
Circulating IL-32 and IL-33 levels in patients with asthma and COPD: a retrospective cross-sectional study
Interleukin-32 (IL-32) and IL-33 are innate cytokines that respond to various external stimuli and show diverse activities depending on the situation (1,2). In inflammatory airway diseases, IL-32 and IL-33 expressed in lung tissues show distinct features between chronic obstructive pulmonary disease (COPD) and asthma. IL-32 expression in the lungs of COPD patients is associated with neutrophilic inflammation and disease severity (3). IL-33 can induce eosinophilic inflammation, and increased IL-33 expression has been observed in the airways of asthma patients (1,4). We hypothesized that plasma IL-32 and IL-33 may be useful for distinguishing between asthma and COPD, and compared the plasma levels of IL-32γ, the most active isoform of IL-32 transcripts, and IL-33 in patients with asthma and COPD.
IL-32γ and IL-33 levels in the plasma of asthma patients (n=103), COPD patients (n=40), and healthy controls (n=51) were evaluated. Asthma was diagnosed by clinicians based on clinical symptoms. The diagnosis was supported by positive investigations for reversible airway obstruction (Appendix 1). Patients with asthma who were current smokers or ex-smokers with >10 pack-years were excluded. COPD was diagnosed by a pulmonologist on the basis of typical symptoms, a smoking history of >10 pack-years, and a pre-bronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity ratio <70%. The Biobank database was analyzed, but data on the post-bronchodilator lung function of COPD patients were not available. The characteristics of the study population are presented in Table S1.
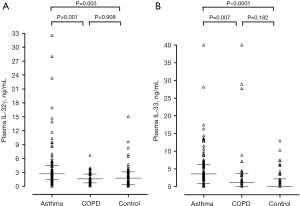
The detection rates of plasma IL-33 were higher in asthmatic patients (80%) than in patients with COPD (65%) and controls (49%). Plasma IL-32γ was detected in most participants in all groups. Plasma levels of IL-32γ and IL-33 were higher in asthma patients than in COPD patients and controls [median (interquartile range): 2.73 (1.42–4.47) vs. 1.61 (0.74–2.68) vs. 1.76 (0.39–3.15) ng/mL, P=0.001; Figure 1A; and 3.56 (0.89–4.47) vs. 1.61 (0.00–3.78) vs. 0.00 (0.00–2.19) ng/mL, P<0.0001; Figure 1B] (Figure S1). Similar results were observed for log transformation of the data. Plasma levels of IL-32γ differed between patients with asthma and COPD following adjustment for FEV1 (%) (P=0.004). However, neither cytokine showed significant differences among the three groups after adjustment for sex, age, and smoking history. Plasma IL-32γ and IL-33 levels were not related to the inflammatory or clinical characteristics of either disease (Table S2), except for a weak correlation between FEV1 (%) and plasma IL-33 level in patients with asthma (r=−0.215, P=0.029), and a positive correlation between the plasma levels of IL-32γ and sputum eosinophilia in patients with COPD (r=0.358, P=0.032). There was a negative correlation between plasma IL-32γ levels and age among asthmatic patients, which requires further evaluation in larger studies. It was reported that serum levels of IL-32α and β were higher in asthma patients compared to controls, and that there were no differences in age among asthmatic patients according to the presence of serum IL-32 (5). And, Bang et al. (6) reported lower serum levels of IL-32γ in older asthmatic patients compared to healthy controls.
There were positive correlations between plasma IL-32γ and IL-33 in patients with asthma, patients with COPD, and healthy controls (r=0.720, P<0.0001; r=0.512, P=0.001; and r=0.784, P<0.0001, respectively) (Figure S2). This was unexpected and contrasted with previous reports of the roles of IL-32 and IL-33 in the lungs of asthma and COPD patients. Notably, no studies have evaluated IL-32 and IL-33 together. Immunohistochemical analyses showed that IL-32γ and IL-33 were co-expressed mainly in monocytes and the expression levels of both cytokines increased with the stimulation of GM-CSF and TNF-α (Figure S3).
The results regarding the relationships between the plasma levels of these cytokines and the inflammatory or clinical characteristics of patients have been inconsistent, which contrasts with the consistent results regarding the presence of these cytokines in asthma and COPD lung tissues. In contrast to the relationship between IL-32 expression and neutrophilic inflammation in the lung tissues of COPD patients, plasma IL-32γ levels in COPD patients were positively correlated with sputum eosinophilia in this study. As one of the diverse features of IL-32, IL-32γ could increase eosinophilic inflammation in response to infectious stimuli (7). The study by Meyer et al. (5) showed that serum IL-32 levels in asthma patients correlated with the responsiveness to asthma therapy, as reflected in improved FEV1 and reduced blood eosinophil counts. Serum IL-32 levels are higher in patients with acute COPD exacerbations than asthmatic patients (8). However, several studies have reported that plasma IL-32 expression is not elevated in stable COPD patients (9,10). As innate cytokines and alarmins, plasma IL-32 and IL-33 are induced by various stimuli such as infections, cigarette smoke, and pollution, which could serve as common triggers for asthma and COPD exacerbations. Therefore, the plasma expression levels of IL-33 and IL-32 in asthma and COPD patients may be related to inflammatory activity. This is a well-established concept in other inflammatory diseases, such as rheumatoid arthritis and tuberculosis, but has not yet been applied to asthma and COPD.
The levels of both plasma IL-32γ and IL-33 were elevated in asthma patients, and the levels of these cytokines in plasma do not seem to reflect the differences in the pathophysiologies of asthma and COPD. The levels of these innate cytokines in plasma were strongly positively correlated, and were co-expressed in monocytes in peripheral blood mononuclear cells. Plasma IL-32γ and IL-33 levels may be related to inflammatory activity in chronic airway inflammatory diseases.
Acknowledgments
Funding: This research was supported by a Research Program funded by the Korea Centers for Disease Control & Prevention (fund code 2016-ER7402-00) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A3B03034684). The biospecimens and data used for this study were provided by the Biobank of Soonchunhyang University Bucheon Hospital, a member of the Korea Biobank Network. (KBN4_A06).
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-524/coif). All authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Donovan C, Hansbro PM. IL-33 in Chronic Respiratory Disease: From Preclinical to Clinical Studies. ACS Pharmacol Transl Sci 2020;3:56-62. [Crossref] [PubMed]
- Khawar B, Abbasi MH, Sheikh N. A panoramic spectrum of complex interplay between the immune system and IL-32 during pathogenesis of various systemic infections and inflammation. Eur J Med Res 2015;20:7. [Crossref] [PubMed]
- Calabrese F, Baraldo S, Bazzan E, et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;178:894-901. [Crossref] [PubMed]
- Guo Z, Wu J, Zhao J, et al. IL-33 promotes airway remodeling and is a marker of asthma disease severity. J Asthma 2014;51:863-9. [Crossref] [PubMed]
- Meyer N, Christoph J, Makrinioti H, et al. Inhibition of angiogenesis by IL-32: possible role in asthma. J Allergy Clin Immunol 2012;129:964-73.e7. [Crossref] [PubMed]
- Bang BR, Kwon HS, Kim SH, et al. Interleukin-32γ suppresses allergic airway inflammation in mouse models of asthma. Am J Respir Cell Mol Biol 2014;50:1021-30. [Crossref] [PubMed]
- Wong CK, Hu S, Leung KM, et al. NOD-like receptors mediated activation of eosinophils interacting with bronchial epithelial cells: a link between innate immunity and allergic asthma. Cell Mol Immunol 2013;10:317-29. [Crossref] [PubMed]
- Jia TG, Zhao JQ, Liu JH. Serum inflammatory factor and cytokines in AECOPD. Asian Pac J Trop Med 2014;7:1005-8. [Crossref] [PubMed]
- Rong Y, Xiang XD, Li YM, et al. IL-32 was involved in cigarette smoke-induced pulmonary inflammation in COPD. Clin Respir J 2015;9:430-5. [Crossref] [PubMed]
- Greene CM, Low TB, O'Neill SJ, et al. Anti-proline-glycine-proline or antielastin autoantibodies are not evident in chronic inflammatory lung disease. Am J Respir Crit Care Med 2010;181:31-5. [Crossref] [PubMed]


