
Leucine-rich a-2 glycoprotein as a potential biomarker of idiopathic multicentric Castleman disease with pulmonary involvement: a single-center case-control study from Japan
Introduction
Multicentric Castleman disease (MCD) is a multiclonal lymphoproliferative disease characterized by aggregation of plasmacytes with hyperplastic germinal center of the lymphatic node, resulting in systemic inflammation and potentially fatal multiple organ failure, involving pulmonary manifestation. The incidence per million persons is 5.1–5.7 in the United States and 2.4–5.8 in Japan (1,2). Patients with idiopathic MCD (iMCD) account for approximately 30% of patients with MCD (3). Although the etiology of iMCD remains unclear, interleukin-6 (IL-6) plays a key role in the pathogenesis and symptomatology of the disease (4). In this context, monoclonal antibodies directed against the IL-6 receptor (tocilizumab) and IL-6 (siltuximab) have been developed as treatment options for iMCD. However, IL-6 levels are not elevated in all patients with iMCD, and approximately 50% of patients do not benefit from IL-6 inhibition (5). In addition, some patients have low serum IL-6 levels even during disease flare-ups (6,7), suggesting that IL-6-independent pathways may drive disease pathogenesis in a subset of patients with iMCD. In addition to IL-6, several other mediators, such as CXCL13, IL-1, and tumor necrosis factor α (TNF-α), have been reported as candidate pathogenic factors (8). Currently, there are no validated biomarkers for monitoring the disease activity in iMCD. Particularly, it is difficult to evaluate disease activity of pulmonary manifestation with iMCD, which respirologists occasionally treat.
Leucine-rich α2-glycoprotein (LRG) is an approximately 50-kDa glycoprotein, including eight leucine-rich repeat domains (9). LRG is induced by IL-6 and other cytokines such as IL-22 by induction of STAT3 and TNF-α, IL-1β by induction of NFκB (10). Previous studies have demonstrated that LRG may serve as a potential biomarker for inflammation-related diseases such as Crohn’s disease, infection, asthma, and various types of cancer (11-17). In particular, in patients with ulcerative colitis, LRG levels are more strongly correlated with disease activity than C-reactive protein (CRP) levels (18). Additionally, LRG could detect inflammation in patients with rheumatoid arthritis during IL-6 blockade treatment (19). These findings indicate that LRG could reflect both IL-6-dependent and -independent inflammatory changes and thus may have the potential to monitor disease activity in iMCD, which cannot be detected only by CRP. However, data on the role of serum LRG levels in patients with iMCD are lacking.
We hypothesized that LRG could be a biomarker of pulmonary manifestation of iMCD, which has no indicator of disease progression. The present study aimed to evaluate the utility of serum LRG levels for monitoring disease activity in patients with iMCD with pulmonary involvement. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1973/rc).
Methods
Study design
We conducted a retrospective study of 165 patients diagnosed with Castleman Disease at Osaka University Hospital between January 2008 and July 2018 identified via a computer search. Of these, 68 patients had iMCD and 20 patients had iMCD with abnormal shadow in the lung. Finally, five patients with histologically confirmed iMCD diagnosis with pulmonary involvement were examined, whereas 15 patients were excluded because they only had imaging findings but their diagnoses were not histologically confirmed. Longitudinal data of pulmonary function tests such as percent diffusing capacity of the lung for carbon monoxide (%DLco), percent vital capacity of the predicted normal value (%VC) and percent forced expiratory volume in 1 s (FEV1.0%) were collected. In addition, the parameters obtained in clinical practice, such as CRP, hemoglobin, platelets, neutrophil counts, and KL-6 were estimated. Sera from three healthy volunteers without any diagnosed disease were used as samples of healthy controls. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients provided informed consent, and this study was approved by the ethical review board of Osaka University Hospital (#20019).
Evaluation of the serum LRG concentration
LRG was quantified in the sera of the five iMCD patients with pulmonary involvement by latex-enhanced immunoturbidimetric assay in the laboratory of SEKISUI CHEMICAL, Tokyo, Japan. LRG measurements were performed before and during remission (defined as a CRP level of <10 mg/L and fewer than two minor diagnostic criteria (20) after treatment with tocilizumab and/or steroid in the same patients with iMCD). The difference in LRG levels before and after treatment was examined.
Measurement of cytokines and chemokines
We investigated the cytokine and chemokine profiles of patients with iMCD and the relationship between these mediators and the LRG concentration. The serum cytokines TNF-α, IL-13, IL-4, IL-10, IL-6, IL-2, TNF-β, interferon-γ, IL-17A, IL-12p70, a proliferation-inducing ligand (APRIL), B-cell activating factor, and CD40L and serum chemokines monocyte chemoattractant protein-1, CXCL10, eotaxin, CCL17, macrophage inflammatory protein (MIP)-1α, MIP-1β, CXCL9, MIP-3α, CXCL5, CXCL1, CXCL11, and IL-8 were analyzed using a LEGEND plex™ Human B Cell Panel and a LEGEND plex™ Human Proinflammatory Chemokine Panel (BioLegend Inc., San Diego, CA, USA) according to the manufacturer’s instructions. The concentrations of cytokines and chemokines were evaluated using a FACS Canto II Flow Cytometer (BD Bioscience, San Jose, CA, USA). In addition, pathway analysis was performed using selected factors that significantly differed between naïve patients with iMCD and healthy controls.
Statistical analysis
A permutation test was used to compare the serum LRG concentration between patients with iMCD and healthy controls. Pearson’s rank correlation coefficient was estimated to analyze the relationships of parameters of pulmonary faction test and LRG and other serum proteins. Complete case analysis was used for handling missing data. All statistical analyses were performed using R ver. 4.0.2 software (available at http://www.R-project.org; R Foundation for Statistical Computing, Vienna, Austria). Pathway analysis was performed using Cytoscape software with ClueGo plug-in based on the Reactome pathway database. Statistical significance was set at P<0.05.
Results
Patient characteristics
The characteristics of the five patients with iMCD are shown in Table 1. The median age at blood sampling before treatment was 54 years (interquartile range, 43–60 years). One patient was female (20.0%). These characteristics were not different from healthy controls (42 years old, male; 49 years old, female; 78 years old, male). Three patients with iMCD received steroids, while two received tocilizumab. The details of histological findings, baseline chest imaging, and comprehensive pulmonary function test are shown in the Appendix 1.
Table 1
| No. | Sex | Age (years) | PS | Trigger for consultation | Biopsy site | Extra pulmonary symptoms | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 60 | 2 | Dyspnea on exertion | Mediastinal lymph node left upper and lower pulmonary lobe | Moderate anemia, mild hypoalbuminemia | Tocilizumab | Alive (6.6 years) |
| 2 | M | 36 | 1 | Hyperproteinemia | Right upper and inguinal pulmonary lobe and skin | Moderate anemia, severe hypoalbuminemia, renal dysfunction (nephrotic syndrome), skin lesion | Steroid | Alive (2.9 years) |
| 3 | M | 67 | 2 | Abnormal shadow | Mediastinal lymph node | Renal dysfunction (elevated creatinine level) | Tocilizumab | Alive (2.0 years) |
| 4 | F | 43 | 2 | Proteinuria, Hematuria | Right upper and lower pulmonary lobe | Renal dysfunction (mesangial proliferative glomerulonephritis) | Steroid | Alive (3.1 years) |
| 5 | M | 54 | 1 | Rash | Left upper and lower pulmonary lobe | Skin lesion, renal dysfunction | Steroid | Alive (3.0 years) |
PS, performance status; M, male; F, female.
Evaluation of LRG concentrations
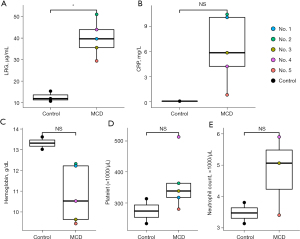
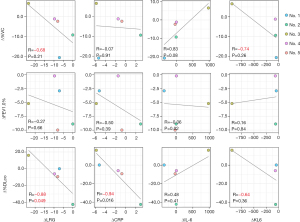
The LRG concentrations were significantly higher in treatment-naïve patients before treatment induction than in the healthy controls (P=0.035). Although the other factors such as CRP level, hemoglobin level, platelet count, and neutrophil count differed between iMCD patients and healthy controls, especially IL-6 dependent factors such as CRP level, hemoglobin level, and platelet count were higher in iMCD patients (Figure 1). Concerning the delta difference (referred to as ∆ below) before treatment and during remission, the ∆LRG concentration was significantly correlated with ∆%DLco (r=−0.88, P=0.049). Additionally, the ∆LRG concentration tended to correlate with ∆%VC (r=−0.68, P=0.21), although not significantly. However, although the ∆CRP concentration was significantly correlated with ∆%DLco (r=−0.94, P=0.016), a relationship between ∆CRP and ∆%VC was not detected. The ∆KL-6 concentration tended to be correlated with ∆%VC and ∆%DLco; however, these relationships were not significant. Further, the ∆IL-6 concentration was not correlated with respiratory function (Figure 2). These results implied that LRG concentration could be a biomarker of MCD-associated pulmonary lesions.
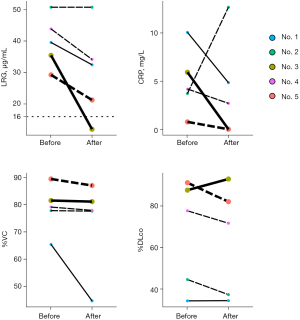
Subsequently, we focused on whether LRG and CRP behaved differently with respect to the clinical course regarding respiratory function. In patient No. 3, both the LRG and CRP levels decreased below the threshold after the induction of tocilizumab, while in patient No. 5, the LRG level did not decrease below the threshold, although the CRP level decreased below the threshold after steroid use. Further, in patient No. 3, the %VC remained steady and the %DLco increased. However, the %VC and %DLco worsened in patient No. 5 (Figure 3). This result suggested that LRG has the potential to detect smoldering inflammation or progressive disease under anti-inflammatory treatment, which could not be detected by CRP estimation due to masking.
Changes were evaluated for biochemical, symptoms, and radiographic findings to evaluate the correlation of LRG and other factors before and after treatment such as steroid and tocilizumab (Table S1, Figure S1). LRG tended to correlate with biochemical values such as hemoglobin, albumin, and CRP, although not with radiological findings and symptoms changes as observed in CRP. Subsequently, we scrutinized the relationships with ∆LRG and ∆CHAP scores, proposed as the scoring system for disease activity (21). The ∆LRG tended to correlate with ∆the CHAP score, although not statistically significant due to the small sample size (Figure S2).
The correlation between LRG levels and iMCD severity based on Castleman’s Disease Collaborative Network Criteria was evaluated (22). Only patient No. 5 was classified as severe iMCD because of renal dysfunction and pulmonary involvement. However, the LRG levels of this patient were not the highest. This may imply that LRG can be a useful individual biomarker but cannot reflect disease severity across individuals.
Cytokine and chemokine quantification
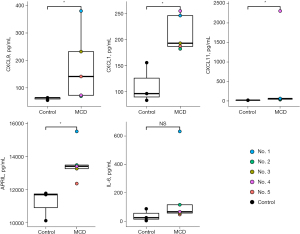
Cytokine and chemokine levels were evaluated in treatment-naïve patients compared to healthy controls. The concentrations of chemokines such as CXCL9, CXCL11, and CXCL1 (all P<0.05) were higher in the patient group than in the healthy control group. In addition, the concentration of APRIL, which is known to be associated with the proliferation of plasma cells, was significantly higher in patients with iMCD than in healthy controls (P<0.05). The IL-6 levels also tended to be higher in patients with iMCD than in the controls, but the difference was not significant (P=0.25) (Figure 4). No other cytokines or chemokines significantly differed between the two groups (Table S2).
Enrichment analysis
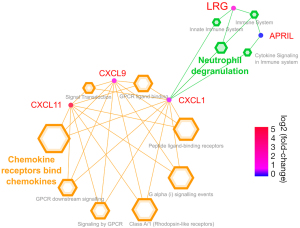
The pathway analysis indicated that LRG and chemokines were linked via neutrophil degranulation (Figure 5).
Discussion
The present study aimed to evaluate the utility of serum LRG levels for monitoring disease activity in patients with iMCD with pulmonary involvement. The results indicated that serum LRG levels could reflect disease activity with respect to respiratory function and have the potential to detect smoldering inflammation under anti-inflammatory treatment. In addition, pathway analysis implied that the pathway of LRG upregulation in patients with iMCD differed from that of CRP upregulation. Enrichment analysis showed a higher LRG level was induced via CXCL1 associated with the neutrophil degranulation pathway. In this study, other chemokines, such as CXCL11 and CXCL9, were significantly increased in patients with iMCD. Previous studies have noted the greater importance of chemokines relative to cytokines for monitoring the disease activity of patients with iMCD. In particular, CXCL13 was identified as a representative of iMCD flares using proteomics. Elevated CXCL13 can trigger B cells to mature into plasma cells, frequently observed in histological samples (23). One interesting finding of the current study was that APRIL levels were significantly higher in patients with iMCD than in controls. This implied that increased plasma cells in patients with iMCD were introduced via CXCL13 and APRIL.
The delta difference in serum LRG levels before and after treatment reflected respiratory function changes, such as % forced vital capacity and %DLco. Regarding ventilatory impairment in iMCD, a previous report demonstrated that iMCD patients with pulmonary involvement presented with obstructive ventilatory impairment (4/13) and impaired gas exchange (12/13) (24). In this study, over 50% (3/5) of the patients presented with the same type of ventilatory impairment. The serum CRP level did not reflect both factors but was correlated with %DLco. Thus, LRG may be a better biomarker of iMCD with pulmonary involvement.
This study has a few limitations. First, in this observational study, bias may have been present in patient selection. Because we could not distinguish pulmonary manifestation of iMCD and lung abnormal shadow of other comorbidities by image findings, we focused on histologically-confirmed iMCD patients with thoracic lesions. However, pulmonary manifestation is not a common manifestation of iMCD. The patients with thoracic lesions who needed biopsy were rare, resulting in selection bias caused by small sample size. Additionally, we could not evaluate patients who were not histologically examined. Patients with subtle pulmonary lesions were also excluded from the study.
Second, because we used serum collected in the biobank, the data of hemoglobin, platelet, and neutrophil count of one of three healthy control patients were missing. These could underestimate the difference between the five iMCD patients and healthy controls. Third, we could not evaluate whether LRG levels can be used to follow patients longitudinally because none of the five patients developed progressive disease based on CDCN response criteria. Fourth, the specificity of LRG for monitoring of pulmonary manifestation of iMCD was not confirmed because iMCD with and without pulmonary involvement or other mimickers such as HHV8 positive MCD and POEMS syndromes were not compared. Fifth, this study was a single-center cohort study conducted in Japan; thus, the results may not completely represent the entire population of patients with iMCD with pulmonary involvement. A multicenter, international prospective study will be needed in future research to resolve these limitations.
Conclusions
LRG could detect inflammation, such as deterioration of pulmonary function, during anti-inflammatory treatment and may be more useful than CRP for monitoring disease activity in patients with iMCD with pulmonary involvement.
Acknowledgments
We thank Mana Nakayama for collecting the samples.
Funding: This work was supported by the Japan Society for the Promotion of Science KAKENHI [JP19K08650 to YT; JP18H05282 to AK]; Center of Innovation program from the Ministry of Education, Culture, Sports, Science and Technology of Japan [to AK]; Japan Agency for Medical Research and Development [J200705023, J200705710, J200705049, JP18cm016335, and JP18cm059042 to AK]; Kansai Economic Federation grant [to AK]; and Mitsubishi Zaidan grants [to AK]. This study was supported in part by SEKISUI CHEMICAL (Tokyo, Japan), which estimated serum LRG levels.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1973/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1973/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1973/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1973/coif). AK reports grants from the Japan Society for the Promotion of Science KAKENHI [JP19K08650 to YT; JP18H05282]; Center of Innovation program from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Japan Agency for Medical Research and Development [J200705023, J200705710, J200705049, JP18cm016335, and JP18cm059042]; Kansai Economic Federation grant; and Mitsubishi Zaidan. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Munshi N, Mehra M, van de Velde H, et al. Use of a claims database to characterize and estimate the incidence rate for Castleman disease. Leuk Lymphoma 2015;56:1252-60. [Crossref] [PubMed]
- Masaki Y, Kawabata H, Fujimoto S, et al. Epidemiological analysis of multicentric and unicentric Castleman disease and TAFRO syndrome in Japan. J Clin Exp Hematop 2019;59:175-8. [Crossref] [PubMed]
- Liu AY, Nabel CS, Finkelman BS, et al. Idiopathic multicentric Castleman's disease: a systematic literature review. Lancet Haematol 2016;3:e163-75. [Crossref] [PubMed]
- Yoshizaki K, Matsuda T, Nishimoto N, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood 1989;74:1360-7. [Crossref] [PubMed]
- Dispenzieri A, Fajgenbaum DC. Overview of Castleman disease. Blood 2020;135:1353-64. [Crossref] [PubMed]
- Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood 2005;106:2627-32. [Crossref] [PubMed]
- van Rhee F, Wong RS, Munshi N, et al. Siltuximab for multicentric Castleman's disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol 2014;15:966-74. [Crossref] [PubMed]
- Fajgenbaum DC. Novel insights and therapeutic approaches in idiopathic multicentric Castleman disease. Hematology Am Soc Hematol Educ Program 2018;2018:318-25. [Crossref] [PubMed]
- Takahashi N, Takahashi Y, Putnam FW. Periodicity of leucine and tandem repetition of a 24-amino acid segment in the primary structure of leucine-rich alpha 2-glycoprotein of human serum. Proc Natl Acad Sci U S A 1985;82:1906-10. [Crossref] [PubMed]
- Naka T, Fujimoto M. LRG is a novel inflammatory marker clinically useful for the evaluation of disease activity in rheumatoid arthritis and inflammatory bowel disease. Immunol Med 2018;41:62-7. [Crossref] [PubMed]
- Serada S, Fujimoto M, Ogata A, et al. iTRAQ-based proteomic identification of leucine-rich alpha-2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann Rheum Dis 2010;69:770-4. [Crossref] [PubMed]
- Ladd JJ, Busald T, Johnson MM, et al. Increased plasma levels of the APC-interacting protein MAPRE1, LRG1, and IGFBP2 preceding a diagnosis of colorectal cancer in women. Cancer Prev Res (Phila) 2012;5:655-64. [Crossref] [PubMed]
- Sandanayake NS, Sinclair J, Andreola F, et al. A combination of serum leucine-rich α-2-glycoprotein 1, CA19-9 and interleukin-6 differentiate biliary tract cancer from benign biliary strictures. Br J Cancer 2011;105:1370-8. [Crossref] [PubMed]
- Andersen JD, Boylan KL, Jemmerson R, et al. Leucine-rich alpha-2-glycoprotein-1 is upregulated in sera and tumors of ovarian cancer patients. J Ovarian Res 2010;3:21. [Crossref] [PubMed]
- Okano T, Kondo T, Kakisaka T, et al. Plasma proteomics of lung cancer by a linkage of multi-dimensional liquid chromatography and two-dimensional difference gel electrophoresis. Proteomics 2006;6:3938-48. [Crossref] [PubMed]
- Honda H, Fujimoto M, Miyamoto S, et al. Sputum Leucine-Rich Alpha-2 Glycoprotein as a Marker of Airway Inflammation in Asthma. PLoS One 2016;11:e0162672. [Crossref] [PubMed]
- Kentsis A, Ahmed S, Kurek K, et al. Detection and diagnostic value of urine leucine-rich α-2-glycoprotein in children with suspected acute appendicitis. Ann Emerg Med 2012;60:78-83.e1. [Crossref] [PubMed]
- Serada S, Fujimoto M, Terabe F, et al. Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm Bowel Dis 2012;18:2169-79. [Crossref] [PubMed]
- Fujimoto M, Serada S, Suzuki K, et al. Leucine-rich α2 -glycoprotein as a potential biomarker for joint inflammation during anti-interleukin-6 biologic therapy in rheumatoid arthritis. Arthritis Rheumatol 2015;67:2056-60. [Crossref] [PubMed]
- Fajgenbaum DC, Uldrick TS, Bagg A, et al. International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood 2017;129:1646-57. [Crossref] [PubMed]
- Fujimoto S, Koga T, Kawakami A, et al. Tentative diagnostic criteria and disease severity classification for Castleman disease: A report of the research group on Castleman disease in Japan. Mod Rheumatol 2018;28:161-7. [Crossref] [PubMed]
- van Rhee F, Voorhees P, Dispenzieri A, et al. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood 2018;132:2115-24. [Crossref] [PubMed]
- Pierson SK, Stonestrom AJ, Shilling D, et al. Plasma proteomics identifies a ‘chemokine storm’ in idiopathic multicentric Castleman disease. Am J Hematol 2018;93:902-12. [Crossref] [PubMed]
- Huang H, Feng R, Li J, et al. Castleman disease-associated diffuse parenchymal lung disease: A STROBE-compliant retrospective observational analysis of 22 cases in a tertiary Chinese hospital. Medicine (Baltimore) 2017;96:e8173. [Crossref] [PubMed]


