis to surgery, days, median [Q1–Q3] | 51 [25–110] | 36 [17–59] | 41 [25–64] | 104 [41–140] | <0.0001abc |
| Surgery to adjuvant treatment, days, median [Q1–Q3] | 61 [43–132] | 64 [43–135] | 61 [43–131] | 57 [42–120] | <0.0001a |
| Surgical approach | <0.0001ac | ||||
| Open | 5,350 (52.3) | 2,174 (50.0) | 618 (53.3) | 2,558 (54.2) | |
| Minimally invasive | 3,252 (31.8) | 1,558 (35.9) | 357 (30.8) | 1,337 (28.3) | |
| Unknown | 1,623 (15.9) | 612 (14.1) | 185 (15.9) | 826 (17.5) | |
| MI converted to open | 522 (16.1) | 221 (14.2) | 65 (18.2) | 236 (17.7) | 0.0202a |
| Outcomes | |||||
| Length of stay, days, median [Q1–Q3] | 5 [3–7] | 5 [3–7] | 5 [3–7] | 5 [3–7] | 0.2767 |
| 30–day mortality | 154 (1.7) | 52 (1.4) | 18 (1.8) | 84 (2.0) | 0.1065 |
| 90–day mortality | 346 (3.9) | 126 (3.4) | 36 (3.7) | 184 (4.4) | 0.0573 |
| 30–day readmission | 399 (4.0) | 185 (4.3) | 51 (4.5) | 163 (3.5) | 0.1037 |
| Lymph node harvest, median [Q1–Q3] | 11 [7–17] | 11 [7–17] | 12 [8–18] | 11 [7–17] | <0.0001bc |
| Lymph node harvest | <0.0001abc | ||||
| ≤ 5 | 1,449 (15.4) | 595 (14.6) | 128 (11.8) | 726 (17.0) | |
| 6–15 | 5,132 (54.4) | 2,295 (56.5) | 576 (52.9) | 2,261 (52.8) | |
| >15 | 2,853 (30.2) | 1,172 (28.9) | 385 (35.4) | 1,296 (30.3) | |
| Number of positive nodes, median [Q1–Q3] | 3 [1–5] | 2 [1–4] | 3 [2–6] | 3 [1–5] | <0.0001abc |
| Resection | 0.0002bc | ||||
| R0 | 9,276 (95.0) | 3,978 (95.3) | 1,009 (92.4) | 4,289 (95.3) | |
| R1 | 475 (4.9) | 195 (4.7) | 78 (7.1) | 202 (4.5) | |
| R2 | 18 (0.2) | 3 (0.1) | 5 (0.5) | 10 (0.2) | |
| Any positive margins | 829 (8.2) | 319 (7.4) | 139 (12.1) | 371 (8.0) | <0.0001bc |
a, cN2 vs. cN0, P<0.05; b, cN2 vs. cN1, P<0.05; c, cN1 vs. cN0, P<0.05.
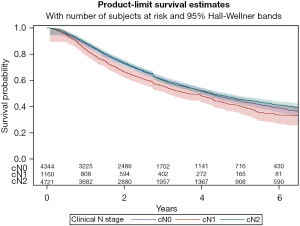
Despite a final pathologic stage of N2 in the entire cohort, 20% of the cN0 group and 18% of the cN1 group did not receive any systemic therapy. This is compared to just 9% of the cN2 cohort. Positive margins were highest in the cN1 group (12%), and this group ultimately also had the worst overall survival (median 3.7 years, 5-year OS 40%) (Figure 2, Table 2).
Table 2
| Variable | All | cN0 | cN1 | cN2 | P value |
|---|---|---|---|---|---|
| Follow-up months, median [Q1–Q3] | 30 [13–51] | 29 [12–50] | 26 [10–46] | 32 [15–53] | <0.0001abc |
| Overall survival | 0.0002bc | ||||
| Median years (Q1–Q3) | 4.2 (1.8–9.2) | 4.2 (4.0–4.4) | 3.7 (3.2–4.2) | 4.3 (1.9–9.2) | |
| 1 year | 87.8% | 88.0% | 83.6% | 88.7% | |
| 3 years | 59.7% | 60.7% | 55.6% | 59.9% | |
| 5 years | 44.4% | 44.3% | 40.2% | 45.4% |
a, cN2 vs. cN0, P<0.05; b, cN2 vs. cN1, P<0.05; c, cN1 vs. cN0, P<0.05.
Analysis of clinical N2 patients
A total of 4,721 patients were found to have clinical N2 disease. Of this cohort, 404 patients received no systemic therapy (8%), 2,626 received neoadjuvant (56%), and 1,691 received adjuvant (36%). Patient demographics and tumor characteristics of the cN2 group are seen in Table 3. Patients with neoadjuvant therapy were more likely to have an R0 resection than patients undergoing upfront surgery. 30-day and 90-day mortality rates were similar among patients receiving neoadjuvant therapy (2.3%, 4.9%) vs. patients undergoing upfront surgery, regardless of systemic therapy (1.6%, 3.8%). However, patients who received no systemic therapy had a much higher 30-day and 90-day mortality (7.8%, 17.2%) compared with patients who received neoadjuvant therapy and those patients who received adjuvant systemic therapy (0.2%, 0.7%).
Table 3
| Variable | All, N (%) | No therapy, N (%) | Neoadjuvant, N (%) | Adjuvant, N (%) | P value |
|---|---|---|---|---|---|
| Total patients, N | 4,721 | 404 | 2,626 | 1,691 | |
| Demographics | |||||
| Age, years [mean ± SD] | 64±10 | 71±10 | 62±9 | 65±9 | <0.0001abc |
| Sex | 0.0020a | ||||
| Female | 2,460 (52.1) | 177 (43.8) | 1,397 (53.2) | 886 (52.4) | |
| Male | 2,261 (47.9) | 227 (56.2) | 1,229 (46.8) | 805 (47.6) | |
| Race | 0.00061c | ||||
| Caucasian | 3,879 (82.2) | 329 (81.4) | 2,166 (82.5) | 1,384 (81.8) | |
| African American | 473 (10.0) | 36 (8.9) | 251 (9.6) | 186 (11.0) | |
| Hispanic | 134 (2.8) | 15 (3.7) | 66 (2.5) | 53 (3.1) | |
| Asian/Pacific Islander | 172 (3.6) | 10 (2.5) | 113 (4.3) | 49 (2.9) | |
| Other/Unknown | 63 (1.3) | 14 (3.5) | 30 (1.1) | 19 (1.1) | |
| Insurance Status | <0.0001abc | ||||
| Private | 1,919 (40.6) | 83 (20.5) | 1,229 (46.8) | 607 (35.9) | |
| Medicare | 2,277 (48.2) | 283 (70.0) | 1,111 (42.3) | 883 (52.2) | |
| Medicaid/other government | 400 (8.5) | 23 (5.7) | 218 (8.3) | 159 (9.4) | |
| None/other | 125 (2.6) | 15 (3.7) | 68 (2.6) | 42 (2.5) | |
| Income, USD | <0.0001abc | ||||
| <$38,000 | 673 (14.3) | 76 (18.8) | 327 (12.5) | 270 (16.0) | |
| $38,000–$62,999 | 2,110 (44.7) | 198 (49.0) | 1,114 (42.4) | 798 (47.2) | |
| ≥$63,000 | 1,464 (31.0) | 99 (24.5) | 910 (34.7) | 455 (26.9) | |
| Unknown | 474 (10) | 31 (7.7) | 275 (10.5) | 168 (9.9) | |
| Location | <0.0001bc | ||||
| Metro/Suburban | 3,792 (80.3) | 314 (77.7) | 2,141 (81.5) | 1,337 (79.1) | |
| Urban | 654 (13.9) | 70 (17.3) | 324 (12.3) | 260 (15.4) | |
| Rural | 81 (1.7) | 11 (2.7) | 27 (1.0) | 43 (2.5) | |
| Unknown | 194 (4.1) | 9 (2.2) | 134 (5.1) | 51 (3.0) | |
| Facility type | <0.0001bc | ||||
| Non-academic | 2,742 (58.1) | 271 (67.1) | 1,361 (51.8) | 1,110 (65.6) | |
| Academic/research program | 1,979 (41.9) | 133 (32.9) | 1,265 (48.2) | 581 (34.4) | |
| Distance to facility, miles, median [Q1–Q3] | 12 [5–28] | 12 [5–30] | 12 [6–30] | 11 [5–26] | 0.0089b |
| Charlson-Deyo comorbidity | <0.0001abc | ||||
| 0 | 2,890 (61.2) | 210 (52.0) | 1,728 (65.8) | 952 (56.3) | |
| 1 | 1,292 (27.4) | 123 (30.4) | 652 (24.8) | 517 (30.6) | |
| ≥2 | 539 (11.4) | 71 (17.6) | 246 (9.4) | 222 (13.1) | |
| Tumor characteristics | |||||
| Clinical T stage | <0.0001bc | ||||
| 1 | 1,581 (33.5) | 146 (36.1) | 808 (30.8) | 627 (37.1) | |
| 2 | 2,191 (46.4) | 183 (45.3) | 1,179 (44.9) | 829 (49.0) | |
| 3–4 | 949 (20.1) | 75 (18.6) | 639 (24.3) | 235 (13.9) | |
| Pathologic T stage | <0.0001abc | ||||
| 1 | 1,915 (40.6) | 102 (25.2) | 1,325 (50.5) | 488 (28.9) | |
| 2 | 2,038 (43.2) | 211 (52.2) | 936 (35.6) | 891 (52.7) | |
| 3 | 768 (16.3) | 91 (22.5) | 365 (13.9) | 312 (18.5) | |
| Tumor size, cm [median (Q1-Q3)] | 3.5 (2.4–5.0) | 3.5 (2.5–5.0) | 3.5 (2.4–5.2) | 3.3 (2.3–5.0) | 0.0083b |
| Tumor size (cm) | <0.0001abc | ||||
| <2.0 | 751 (15.9) | 49 (12.1) | 429 (16.3) | 273 (16.1) | |
| 2.0–3.9 | 1,963 (41.6) | 183 (45.3) | 1,015 (38.7) | 765 (45.2) | |
| 4.0–5.9 | 1,121 (23.7) | 97 (24.0) | 635 (24.2) | 389 (23.0) | |
| ≥6.0 | 819 (17.3) | 71 (17.6) | 492 (18.7) | 256 (15.1) | |
| Unknown | 67 (1.4) | 4 (1.0) | 55 (2.1) | 8 (0.5) | |
| Histology | 0.0019abc | ||||
| Adenocarcinoma | 3,321 (70.3) | 262 (64.9) | 1,859 (70.8) | 1,200 (71.0) | |
| Squamous cell carcinoma | 1,267 (26.8) | 126 (31.2) | 708 (27.0) | 433 (25.6) | |
| Adenosquamous carcinoma | 133 (2.8) | 16 (4.0) | 59 (2.2) | 58 (3.4) | |
| Treatment | |||||
| Radiation | 3,016 (63.9) | 0 (0.0) | 2,005 (76.4) | 1,011 (59.8) | <0.0001abc |
| Diagnosis to first treatment, days, median [Q1–Q3] | 30 [15–49] | 34 [9–61] | 31 [18–47] | 29 [11–50] | 0.0218 |
| Diagnosis to surgery, days, median [Q1–Q3] | 104 [41–140] | 37 [15–62] | 132 [111–160] | 35 [17–60] | <0.0001bc |
| Surgery to adjuvant treatment, days, median [Q1–Q3] | 57 [42–120] | – | – | 61 [42–132] | – |
| Surgical approach | <0.0001abc | ||||
| Open | 2,558 (54.2) | 249 (61.6) | 1,440 (54.8) | 869 (51.4) | |
| Minimally invasive | 1,337 (28.3) | 130 (32.2) | 744 (28.3) | 463 (27.4) | |
| Unknown | 826 (17.5) | 25 (6.2) | 442 (16.8) | 359 (21.2) | |
| MI converted to open | 236 (17.7) | 25 (19.2) | 134 (18.0) | 77 (16.6) | 0.7331 |
| Outcomes | |||||
| Length of stay, days, median [Q1–Q3] | 5 [3–7] | 7 [4–10] | 5 [3–7] | 5 [3–7] | <0.0001ac |
| 30-day mortality | 84 (2.0) | 27 (7.8) | 54 (2.3) | 3 (0.2) | <0.0001abc |
| 90-day mortality | 184 (4.4) | 59 (17.2) | 115 (4.9) | 10 (0.7) | <0.0001abc |
| 30-day readmission | 163 (3.5) | 24 (6.0) | 92 (3.6) | 47 (2.8) | 0.0086ac |
| Lymph node harvest, median [Q1–Q3] | 11 [7–17] | 11 [7–16] | 11 [7–18] | 11 [7–17] | 0.7120 |
| Lymph node harvest | 0.0055bc | ||||
| ≤5 | 726 (17.0) | 59 (15.4) | 424 (18.3) | 243 (15.4) | |
| 6-15 | 2,261 (52.8) | 223 (58.4) | 1,169 (50.4) | 869 (55.0) | |
| >15 | 1,296 (30.3) | 100 (26.2) | 727 (31.3) | 469 (29.7) | |
| Number of positive nodes, median [Q1–Q3] | 3 [1–5] | 3 [1–5] | 2 [1–5] | 3 [2–5] | <0.0001b |
| Resection | 0.0009b | ||||
| R0 | 4,289 (95.3) | 368 (95.1) | 2,432 (96.4) | 1,489 (93.6) | |
| R1 | 202 (4.5) | 19 (4.9) | 89 (3.5) | 94 (5.9) | |
| R2 | 10 (0.2) | 0 (0.0) | 3 (0.1) | 7 (0.4) | |
| Any positive margins | 371 (8.0) | 33 (8.2) | 161 (6.2) | 177 (10.6) | <0.0001b |
| Reason for no chemotherapy | – | ||||
| None, not part of the first course of treatment | – | 192 (47.5) | – | – | |
| Contraindicated | – | 48 (11.9) | – | – | |
| Died prior to planned/recommended therapy | – | 10 (2.5) | – | – | |
| Recommended but not administered, unknown reason | – | 54 (13.3) | – | – | |
| Recommended but patient refused | – | 100 (24.8) | – | – | |
| Follow-up months, median [Q1–Q3] | 32 [15–53] | 17 [4–34] | 33 [17–55] | 32 [15–53] | <0.0001bc |
| Overall survival | <0.0001abc | ||||
| Median years (Q1–Q3) | 4.3 (1.9–9.2) | 2.0 (1.7–2.3) | 5.0 (2.1–8.9) | 3.9 (3.5–4.2) | |
| 1 year | 88.7% | 67.9% | 90.7% | 90.2% | |
| 3 years | 59.9% | 35.3% | 65.0% | 57.5% | |
| 5 years | 45.4% | 24.5% | 50.1% | 42.7% |
a, Adjuvant vs. No Therapy, P<0.05; b, Adjuvant vs. Neoadjuvant, P<0.05; c, Neoadjuvant vs. No Therapy, P<0.05.
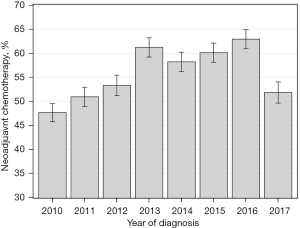
The NCCN Guidelines recommend neoadjuvant systemic therapy prior to lobectomy for clinical N2 NSCLC. During this time period, the rate of guideline concordance rose from 48% in 2010 to 63% in 2016. In 2017, only 52% of cN2 patients received neoadjuvant therapy (Figure 3). For this group, being treated at an academic center was correlated with receiving neoadjuvant therapy versus upfront lobectomy for cN2 disease (academic 64% vs. non-academic 50%, P<0.0001). Survival differed based on receipt and timing of systemic therapy. In univariate analysis, median survival was 2.0 years for no systemic therapy, 5.0 years for neoadjuvant, and 3.9 years for adjuvant (P<0.0001). Five-year survival was 25%, 50%, and 43% respectively.
On multivariable analysis, both neoadjuvant and adjuvant systemic therapy showed an overall survival benefit compared with no systemic therapy (HR 0.54, P<0.0001 for neoadjuvant, and HR 0.57, P<0.0001 for adjuvant). There was no difference in survival when comparing adjuvant vs. neoadjuvant (Table 4).
Table 4
| Variable | Survival analysis | 30-day mortality | 90-day mortality | 30-day readmission | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age, years | |||||||||||
| 55–64 vs. <55 | 1.22 (1.06–1.41) | 0.0046 | 2.33 (0.95–5.76) | 0.0661 | 1.37 (0.76–2.48) | 0.2991 | 0.55 (0.35–0.88) | 0.0122 | |||
| 65–74 vs. <55 | 1.31 (1.12–1.55) | 0.0009 | 3.27 (1.24–8.65) | 0.0169 | 1.93 (1.02–3.65) | 0.0445 | 0.86 (0.51–1.45) | 0.5678 | |||
| ≥75 vs. <55 | 1.68 (1.39–2.01) | <0.0001 | 4.00 (1.40–11.41) | 0.0096 | 2.14 (1.06–4.32) | 0.0332 | 1.02 (0.55–1.88) | 0.9508 | |||
| Sex, male vs. female | 1.31 (1.20–1.43) | <0.0001 | 1.57 (1.00–2.46) | 0.0494 | 1.49 (1.08–2.05) | 0.0145 | 1.27 (0.93–1.74) | 0.1379 | |||
| Facility type, academic/research vs. non-academic | 0.87 (0.80–0.96) | 0.0031 | 0.75 (0.48–1.18) | 0.2120 | 0.62 (0.45–0.86) | 0.0039 | 0.86 (0.62–1.18) | 0.3418 | |||
| Charlson-Deyo comorbidity | |||||||||||
| 1 vs. 0 | 1.10 (1.00–1.21) | 0.0573 | 0.68 (0.40–1.17) | 0.1615 | 1.04 (0.72–1.49) | 0.8376 | 1.26 (0.89–1.79) | 0.1907 | |||
| ≥2 vs. 0 | 1.30 (1.14–1.49) | <0.0001 | 1.14 (0.63–2.05) | 0.6721 | 1.66 (1.09–2.51) | 0.0180 | 1.34 (0.84–2.13) | 0.2209 | |||
| Tumor characteristics | |||||||||||
| Pathologic T stage | |||||||||||
| 2 vs. 1 | 1.26 (1.15–1.39) | <0.0001 | 0.94 (0.58–1.52) | 0.7959 | 1.22 (0.86–1.72) | 0.2719 | 1.08 (0.76–1.53) | 0.6821 | |||
| 3 vs. 1 | 1.48 (1.31–1.68) | <0.0001 | 1.06 (0.58–1.93) | 0.8551 | 1.39 (0.90–2.14) | 0.1330 | 1.28 (0.83–1.97) | 0.2680 | |||
| Treatment | |||||||||||
| Treatment timing | |||||||||||
| Neoadjuvant vs. no therapy | 0.54 (0.47–0.63) | <0.0001 | 0.37 (0.22–0.64) | 0.0003 | 0.40 (0.27–0.59) | <0.0001 | 0.75 (0.46–1.23) | 0.2587 | |||
| Adjuvant vs. no therapy | 0.57 (0.49–0.66) | <0.0001 | 0.03 (0.01–0.10) | <0.0001 | 0.04 (0.02–0.08) | <0.0001 | 0.53 (0.32–0.87) | 0.0131 | |||
| Adjuvant vs. neoadjuvant | 1.05 (0.95–1.15) | 0.3285 | 0.09 (0.03–0.25) | <0.0001 | 0.10 (0.05–0.19) | <0.0001 | 0.70 (0.49–1.00) | 0.0532 | |||
Timing and response to systemic therapy in cN2 patients
A survival analysis was performed based on receipt, timing, and nodal response to systemic therapy. Patients were divided into one of five groups: (I) complete response: cN2-> neoadjuvant-> pN0; (II) partial response: cN2-> Neoadjuvant-> pN1; (III) no response: cN2-> Neoadjuvant-> pN2; (IV) adjuvant: cN2-> pN2-> adjuvant; (V) no systemic therapy: cN2-> pN2. Complete responders showed a significantly improved survival compared to the rest of the group, while those without any systemic therapy showed a significantly worse survival (median survival: complete 6.2 years, partial 4.7 years, no response 4.2 years, adjuvant 3.9 years, no systemic therapy 2.0 years, P<0.0001) (Figure 4, Table 5).
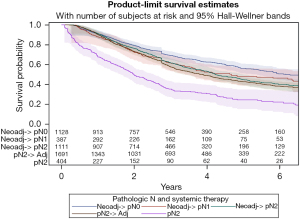
Table 5
| Variable | Neoadj-> pN0 | Neoadj-> pN1 | Neoadj-> pN2 | pN2-> Adj | pN2 | P value |
|---|---|---|---|---|---|---|
| Overall survival | <0.0001a | |||||
| Median years (Q1–Q3) | 6.2 (2.4–8.6) | 4.7 (1.9–6.4) | 4.2 (2.1–8.9) | 3.9 (1.9–8.8) | 2.0 (0.8–4.8) | |
| 1 year | 91.0% | 87.9% | 91.3% | 90.2% | 67.9% | |
| 3 years | 69.3% | 61.5% | 61.7% | 57.5% | 35.3% | |
| 5 years | 56.1% | 48.0% | 44.7% | 42.7% | 24.5% |
a, all pairwise comparisons P<0.05 except between Neoadj-> pN1 vs. Neoadj-> pN2, Neoadj-> pN1 vs. pN2-> Adj, and Neoadj-> pN2 vs. pN2-> Adj. Middle columns similar to each other, but different from outer (Neoadj-> pN0 and pN2).
Discussion
Using the most recent National Cancer Database registry data, we find that 54% of all patients with pathologic N2 disease were initially understaged as cN0 or cN1 and discovered to have involved mediastinal nodes after surgical resection. Of those that were clinically staged as N2 and who underwent lobectomy, only 56% received neoadjuvant therapy. This is despite clear NCCN recommendations for induction chemotherapy, plus or minus radiation, in cN2 patients (4). While there has been literature promoting upfront surgical resection for single station N2 disease (12,13), it is a rare entity and there is no consensus guideline advocating for surgery followed by adjuvant therapy wherein we would expect 35.8% of clinically staged N2 patients to follow that treatment algorithm, as seen in this cohort.
Approximately 9% of the 4,721 cN2pN2 patients had resection without any systemic therapy. In 25% of these patients, it was due to patient refusal. Our assumption is that the remaining 75% had been planned for adjuvant systemic therapy, but were deemed either too frail or did not recover well enough from surgical resection to tolerate chemotherapy. This is partially confirmed by our data, as patients who received no systemic therapy had substantially higher 30- and 90-day mortality and 30-day readmission compared with both the neoadjuvant and adjuvant chemotherapy cohorts.
Over the 7 years of data analyzed, the rate of guideline concordance (neoadjuvant therapy for cN2 disease) slowly increased from 47.6% in 2010 to 62.9% in 2016. In 2017, there was a sudden dip in guideline concordance to 51.8%. We cannot provide a clear explanation why this occurred, as there were no major changes in guidelines that year. The only variation in the lung cancer landscape was the FDA approval of pembrolizumab (Keytruda, Merck and Co, Inc) in May of 2017 for metastatic non-squamous NSCLC (14,15). Overall, there is no obvious reason why guideline concordance is not higher, but it is reassuring to see the rates generally increasing.
Despite increasingly advanced diagnostic technology, the inaccuracy of clinical stage has been a continued problem for lung cancer (16,17). We specifically found that clinical accuracy of T-stage is quite poor, regardless of nodal stage. Pathologic N2 patients are therefore also being substantially understaged by tumor characteristics, or the tumors are growing rapidly from time of clinical staging to surgery (median 35 days).
To compare the timing of therapy in similar clinical cohorts, we looked at cN2 patients only stratified by timing of systemic treatment. Patients who received neoadjuvant chemotherapy had superior 5-year overall survival (50%) compared with patients who received adjuvant treatment (43%), with a median survival difference of 1.1 years, though in multivariable analysis, there was not a significant difference in hazard ratios between these two groups. The true benefit of neoadjuvant therapy may be in in the prognostic value it provides. Our data shows that tumor responsiveness to chemotherapy plays an important role in regards to survival. Clinical N2 patients receiving neoadjuvant therapy with a complete pathologic response to pN0 had the best 5-year survival of 56%. As expected, cN2 patients who received no systemic therapy before or after surgery had the worst 5-year survival of 25%. Clinical N2 patients that were either partially downstaged to pN1 by neoadjuvant therapy, remained pN2 despite neoadjuvant therapy, or had upfront surgery followed by adjuvant systemic therapy had statistically similar 5-year survivals ranging from 42.7% to 48.0%. While currently this information is merely prognostic, with new data emerging on consolidative immunotherapy and/or targeted therapy, response to neoadjuvant therapy may, in the very near future, prove predictive, helping guide who receives these additional therapies and who does not (18). Such a treatment paradigm has already been proven in the treatment of esophageal cancer (19), and a similar strategy for lung cancer is likely not far behind.
There are important limitations to this study. The survival comparison between the different clinical nodal stages is hard to interpret with this data. Unfortunately, the NCDB does not provide information on diagnostic mediastinal staging prior to surgical resection. We do not know how patients were clinically staged. This includes lack of information on endobronchial ultrasound (EBUS), mediastinoscopy, or even positron emission tomography (PET) scan. We therefore cannot make any valid conclusions about the accuracy of these diagnostic modalities or if patients were clinically staged appropriately. With that caveat, median and 5-year overall survival were similar between cN0pN2 and cN2pN2 patients, with most cN0 patients receiving adjuvant therapy while most cN2 patients received neoadjuvant. Both of these groups had a significant survival advantage of approximately 6 months and 5% at 5 years over the cN1pN2 patients, who like cN0, mostly received adjuvant therapy. When we performed a sub-analysis of only patients receiving adjuvant therapy, there was a moderate survival benefit for lower clinical stage. cN0 patients had a 5-year survival of 48.7%, compared with 44.9% and 42.7% for cN1 and cN2.
As it is a hospital-based study of a national dataset, granular information is not available on each individual patient. Mentioned earlier, information regarding diagnostic modality for clinical stage is not incorporated into the NCDB. While the lack of appropriate mediastinal staging likely leads to clinical understaging, it can also lead to overstaging of non-biopsy proven mediastinal disease. This would cause a false improvement in survival in the cN2pN1 and cN2pN0 cohorts, although we countered this by removing any downstaged patients who did not receive neoadjuvant therapy. Specific chemotherapy regimen, and whether a patient was planned for resection but did not make it to the operation is not included. A high percentage of patients receiving neoadjuvant chemotherapy but not making it to surgical resection would have significant implications on treatment recommendations. The lack of recurrence data and disease free survival prevent us from including them into our analysis. Finally, the NCDB does not separate bulky from non-bulky N2 disease, or single station from multi station. These are important clinical variables that play decisive roles in treatment algorithms.
Conclusions
There appears to be a high rate of clinical nodal under staging nationally. While guideline concordance is generally improving, a large number of patients with clinically involved mediastinal nodes are not receiving recommended neoadjuvant therapy. The benefits of timing of therapy (neoadjuvant vs. adjuvant) on long-term survival remain unclear. What is clear is that tumor responsiveness to chemotherapy is a major predictor of survival, and may help guide additional treatment decisions. Our study indicates the need for future clinical trials incorporating effective use of diagnostic modalities for mediastinal staging and assessing specific chemotherapy regimens to help determine the best treatment strategy for lung cancer patients with clinically positive nodal disease.
Acknowledgments
We thank the Huntsman Cancer Institute and NorthShore University Health System for statistical support and resources to advance research in management of lung cancer. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1845/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1845/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1845/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Cascone T, William WN Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504-14. [Crossref] [PubMed]
- Pechoux CL, Pourel N, Barlesi F, et al. LBA3_PR An international randomized trial, comparing post-operative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non-small cell lung cancer (NSCLC) and mediastinal N2 involvement: Primary end-point analysis of LungART (IFCT-0503, UK NCRI, SAKK). Ann Oncol 2020;31:S1178. [Crossref]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 5.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed July 19, 2021.
- Boffa DJ, Hancock JG, Yao X, et al. Now or later: evaluating the importance of chemotherapy timing in resectable stage III (N2) lung cancer in the National Cancer Database. Ann Thorac Surg 2015;99:200-8. [Crossref] [PubMed]
- Obiols C, Call S, Rami-Porta R, et al. Survival of patients with unsuspected pN2 non-small cell lung cancer after an accurate preoperative mediastinal staging. Ann Thorac Surg 2014;97:957-64. [Crossref] [PubMed]
- Tsitsias T, Boulemden A, Ang K, et al. The N2 paradox: similar outcomes of pre- and postoperatively identified single-zone N2a positive non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:882-7. [Crossref] [PubMed]
- Yang CF, Kumar A, Gulack BC, et al. Long-term outcomes after lobectomy for non-small cell lung cancer when unsuspected pN2 disease is found: A National Cancer Data Base analysis. J Thorac Cardiovasc Surg 2016;151:1380-8. [Crossref] [PubMed]
- Krantz SB, Howington JA, Wood DE, et al. Invasive Mediastinal Staging for Lung Cancer by The Society of Thoracic Surgeons Database Participants. Ann Thorac Surg 2018;106:1055-62. [Crossref] [PubMed]
- Jin Y, Chen M, Yu X. Comparison of the 7(th) and proposed 8(th) editions of the AJCC/UICC TNM staging system for non-small cell lung cancer undergoing radical surgery. Sci Rep 2016;6:33587. [Crossref] [PubMed]
- ICD-0-3 SEER Site/Histology Validation List (Version 6/7/2021). Available online: https://seer.cancer.gov/icd-o-3/sitetype.icdo3.20210607.pdf. Accessed July 19, 2021.
- Rocco G, Nason K, Brunelli A, et al. Management of stage IIIA (N2) non-small cell lung cancer: A transatlantic perspective. J Thorac Cardiovasc Surg 2016;151:1235-8. [Crossref] [PubMed]
- Tsitsias T, Boulemden A, Ang K, et al. The N2 paradox: similar outcomes of pre- and postoperatively identified single-zone N2a positive non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:882-7. [Crossref] [PubMed]
- Pembrolizuman (Keytruda) 5-10-2017. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda-5-10-2017. Accessed 4/1/2021.
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomized, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Navani N, Fisher DJ, Tierney JF, et al. The Accuracy of Clinical Staging of Stage I-IIIa Non-Small Cell Lung Cancer: An Analysis Based on Individual Participant Data. Chest 2019;155:502-9. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Ojha B, et al. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg 2005;80:1207-13; discussion 1213-4. [Crossref] [PubMed]
- Moding EJ, Liu Y, Nabet BY, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nature Cancer. 2020;1:176-83. [Crossref] [PubMed]
- Kelly RJ, Lockhart AC, Jonker DJ, et al. CheckMate 577: A randomized, double-blind, phase 3 study of nivolumab (Nivo) or placebo in patients (Pts) with resected lower esophageal or gastroesophageal junction (GEJ) cancer. J Clin Oncol 2017;35. [Crossref]