Predilection of contralateral upper lung metastasis in upper lobe lung adenocarcinoma patients
Introduction
Some types of cancer occur in certain regions and tend to spread to predilection organs or locations. Lung cancer occurs predominantly in the upper lobes and the prevalence of lung cancer is increasing (1). A recent finding demonstrated that the majority of invasive lung adenocarcinomas occur and are recurrent in the upper lobes (2). Lung cancer with lung to lung metastasis is frequently encountered in daily practice and owing to the availability of computed tomography (CT) scan it can be readily detected. Due to systemic spreading to the lungs through the bloodstream, the distribution can be similar to pulmonary metastases from other non-lung cancer malignancies. That is to say, on CT scans, lung to lung metastases by lung cancer will be most commonly seen in the outer third of the lungs, especially the subpleural regions of the lower lobes (3-5). However, in a study by Onuigbo WI, one lung cancer patient had contralateral lung metastases confined to the upper lobe after necropsy (6).
In East Asia, there are several unique characteristics of lung cancer (7), including the predominance of adenocarcinoma over other cell types, as well as a large proportion of never smokers and female patients. Moreover, the incidences of activating mutations of oncogenic drivers in Western and Asian populations are different, especially the epidermal growth factor receptor (EGFR) and KRAS mutations (8-10).
Although important advances have been made in our understanding of the molecular mechanisms underlying lung cancer, few recent studies have investigated the patterns and mechanisms of contralateral lung metastasis in lung adenocarcinoma.
The main objective of this study was to investigate the association among the distribution of contralateral lung metastases versus primary lung tumor, the clinical characteristics of affected patients, and EGFR mutation status.
Methods
Study population
This retrospective study included patients with lung adenocarcinoma who were diagnosed between January 2012 and December 2013 at Taichung Veterans General Hospital (TCVGH), Taiwan. To be eligible for the study, patients were required to have treatment-naïve and pathologically confirmed stage IV lung adenocarcinoma with contralateral lung metastases according to the 7th edition of the American Joint Committee for Cancer (AJCC) staging system (11) and available tumor specimens for EGFR mutation genetic analysis. Patients were excluded if they had lung cancer with histology other than adenocarcinoma, including those with carcinoma not otherwise specified (NOS), synchronous multiple primary lung cancers [according to Martini and Melamed’s criteria (12)], or other active malignancy. Chest CT studies, including the liver and adrenal glands, were reviewed by two chest physicians. Lung cancer histology was classified according to the World Health Organization criteria (13). Demographic characteristics and clinical data, including age, gender, tumor stage, primary tumor location, performance status, smoking status, and EGFR mutation status were collected for analysis. A never smoker was defined as someone who had never smoked or smoked less than 100 cigarettes in his or her lifetime. This study was approved by the Institutional Review Board (IRB) of TCVGH, Taiwan. Written informed consent for genetic testing and clinical data records were obtained from all patients.
EGFR mutation test
EGFR mutations were tested by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS), as described in our previous report (14). All tests were performed at the ISO15189-certified TR6 Pharmacogenomics Lab (PGL), as part of the National Research Program for Biopharmaceuticals (NRPB), in the National Center of Excellence for Clinical Trial and Research of NTUH.
Classification of primary tumor location and contralateral lung metastases
Chest CT scans were reviewed to identify the location of the primary tumor. Primary lung tumors in the right upper, right middle and left upper lobes (LUL) were classified as the “upper lobe” group. Those in the right lower and left lower lobes (LLL) were included in “lower lobe” group.
The distributions of contralateral lung metastases were divided into three patterns according to the findings of the chest CT scans: upper lung predominance when the numbers of metastases of the upper lobes were more than those of the lower lobes; lower lung predominance when the numbers of metastases of the lower lobes were more than those of upper lobes; and equal distributions were denoted when the numbers of metastases were equally distributed between the upper and lower lobes (Figure 1).
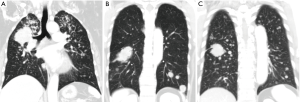
Statistical analyses
Statistical analysis was used to identify the different factors associated with lobar distribution of contralateral lung metastases. Clinical data for analysis included patients’ age, gender, tumor stage, primary tumor location, performance status, smoking status, and EGFR mutation status. Tumor, node, and metastases (TNM) staging was done according to the 7th edition of the AJCC staging system (11). Associations between patients’ characteristics and pattern of lung metastases were assessed using Pearson’s Chi-square test or Fisher’s exact test. Multivariate analyses of the association between primary tumor location and pattern of contralateral lung metastases were performed using a logistic regression model. All statistical tests were done with SPSS 15.0 (SPSS, Chicago, IL, USA). Two-tailed tests were used and P values <0.05 were considered significant.
Results
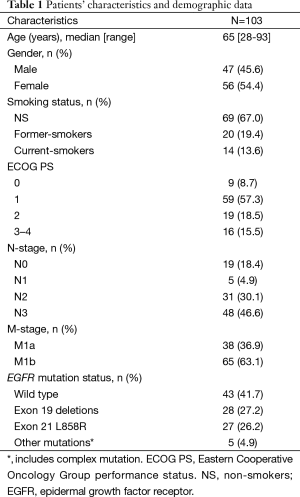
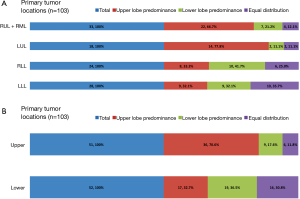
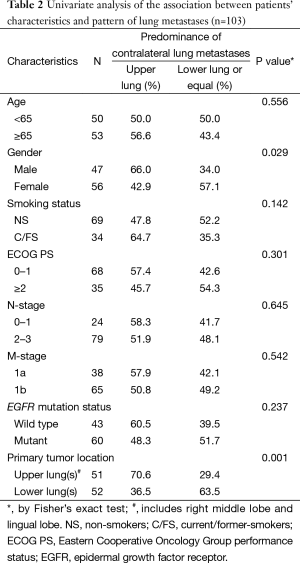
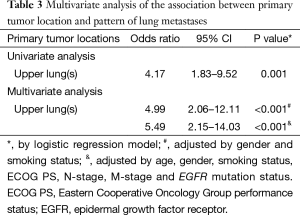
From January 2012 to December 2013, a total of 103 patients with treatment-naïve lung adenocarcinoma and contralateral lung metastases were enrolled after excluding lung cancer with histology other than adenocarcinoma, including those with carcinoma NOS, synchronous multiple primary lung cancers, or other active malignancy. The baseline characteristics are shown in Table 1. Briefly, the median age was 65 years (range, 28–93 years), 47 patients (45.6%) were male, 69 patients (67.0%) were non-smokers (NS), 68 patients (66.0%) had Eastern Cooperative Oncology Group performance status (ECOG PS) 0–1, 38 patients (38.9%) were M1a, and 60 patients (58.3%) had EGFR mutation. The primary tumor locations and the distribution of contralateral lung metastases are summarized in Figure 2. The numbers of contralateral lung metastases ranges from five to numerous. There were 51 (49.5%) lung cancer patients with primary tumors located over the upper lobes. Among them, 36 (70.6%) had contralateral upper lung predominance metastasis, 9 (17.6%) had lower lung predominance, and 6 (11.8%) had equal distribution; while among the 52 patients with primary tumors located over lower lobes, 17 (32.7%) had contralateral upper lung predominance metastasis, 19 (36.5%) had lower lung predominance, and 16 (30.8%) had equal distribution. The univariate analysis of the association between patients’ characteristics and patterns of contralateral lung metastases is shown in Table 2. Among the variables, only male gender and primary tumor location over the upper lobes were significantly associated with contralateral metastases with upper lung predominance. The multivariate analysis revealed that only primary tumor location over the upper lobes was significantly associated with the contralateral upper lung predominance metastases (adjusted OR 5.49, 95% CI, 2.15–14.03, P<0.001) (Table 3).
Full table
Full table
Full table
Discussion
In this study, “upper lobe” lung adenocarcinoma was significantly associated with contralateral upper lung predominance metastases. This result was in contrast to the distribution of pulmonary metastases from other non-lung cancer malignancies which are predominantly found over the subpleural regions of the lower lobes (3-5). There are few studies on contralateral lung metastases from lung cancer (15,16). Feld et al. analyzed 390 stages I NSCLC patients receiving postoperative adjuvant treatment in the 1980s. Among them, 158 patients had disease recurrence and 20 patients (13%) had contralateral lung metastases with more in non-squamous/mixed subtypes (15). Mountain et al., in a series of 396 patients undergoing complete resection of primary lung cancer between 1965 and 1976, observed that 151 patients (38%) had recurrence, with contralateral lung metastases in 25 patients (16.6%) (16). However, there were no data in the aforementioned studies on the association of the primary tumor location with contralateral lung metastases patterns.
Lung metastasis is a multistage process in which the lung cancer tumor progresses and spreads to distant organs, most commonly through the systemic veins and pulmonary arteries. Lung adenocarcinomas typically metastasize to a range of organs, including brain, bone, lung, liver, and adrenal gland (15,17). Moreover, lung adenocarcinoma metastasis in these various sites frequently occur concomitantly (18). The phenomenon is consistent with our findings which showed multiple metastases in patients with contralateral lung metastases. A number of general mechanisms of dissemination enable lung cancer tumor cells to leave the primary tumor and reach distal organs, and one such mechanism might be influenced in part by circulation patterns. However, in addition to the influence of hematogenous dynamics, the lung cancer cells preferentially metastasize to specific organs, which suggests the existence of specific molecular interactions that favor the retention of tumor cells in these organs. Lung capillaries are lined with endothelial cells that are surrounded by a basement membrane and adjacent alveolar cells. The basement membrane is an obstacle that circulating tumor cells can bypass only by expressing specific mediators of transendothelial migration (19,20).
Stephen Paget’s seed and soil hypothesis and the following phenomenon suggested that the location of metastases was not random, and indicated that “fertile soil”, “inflammatory oncotaxis”, and microvascular injury are the keys for circulating metastatic cells to allow colony establishment (21,22). However, why is the upper lobe predominance contralateral lung metastases in upper lobe lung cancer? The majority of invasive lung adenocarcinomas occur and are recurrent in the upper lobes even in never-smokers (2). The relatively poor perfusion of the upper lung regions results in slower lymphatic drainage along the peribronchial system. Gurney et al. postulated that this process might result in higher particle concentrations, subsequently predisposing those regions to lung disease (23). Alternatively, physiologic variables such as differences in perfusion could lead to a variation in immune surveillance, angiogenesis, or other mechanisms that affect early tumor development and invasion (2). Inhaled air pollutants such as particulate matter measuring 2.5 to 0.5 micrometers in size (PM2.5 or PM0.5) can damage pulmonary epithelial cells and vascular endothelial cells (24-26). Particulate matter can induce marked increases in vascular permeability via ROS-mediated calcium leakage, via activated TRPM2, and via ZO-1 degradation by activated calpain (26). In humans, damage to alveolar epithelial cells, macrophages, vascular endothelial cells, and the blood-gas barrier by nanomaterials was observed using electron microscopy and energy dispersive X-ray analysis (27). Particulate matter and nanomaterials can induce pulmonary inflammation, and the expression of adhesion molecules by vascular endothelial cells could be differentially affected by regions within the body. These adhesion molecules include intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1), and P or E-selectin (28-32). Previous evidence indicated that the upper lobes of the lungs of smokers contain higher extracellular concentrations of ferritin-bound iron and decreased concentrations of transferring (29). Age-long inhaled particles, chemicals, or/and other injuries may trigger differentially and accumulated responses between the upper lobes and lower lobes and cause damage-induced microvascular leakage, inflammation and adhesion molecules (P- or E-seletin, ICAM, VCAM), which may provide the niche, inflammatory oncotaxis, and promote the recruitment of circulating tumor cells to the lung (33-37).
Our study had several limitations. The predilection for the upper lung regions may not be limited to lung adenocarcinomas, as other invasive histology subtypes of lung cancer may also exhibit this phenomenon. Larger sample sizes are needed for further analysis of the distribution of contralateral lung metastases. EGFR mutation and smoking status were not associated with the distribution of contralateral lung metastasis. This finding requires further study.
Conclusions
Our findings indicate that upper lobe lung adenocarcinoma was significantly associated with contralateral upper lung predominance metastases. Further research is needed to gain a better understanding of the underlying mechanisms.
Acknowledgements
We would like to thank the Comprehensive Cancer Center of Taichung Veterans General Hospital for its assistance with the collection and management of the data.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Byers TE, Vena JE, Rzepka TF. Predilection of lung cancer for the upper lobes: an epidemiologic inquiry. J Natl Cancer Inst 1984;72:1271-5. [PubMed]
- Kinsey CM, Estepar RS, Zhao Y, et al. Invasive adenocarcinoma of the lung is associated with the upper lung regions. Lung Cancer 2014;84:145-50. [PubMed]
- Crow J, Slavin G, Kreel L. Pulmonary metastasis: a pathologic and radiologic study. Cancer 1981;47:2595-602. [PubMed]
- Davis SD. CT evaluation for pulmonary metastases in patients with extrathoracic malignancy. Radiology 1991;180:1-12. [PubMed]
- Scholten ET, Kreel L. Distribution of lung metastases in the axial plane. A combined radiological-pathological study. Radiol Clin (Basel) 1977;46:248-65. [PubMed]
- Onuigbo WI. Contralateral pulmonary metastases in lung cancer. Thorax 1974;29:132-3. [PubMed]
- Bell DW, Brannigan BW, Matsuo K, et al. Increased prevalence of EGFR-mutant lung cancer in women and in East Asian populations: analysis of estrogen-related polymorphisms. Clin Cancer Res 2008;14:4079-84. [PubMed]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [PubMed]
- Seo JS, Ju YS, Lee WC, et al. The transcriptional landscape and mutational profile of lung adenocarcinoma. Genome Res 2012;22:2109-19. [PubMed]
- Serizawa M, Koh Y, Kenmotsu H, et al. Assessment of mutational profile of Japanese lung adenocarcinoma patients by multitarget assays: a prospective, single-institute study. Cancer 2014;120:1471-81. [PubMed]
- Edge SB, Byrd DR, Compton CC, et al., editors. Cancer Staging Manual, 7th Ed. New York, NY: Springer, 2010.
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [PubMed]
- Travis WD, Brambilla E, Muller-Hermelink HK, et al., editors. World Health Organization Classification of tumours, pathology and genetics of tumours of the lung, pleura, thymus, and heart. Lyon, France: IARC Press, 2004.
- Hsu KH, Ho CC, Hsia TC, et al. Identification of five driver gene mutations in patients with treatment-naïve lung adenocarcinoma in Taiwan. PLoS One 2015;10:e0120852. [PubMed]
- Feld R, Rubinstein LV, Weisenberger TH. Sites of recurrence in resected stage I non-small-cell lung cancer: a guide for future studies. J Clin Oncol 1984;2:1352-8. [PubMed]
- Mountain CF, McMurtrey MJ, Frazier OH, et al. Present status of postoperative adjuvant therapy for lung cancer. Cancer Bulletin 1980.108-12.
- Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet 2000;355:479-85. [PubMed]
- Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer 2006;106:1624-33. [PubMed]
- Gupta GP, Nguyen DX, Chiang AC, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 2007;446:765-70. [PubMed]
- Weis S, Cui J, Barnes L, et al. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol 2004;167:223-9. [PubMed]
- Mendoza M, Khanna C. Revisiting the seed and soil in cancer metastasis. Int J Biochem Cell Biol 2009;41:1452-62. [PubMed]
- Orr FW, Adamson IY, Young L. Promotion of pulmonary metastasis in mice by bleomycin-induced endothelial injury. Cancer Res 1986;46:891-7. [PubMed]
- Gurney JW, Schroeder BA. Upper lobe lung disease: physiologic correlates. Radiology 1988;167:359-66. Review. [PubMed]
- Inoue K, Takano H, Sakurai M, et al. Pulmonary exposure to diesel exhaust particles enhances coagulatory disturbance with endothelial damage and systemic inflammation related to lung inflammation. Exp Biol Med (Maywood) 2006;231:1626-32. [PubMed]
- Wang T, Chiang ET, Moreno-Vinasco L, et al. Particulate matter disrupts human lung endothelial barrier integrity via ROS- and p38 MAPK-dependent pathways. Am J Respir Cell Mol Biol 2010;42:442-9. [PubMed]
- Wang T, Wang L, Moreno-Vinasco L, et al. Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Part Fibre Toxicol 2012;9:35. [PubMed]
- Song Y, Li X, Wang L, et al. Nanomaterials in humans: identification, characteristics, and potential damage. Toxicol Pathol 2011;39:841-9. [PubMed]
- Lee CC, Huang SH, Yang YT, et al. Motorcycle exhaust particles up-regulate expression of vascular adhesion molecule-1 and intercellular adhesion molecule-1 in human umbilical vein endothelial cells. Toxicol In Vitro 2012;26:552-60. [PubMed]
- Nelson ME, O'Brien-Ladner AR, Wesselius LJ. Regional variation in iron and iron-binding proteins within the lungs of smokers. Am J Respir Crit Care Med 1996;153:1353-8. [PubMed]
- Ramos-Godínez Mdel P, González-Gómez BE, Montiel-Dávalos A, et al. TiO2 nanoparticles induce endothelial cell activation in a pneumocyte-endothelial co-culture model. Toxicol In Vitro 2013;27:774-81. [PubMed]
- Rui W, Guan L, Zhang F, et al. PM2.5 -induced oxidative stress increases adhesion molecules expression in human endothelial cells through the ERK/AKT/NF-κB-dependent pathway. J Appl Toxicol 2016;36:48-59. [PubMed]
- Vesterdal LK, Mikkelsen L, Folkmann JK, et al. Carbon black nanoparticles and vascular dysfunction in cultured endothelial cells and artery segments. Toxicol Lett 2012;214:19-26. [PubMed]
- Walter ND, Rice PL, Redente EF, et al. Wound healing after trauma may predispose to lung cancer metastasis: review of potential mechanisms. Am J Respir Cell Mol Biol 2011;44:591-6. [PubMed]
- Coupland LA, Chong BH, Parish CR. Platelets and P-selectin control tumor cell metastasis in an organ-specific manner and independently of NK cells. Cancer Res 2012;72:4662-71. [PubMed]
- DeLisser H, Liu Y, Desprez PY, et al. Vascular endothelial platelet endothelial cell adhesion molecule 1 (PECAM-1) regulates advanced metastatic progression. Proc Natl Acad Sci U S A 2010;107:18616-21. [PubMed]
- Gassmann P, Kang ML, Mees ST, et al. In vivo tumor cell adhesion in the pulmonary microvasculature is exclusively mediated by tumor cell--endothelial cell interaction. BMC Cancer 2010;10:177. [PubMed]
- Taranova AG, Maldonado D 3rd, Vachon CM, et al. Allergic pulmonary inflammation promotes the recruitment of circulating tumor cells to the lung. Cancer Res 2008;68:8582-9. [PubMed]


