Clinical pathway for surgical treatment of primary lung cancer (2012 Edition)
I. Clinical pathway for surgical treatment of primary lung cancer: standard hospitalization
(A) Subjects applicable
1. Patients with the first diagnosis as primary lung cancer (ICD-10: C34/D02.2).
2. Patients with stage I, stage II, or completely resectable stage IIIA non-small cell lung cancer (NSCLC) (UICC 2009).
3. Patients with T1-2N0 M0 small cell lung cancer (UICC 2009).
4. Patients undergoing partial pneumonectomy, lobectomy, total pneumonectomy, or exploratory thoracotomy (ICD-9-CM-3:32.29/32.3-32.5).
(B) Diagnosis
Diagnosis is based on the “Diagnosis and Treatment Practices for Primary Lung Cancer (2011)” and the “Diagnosis Practices for Primary Lung Cancer (2011)” released by the Ministry of Health of China:
1. High risk factors: smoking index >400 cigarettes/year; >45 years of age; and family history of lung cancer.
2. Clinical symptoms: The early symptoms are often non-specific. The common symptoms include irritable cough, hemosputum or hemoptysis, chest pain, shortness of breath, and fever.
3. Auxiliary examination: chest radiography, blood tumor marker determination, sputum cytology, and fiberoptic bronchoscopy.
4. Definitive diagnosis is based on pathological findings (cytologic or histologic findings).
(C) Selection of treatment options
Treatment is according to the “Diagnosis and Treatment Practices for Primary Lung Cancer (2011)” released by the Ministry of Health of China:
1. Partial pneumonectomy (including pulmonary wedge resection and segmentectomy).
2. Lobectomy (including composite lobectomy and bronchial sleeve resection and reconstruction of pulmonary artery).
3. Total pneumonectomy.
4. Systematic lymph node dissection or sampling should be performed during the above procedures.
Comprehensive treatment plan and imaging examinations (for clinical staging) should be completed prior to non-emergency surgical treatment. The possibility of surgical resection should be thoroughly evaluated and a surgical plan should be developed accordingly.
The principle for surgery is to achieve the complete resection of the tumor and regional lymph nodes and meanwhile retain the healthy functional lung tissue as much as possible. Video-assisted thoracoscopic surgery (VATS) is mainly feasible for stage I - II lung cancers.
(D) The standard hospital stay is ≤21 days
(E) Criteria for clinical pathway
1. The first diagnosis complies with ICD-10: C34/D02.2 (diagnosis code for lung disease).
2. The heart, lung, liver, kidney, and other organs can tolerate thoracotomy under general anesthesia.
3. Any comorbidity, if exists, does not require special treatment and/or will not affect the implementation of the clinical pathway for the first diagnosis.
(F) Preoperative preparations (≤6 days)
1. Required items for testing:
(1) Routine blood, urine, and stool tests;
(2) Coagulation function, blood group, liver function, kidney function, electrolytes, and screening for infectious diseases such as hepatitis B, hepatitis C, HIV/AIDS, and syphilis;
(3) Pulmonary function test, ECG, and arterial blood gas analysis;
(4) Sputum cytology and fiberoptic bronchoscopy;
(5) Imaging examinations: chest X-ray, chest CT (plain scan/enhanced scan), abdominal ultrasound/abdominal CT, whole body bone scan, cranial MRI, or enhanced CT.
2. The following examinations can be selected according to the patient’s condition:
(1) Mediastinoscopy or EBUS;
(2) Percutaneous needle lung biopsy;
(3) Echocardiography and 24 h dynamic electrocardiogram (Holter);
(4) Tumor markers; and
(5) Examinations for cardiovascular and cerebrovascular diseases.
3. Preoperative risk assessment.
(G) Selection and administration of prophylactic antibiotics
Antimicrobial agents should be used in compliance with “Guiding Principles for Clinical Application of Antimicrobial Agents” (MoH Medical File No.285 [2004]). Antimicrobial prophylaxis should be given 30 minutes preoperatively.
(H) Operation date: within 7 days after admission
1. Mode of anesthesia: endotracheal intubation combined with intravenous general anesthesia.
2. Surgical consumables: Surgical stapler, cutting and stapling devices, vascular clamp, hemostasis materials, etc.
3. Intraoperative medications: antimicrobial agents.
4. Blood transfusion: based on intraoperative blood loss.
5. Pathology: frozen sections.
(I) Postoperative hospital rehabilitation: ≤14 day after surgery
1. Required examination items:
(1) Routine blood tests and tests for liver function, kidney function, and electrolytes;
(2) Chest radiography (on the first postoperative day and before the removal of the chest tube) and chest CT (if necessary).
(3) Pathological examinations are performed according to the “Diagnosis and Treatment Practices for Primary Lung Cancer (2011)” released by the Ministry of Health of China.
2. Postoperative prophylactic use of antimicrobial agents should be in accordance with “Guiding Principles for Clinical Application of Antimicrobial Agents” [MoH Medical File No.285 (2004)].
3. The days and types of antimicrobial drugs can be adjusted based on the disease condition.
(J) Discharge criteria
1. The wounds heal well, or the slow healing wounds can be managed in outpatient services.
2. The vital signs are stable.
(K) Variations and causes
1. Comorbidities that may affect the surgery and need appropriate diagnosis and treatment before surgery.
2. Postoperative pulmonary infection, respiratory failure, heart failure, bronchopleural fistula, or other complication that requires prolonged treatment or whose budget exceeds the reference cost.
3. The cause that has been recognized by a senior physician.
4. Causes from the patient and other aspects.
(L) Reference cost
30,000-50,000 RMB Yuan (VATS: 40,000-60,000 RMB Yuan).
II. Clinical pathway for bronchogenic carcinoma
Subjects applicable
Patients with the first diagnosis as bronchogenic carcinoma (ICD-10: C34/D02.2).
Partial pneumonectomy/lobectomy/total pneumonectomy plus systematic lymph node dissection and exploratory thoracotomy (ICD-9-CM-3:32.29/32.3-32.5).
Name: _________Gender: _________Age: _________Outpatient number: _________Inpatient number: _________
Date of admission: _________YYYY _________MM _________DD
Date of discharge: _________YYYY _________MM _________DD
Standard hospital stay: 12-21 days (Table 1)
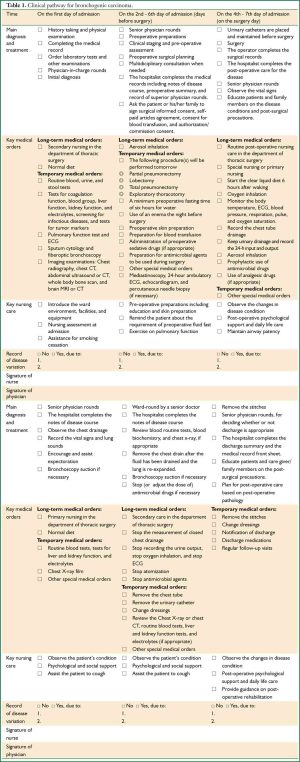
Full table
Acknowledgements
We would like to thank Secretary of the expert panel, Dr Dong-hong Chen, MD, PhD, Xuanwu Hospital of Capital Medical University, Beijing, China.
Disclosure: The authors declare no conflict of interest.


