
Clinical, imaging and functional determinants of sarcoidosis phenotypes in a Greek population
Introduction
Almost 150 years after it was first described (1), sarcoidosis remains a multi system granulomatous disease of unknown etiology since its exact pathogenesis is yet to be elucidated (2). The identification of certain sarcoidosis phenotypes is an emerging field of research (3). Current phenotype definition is based on natural history, clinical and laboratory findings along with the response to therapeutic protocols (4). Identifying specific clinical phenotypes for a disorder with such heterogeneity is particularly important. The first proposals for providing validated algorithms were made by Dr Loefgren (5), Wurm (6) and Scadding (7) in the early 50s and 60s. Since then, a number of algorithms to identify clinical phenotypes, such as the A Case Control Etiologic Study of Sarcoidosis (ACCESS) study in the 90s and the World Association of Sarcoidosis and other Granulomatous Disorders (WASOG) study afterwards, have been suggested (8,9). Schupp et al. study, published in 2018 included only Caucasian patients (10) and more recently, in 2020 the Delphi expert consensus panel published guidelines about clinical phenotyping and management in sarcoidosis (11,12) based on the WASOG Clinical Outcome Status (WASOG COS) (13).
Sarcoidosis is the most common interstitial lung disease (ILD) in Greece, accounting for 34% of ILD cases (14). Nevertheless, published data about sarcoidosis phenotypes, type of onset and outcomes concerning Greek patients are limited. Therefore, the present study aims at phenotyping sarcoid patients coming from the area of Northern Greece, according to the most recently published phenotyping classifications (11). In addition, type of onset, disease course as well as specific clinical, radiological and spirometric characteristics prevalent to each phenotype were also studied. We used the Delphi expert consensus panel clinical phenotyping staging system (12) along with the Scadding radiographic criteria (7) and the WASOG COS system (13). Furthermore, we categorized our patients according to the type of disease onset and we proposed potential characteristics with prognostic value for disease outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1760/rc).
Methods
Study design and patients
In the present longitudinal study, we prospectively collected data from a cohort of patients who were already under follow up in the Sarcoidosis Outpatient Clinic of the Pulmonary Department, Aristotle University of Thessaloniki, covering the region of Northern Greece. Patients were included in the study if they had consecutive visits for at least twice a year during a 5-year period, starting from 2012 and filled in an informed consent. Out of 350 individuals regularly followed up in our Clinic, 147 Caucasian patients were included in the final analysis. The diagnosis of sarcoidosis had been set according to the proposed criteria (15). All our patients presented compatible imaging and clinical findings. Noncaseating granulomas were found in tissue biopsies. Lung biopsies (bronchial, transbronchial, lymph node) were obtained by bronchoscopy, while biopsies of other organs were obtained surgically, if necessary. Bronchial lavage analysis was also available for all the patients. Other diseases with similar histological findings of granuloma were excluded (16,17).
Patients were divided according to age by the threshold of 40 years of age. All patients were thoroughly examined for any clinical findings of pulmonary or extra-pulmonary manifestations of the disease, according to the ACCESS and WASOG recommendations (8,9). If diagnosis was not certain or an extra-pulmonary manifestation could not be characterized as disease related, a multidisciplinary approach was followed, in collaboration with radiologists, pathologists and rheumatologists. For categorization according to the type of disease onset we used the proposed classification by Schupp et al., since there are demographic similarities with our cohort (10).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study protocol was reviewed and approved by the Aristotle University of Thessaloniki Ethics Committee (No. 83/2014) and all patients filled in an informed consent form.
Assessments
Upon diagnosis, all patients had undergone chest radiography (CXR) and high-resolution computed tomography (HRCT) of the thorax. At every scheduled visit, laboratory tests included peripheral blood and urine samples for identifying liver and kidney function abnormalities and pulmonary function tests, including spirometry, lung volumes and diffusing capacity for carbon monoxide (DLCO) assessment (PFTs). PFTs were assessed by Masterscreen PFT, Jaeger, Wurzburg, Germany spirometer. Additional imaging such as abdomen ultrasound or positron emission tomography (PET) scan were performed, when required (18).
Staging was assessed using both the radiographic Scadding criteria upon diagnosis (7) and the WASOG COS criteria (13). For patient phenotyping, we used the classification proposed by Baughman et al., according to which our patients were classified into four categories: asymptomatic, acute, chronic and advanced (11). The prevalence of the six extra-pulmonary manifestations suggested by Baughman et al. (11) was assessed for each clinical phenotype. Clinical, PFTs and imaging findings were correlated to the above phenotypes.
Based on Schupp et al. (10), patients were divided according to the type of disease onset. Regarding the disease onset, patients with acute onset presented with fever, fatigue, weight loss and Löfgren syndrome (LS) (erythema nodosum, bihilar lymphadenopathy, arthritis) and those with subacute onset presented with cough, dyspnoea and chest pain. Finally, we assessed spontaneous remission and relapse after remission in correlation to clinical, radiological and spirometry findings and treatment parameters.
At the time of the present study, the recent guidelines for treatment options had not been published (12,19), therefore, all patients were treated according to the previous recommendations (20). Approval was obtained by the Aristotle University of Thessaloniki Ethics Committee and all patients filled in an informed consent.
Statistical analysis
Descriptive data were presented as mean and standard deviation for continuous variables and numbers and percentages for categorical variables. Normality of distributions was checked with the Kolmogorov-Smirnov test. Comparisons between groups were performed using t-tests or one-way analysis of variance (ANOVA) for normally distributed data and Mann-Whitney U-tests or Kruskal-Wallis tests for non-parametric data. Chi-square test (Monte Carlo) was used for categorical data. A P value <0.05 was considered statistically significant.
Results
Patient characteristics
Our cohort, followed up for 5 years, consisted of 147 patients, 92 women (62.6%) and 55 men (37.4%), mean age 48.7±13.2 years. Age of diagnosis was higher for female patients (50±11.2 vs. 46.5±15.7 years for male, P=0.001). Regarding smoking history, 76.9% of the patients were never smokers, 15.6% ex-smokers and 7.5% current smokers. Mean body mass index (BMI) was 29.5±5.2 kg/m2. The symptoms at onset and extra-pulmonary manifestations are presented in Table 1 and Figure 1.
Table 1
| Parameters | Values (n=147) |
|---|---|
| Age (years) | 48.7±13.2 |
| Gender, n (%) | |
| Male | 55 (37.4) |
| Female | 92 (62.6) |
| Symptoms, n (%) | |
| Dyspnoea | 97 (66.6) |
| Cough | 61 (41.5) |
| Fever | 34 (23.1) |
| Erythema nodosum | 27 (18.4) |
| LS | 9 (6.0) |
| Scadding stage, n (%) | |
| 0 | 1 (0.7) |
| I | 60 (40.8) |
| II | 64 (43.5) |
| III | 20 (13.6) |
| IV | 2 (1.4) |
| Treatment, n (%) | 78 (53.1) |
| Duration of treatment (months) | 27±40.3 |
The values are presented as the mean and standard deviation unless otherwise indicated. LS, Loefgren syndrome.
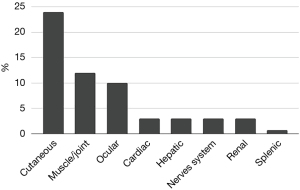
Imaging
Parenchymal involvement was found in 72.1% of the patients on the HRCT. Lymph node enlargement was the most prevalent HRCT finding (Table 2). Even though only two patients presented with stage 4 disease based on CXR, fibrosis of various extent was detected in 39% of the patients on HRCT (18% of stage I, 43% of stage II and 60% of stage III). Fibrosis was more common for men (45.5% vs. 34.8% for women, P=0.199) and patients over 40 years old (42.6% vs. 30.4% under 40 years old, P=0.161).
Table 2
| Parameters | Asymptomatic, n (%) | Acute, n (%) | Chronic, n (%) | Advanced, n (%) | P values |
|---|---|---|---|---|---|
| Patients | 58 (39.5) | 21 (14.3) | 19 (12.9) | 49 (33.3) | – |
| Scadding stage | 0.424 | ||||
| 0 | 1 (1.7) | 0 | 0 | 0 | |
| I | 30 (51.7) | 9 (42.9) | 7 (36.8) | 14 (28.6) | |
| II | 21 (36.2) | 10 (47.6) | 8 (42.1) | 25 (51.0) | |
| III | 6 (10.4) | 2 (9.5) | 4 (21.1) | 8 (16.3) | |
| IV | 0 | 0 | 0 | 2 (4.1) | |
| HRCT | |||||
| Normal | 0 | 0 | 0 | 0 | – |
| Abnormal LN | 52 (89.7) | 19 (90.5) | 18 (94.7) | 43 (87.8) | 0.880 |
| Abnormal no fibrosis | 33 (56.9) | 15 (71.4) | 17 (89.5) | 41 (83.7) | 0.005 |
| Abnormal fibrosis | 8 (13.8) | 8 (38.1) | 11 (57.9) | 30 (61.2) | <0.001 |
| PFT’s | |||||
| FVC | 0.002* | ||||
| <70% | 0 | 1 (4.8) | 1 (5.3) | 10 (20.4) | |
| 70–80% | 3 (5.2) | 2 (9.5) | 1 (5.3) | 5 (10.2) | |
| >80% | 55 (94.8) | 18 (85.7) | 17 (89.4) | 34 (69.4) | |
| DLCO | 0.007* | ||||
| <70% | 2 (3.4) | 3 (14.3) | 6 (31.6) | 16 (32.7) | |
| 70–80% | 13 (22.4) | 5 (23.8) | 4 (21.0) | 8 (16.3) | |
| >80% | 43 (74.2) | 13 (61.9) | 9 (47.4) | 25 (51.0) | |
| Symptoms | |||||
| Fever | 13 (22.4) | 7 (33.0) | 5 (26.3) | 9 (18.4) | 0.577 |
| Cough | 19 (33.0) | 10 (47.6) | 8 (42.1) | 24 (49.0) | 0.350 |
| Erythema nodosum | 9 (15.5) | 7 (33.0) | 4 (21.0) | 7 (14.0) | 0.259 |
| Dyspnoea mMRC | 0.027 | ||||
| 0 | 28 (48.3) | 5 (23.8) | 5 (26.3) | 10 (20.4) | |
| 1 | 8 (13.8) | 8 (38.1) | 8 (42.1) | 11 (22.4) | |
| 2 | 13 (22.4) | 6 (28.6) | 5 (26.3) | 19 (38.8) | |
| 3 | 9 (15.5) | 2 (9.5) | 1 (5.3) | 9 (18.4) | |
| 4 | 0 | 0 | 0 | 0 | |
| Extrapulmonary manifestations | |||||
| Heart | 0 | 0 | 1 (5.3) | 4 (8.2) | 0.070 |
| Nervous system | 0 | 0 | 1 (5.3) | 3 (6.1) | 0.196 |
| Eye | 3 (5.2) | 2 (9.5) | 2 (10.5) | 7 (14.3) | 0.480 |
| Skin | 15 (25.9) | 8 (38.0) | 2 (10.5) | 10 (20.4) | 0.199 |
| Liver | 2 (3.4) | 1 (4.8) | 0 | 2 (4.1) | 0.942 |
| Renal | 2 (3.4) | 0 | 0 | 7 (14.3) | 0.032 |
*, Mann-Whitney test. HRCT, high resolution computed tomography; LN, lymph node; PFT’s, pulmonary function tests; FVC, forced vital capacity; DLCO, diffusing capacity for carbon monoxide; mMRC, modified Medical Research Council.
PFTs
The majority of patients in our cohort presented only mildly impaired lung function, as indicated by the mean values of forced vital capacity (FVC) and DLCO predicted (96.4%±17.6% and 85%±18.7% respectively). The percentage of DLCO at the time of diagnosis was positively correlated with FVC% (r=0.523, P<0.001) (Figure 2A) and negatively correlated with the period of treatment in months (r=−0.2, P=0.015) (Figure 2B). Lung function upon diagnosis varied among different phenotypes. Severity and chronicity were in line with lung function decline (Figure 3A,3B, Table 2).
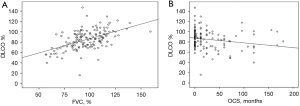
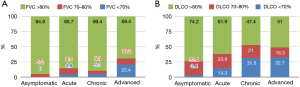
Treatment
None of our patients received second line therapy or corticosteroid sparing treatment throughout the observation period.
Type of disease onset and course
Cutaneous manifestations were more common in the acute type of onset (Table 3). Spontaneous remission was more common for stage I patients (stage 0: 2.2%, stage I: 52.2%, stage II: 37%, stage III: 8.6%, stage IV 0%, P=0.118). Patients with fibrosis on HRCT presented remission less often than those without fibrosis (19.6% vs. 80.4% non-remitting, P<0.001). Spontaneously remitting patients had FVC% >80% at diagnosis more often (95.7% vs. 4.3% non-remitting, P=0.037). Cutaneous involvement was more common in this group (Table 3).
Table 3
| Extrapulmonary manifestations | Acute onset (n=49) | Subacute onset (n=98) | P values | Spontaneous remission (n=46) | No Spontaneous remission (n=101) | P values | Relapse (n=42) | No relapse (n=105) | P values |
|---|---|---|---|---|---|---|---|---|---|
| Cardiac | 2 | 3 | 0.473 | 0 | 5 | 0.125 | 4 | 1 | 0.055 |
| Cutaneous | 25 | 10 | <0.001 | 17 | 18 | 0.0012 | 11 | 25 | 1 |
| Hepatic | 3 | 2 | 0.478 | 2 | 3 | 0.669 | 2 | 3 | 0.638 |
| Nerves system | 2 | 2 | 1 | 1 | 3 | 0.783 | 2 | 2 | 0.587 |
| Ocular | 4 | 10 | 0.691 | 2 | 12 | 0.149 | 9 | 5 | <0.001 |
| Renal | 5 | 4 | 1 | 2 | 7 | 0.545 | 4 | 5 | 0.476 |
Relapses were more common for stage II patients (stage 0: 0%, stage I: 23.8%, stage II: 54.8%, stage III: 19%, stage IV: 2.4%, P=0.041). Patients with fibrosis on HRCT relapsed more often than those without (69% vs. 31% non-relapsing, P<0.001). Regarding PFTs, 49.5% of the patients with DLCO <80% relapsed. Ocular involvement was mostly diagnosed in this group of patients (Table 3) The need for treatment upon diagnosis was observed more often for relapsing patients (69% vs. 31% untreated patients, P=0.023).
Staging
The most common radiographic stages in our cohort were stages I and II (40.8% and 43.5% respectively). The classification according to the COS instrument is presented in Table 4.
Table 4
| COS | Patients (n=147) (%) |
|---|---|
| Resolved | |
| 1. Never treated | 11 (7.5) |
| 2. No therapy >1 year | 14 (9.5) |
| Minimal disease | |
| 3. Never treated | 26 (17.7) |
| 4. No therapy >1 year | 13 (8.8) |
| Persistent | |
| No current therapy | |
| 5. Never treated | 21 (14.3) |
| 6. No therapy >1 year | 13 (8.8) |
| Current therapy | |
| No worsening prior year | |
| 7. Asymptomatic | 17 (11.6) |
| 8. Symptomatic | 24 (16.3) |
| 9. Worsening in prior year | 8 (5.5) |
COS, clinical outcome status.
Disease phenotyping
The asymptomatic phenotype was the most common in our cohort (39.5%). A proportion of 51.7% of patients of this phenotype were categorized as Scadding stage I. The absence of dyspnoea [Modified Medical Research Council Dyspnea Scale (mMRC) 0: dyspnea only with strenuous exercise] was more common for this phenotype compared to the other 3 phenotypes (asymptomatic: 48.3%, acute: 23.8%, chronic: 26.3%, advanced: 20.4%). Fibrosis was less common in the asymptomatic phenotype compared to the other ones (asymptomatic: 13.8%, acute: 38.1%, chronic: 57.9%, advanced: 61.2%, P<0.001) (Table 2).
The advanced phenotype was the second most common in our cohort (33.3%). Fifty-one percent of the patients were stage II. The most common presenting symptoms were cough (49%) and dyspnea mMRC scale 2 (39%). Renal involvement was more frequently diagnosed (Table 2).
A proportion of 14.3% of our patients were categorized in the acute phenotype and presented more symptoms upon diagnosis than those who belonged to the other phenotypes. Prevalent manifestations were cough (48%), dyspnea mMRC scale 1 (38%), fever (33%) and erythema nodosum (33%). Skin involvement was mostly found in this phenotype (38%) (Table 2).
Finally, the chronic phenotype included 12.9% of patients. Stage II was the most common stage in this phenotype (42%). Cough (42%) and dyspnoea mMRC scale 1 (42%) were more frequently diagnosed. Treatment duration in months for patients of the chronic phenotype was longer compared to the other phenotypes (72±46.5 months) (Table 2).
Discussion
In the present study we aimed to categorize our patients according to the latest phenotyping classification. Furthermore, we propose specific patient characteristics that could be potentially related to each clinical phenotype, sarcoidosis onset and disease outcomes. To our knowledge, our study is the first evolving such relations regarding phenotyping. Patients of various phenotypes presented different functional, radiological and extra-pulmonary manifestations upon diagnosis. Interestingly, clinical phenotypes were not found to be related to the Scadding staging system. The asymptomatic phenotype was characterized by lack of dyspnea (mMRC: 0), HRCT without fibrosis and relatively normal lung function. The most severe phenotypes were related to worse PFT and HRCT findings, with DLCO% <80% predicted and radiographic fibrosing lesions, upon diagnosis, being prevalent in the chronic and advanced phenotypes. Patients with fibrosis upon diagnosis relapsed more often and on the contrary, lack of fibrosis was related to spontaneous remission. Cutaneous involvement was prevalent in patients with acute onset and spontaneous disease remission. Renal involvement was shown to be related to the advanced phenotype. Ocular manifestations as well as the need for treatment upon diagnosis appeared in relapsing patients.
Every published study focusing on sarcoidosis phenotypes is based on different ways of classification of PFT results, different imaging techniques (simple CT scan, HRCT, PET scan), different tools for the assessment of extra-pulmonary manifestation and treatment options. Wasfi et al. proposed in 2006 a scoring system for the severity assessment in sarcoidosis, using clinical parameters as well as PFT’s, extra-pulmonary manifestations and treatment options (21). The authors of the study, bearing the limitations of a single center patient cohort and cross-sectional assessment, concluded that patients with the most severe clinical phenotype shared some common findings: abnormal FVC and DLCO, cardiac and neurologic manifestations and necessity for treatment other than OCS. Regarding extra-pulmonary manifestations for the advanced disease, our findings were not compatible with the results of Wasfi et al. (21) However, in our study cardiac and nervous system MRI were performed only in patients with suggestive clinical findings. Prasse et al. proposed a clinical phenotype classification system that was based mainly on the clinical course of the disease and divided their cohort in acute (40%) and non-acute (60%) sarcoidosis. In this study, 71.3% of the patients with acute disease suffered from extra-pulmonary involvement (22). In the present study 63.2% of patients with acute onset based on the Schupp criteria and 52.4% of patients with acute phenotype based on Baughman study (11) respectively presented with extra pulmonary manifestations upon diagnosis. Furthermore, the main manifestation in the acute group in the cohort described by Prasse was extensive adenopathy, arthritis, kidney and liver involvement, in contrast to the cutaneous, ocular and liver manifestations in our cohort. Papiris et al. in 2019 proposed another way of phenotyping sarcoid patients using FDG-PET scan. The study included Greek patients and proposed four different clusters of organ involvement in sarcoidosis based on imaging. Clinical manifestations were not included (23).
In our cohort, no correlation was found between the radiographic stages and the clinical phenotypes. The aforementioned outcome was expected, since the Scadding criteria do not include current precise radiographic, laboratory and clinical investigations that are now routine for most Sarcoidosis Clinics and are consistent with the recommendations of sarcoidosis experts (3). Stages I and II were the most common in our cohort. This result is relevant to a recent review of sarcoidosis epidemiology in Southern Europe (24).
LS was diagnosed only in 9 patients (6%) in our study, in contrast to the increased incidence of LS in Northern Europe and correspondingly to the results of one of our previously published study (5,25). Papiris et al. reported a higher incidence of LS percentage (29 out of 195 patients, 14.5%) (23). Published data about LS incidence in Greek patients are scarce, therefore we cannot make firm conclusions about LS incidence in the Greek population.
The classification using the COS instrument used in our study, has not been related to objective findings as PFTs and HRCT results so far. Kampstra et al. have proposed COS as one of the seven parameters (COS, mortality, pulmonary function, soluble interleukin-2 receptor change, weight gain, quality of life, osteoporosis) of a standard set of outcome measures for pulmonary sarcoidosis in everyday practice in specific centers (26). We believe that the combination of COS with other determinants could create a practical diagnostic and prognostic tool for patients and researchers. Our study was an attempt to relate those results to clinical, imaging and PFT findings.
The study bears the limitations of a single center study including patients from the same nationality and geographic region of Greece. The diagnosis and follow up was orchestrated by pulmonologists, therefore every patient recruited had pulmonary involvement, excluding those with exclusively extra-pulmonary disease. The strength of the study is that the patients were thoroughly examined at least twice a year for 5 consecutive years. We believe that our study is clinically relevant since we used the latest proposed sarcoidosis clinical phenotype recommendations by the DELPHI consensus, which are easy to apply and can be a common way of communication among sarcoidosis centers worldwide. The future perspective of this study is a cohort that includes patients from other geographic regions of Greece.
Conclusions
It is important to use effective and efficient algorithms for staging and phenotyping sarcoidosis patients, which could be applied by Sarcoidosis Clinics for monitoring patients and delivering proper therapeutic regimens. We came to the conclusion that sarcoidosis clinical phenotypes have certain clinical, imaging and functional characteristics, at initial diagnosis of the disease, which could be assessed in everyday practice.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1760/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1760/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1760/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1760/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study protocol was reviewed and approved by the Aristotle University of Thessaloniki Ethics Committee (No. 83/2014) and all patients filled in an informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sharma OP. Definition and history of sarcoidosis. Eur Respir Mon 2005;32:1-12.
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:149-73. [PubMed]
- Culver DA, Baughman RP. It's time to evolve from Scadding: phenotyping sarcoidosis. Eur Respir J 2018;51:1800050. [Crossref] [PubMed]
- Pereira CA, Dornfeld MC, Baughman R, et al. Clinical phenotypes in sarcoidosis. Curr Opin Pulm Med 2014;20:496-502. [Crossref] [PubMed]
- Grunewald J, Eklund A. Löfgren's syndrome: human leukocyte antigen strongly influences the disease course. Am J Respir Crit Care Med 2009;179:307-12. [Crossref] [PubMed]
- Wurm K. The significance of stage classification of sarcoidosis (Boeck's disease. Dtsch Med Wochenschr 1960;85:1541-8. [Crossref] [PubMed]
- Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years' observation. Br Med J 1961;2:1165-72. [Crossref] [PubMed]
- Judson MA, Baughman RP, Teirstein AS, et al. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:75-86. [PubMed]
- Judson MA, Costabel U, Drent M, et al. The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis 2014;31:19-27. [PubMed]
- Schupp JC, Freitag-Wolf S, Bargagli E, et al. Phenotypes of organ involvement in sarcoidosis. Eur Respir J 2018;51:1700991. [Crossref] [PubMed]
- Baughman RP, Scholand MB, Rahaghi FF. Clinical phenotyping: role in treatment decisions in sarcoidosis. Eur Respir Rev 2020;29:190145. [Crossref] [PubMed]
- Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev 2020;29:190146. [Crossref] [PubMed]
- Baughman RP, Nagai S, Balter M, et al. Defining the clinical outcome status (COS) in sarcoidosis: results of WASOG Task Force. Sarcoidosis Vasc Diffuse Lung Dis 2011;28:56-64. [PubMed]
- Karakatsani A, Papakosta D, Rapti A, et al. Epidemiology of interstitial lung diseases in Greece. Respir Med 2009;103:1122-9. [Crossref] [PubMed]
- Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2020;201:e26-51. [Crossref] [PubMed]
- Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012;185:1004-14. [Crossref] [PubMed]
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet 2014;383:1155-67. [Crossref] [PubMed]
- Keijsers RGM, Grutters JC. In Which Patients with Sarcoidosis Is FDG PET/CT Indicated? J Clin Med 2020;9:890. [Crossref] [PubMed]
- Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J 2021;58:2004079. [Crossref] [PubMed]
- Pande A, Culver DA. Knowing when to use steroids, immunosuppressants or biologics for the treatment of sarcoidosis. Expert Rev Respir Med 2020;14:285-98. [Crossref] [PubMed]
- Wasfi YS, Rose CS, Murphy JR, et al. A new tool to assess sarcoidosis severity. Chest 2006;129:1234-45. [Crossref] [PubMed]
- Prasse A, Katic C, Germann M, et al. Phenotyping sarcoidosis from a pulmonary perspective. Am J Respir Crit Care Med 2008;177:330-6. [Crossref] [PubMed]
- Papiris SA, Georgakopoulos A, Papaioannou AI, et al. Emerging phenotypes of sarcoidosis based on 18F-FDG PET/CT: a hierarchical cluster analysis. Expert Rev Respir Med 2020;14:229-38. [Crossref] [PubMed]
- Brito-Zerón P, Kostov B, Superville D, et al. Geoepidemiological big data approach to sarcoidosis: geographical and ethnic determinants. Clin Exp Rheumatol 2019;37:1052-64. [PubMed]
- Papakosta D, Kyriazis G, Gioulekas D, et al. Variations in alveolar cell populations, lymphocyte subsets and NK-cells in different stages of active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2005;22:21-6. [PubMed]
- Kampstra NA, Grutters JC, van Beek FT, et al. First patient-centred set of outcomes for pulmonary sarcoidosis: a multicentre initiative. BMJ Open Respir Res 2019;6:e000394. [Crossref] [PubMed]


