
Regional anesthesia and acute perioperative pain management in thoracic surgery: a narrative review
Introduction
Thoracic surgery causes significant postoperative pain, and an effective analgesic strategy in the perioperative period is essential to ensure optimal patient comfort, avoid postoperative complications, and enhance recovery. Disruption of respiratory mechanics by thoracic procedures leads to a restrictive ventilation pattern with decreased functional residual capacity, decreased compliance, and atelectasis. The detrimental effects are amplified in high-risk patients with pre-existing cardiopulmonary disease, obesity, and advanced age. Inadequate analgesia amplifies the negative effects of splinting including reduced tidal volumes, impaired cough and clearance of secretions, and increased atelectasis. Altogether, this may increase the risk of postoperative pulmonary complications including hypoxemia, hypercarbia, pneumonia, possible need for prolonged mechanical ventilatory support, reintubation, and intensive care unit (ICU) admission (1,2). Poorly-controlled acute pain may also increase the risk of chronic post-thoracotomy pain syndrome (CPTPS) after thoracotomy and after video-assisted thoracic surgery (VATS), which can be difficult to treat and have significant impacts on a patient’s quality of life for months to years (3).
Thoracotomy requires a long incision anteriorly or posterolaterally. It may involve division of the latissimus dorsi, serratus anterior, and intercostal muscles with separation and sometimes resection of ribs. This causes traumatic injury to the chest wall and excess pressure on the neurovascular bundle during retraction. Thoracoscopic approaches including VATS and robot-assisted thoracic surgery (RATS) involve smaller incisions and the use of trocars for video and instrument access to the chest cavity, and oftentimes mini-thoracotomies for access and specimen removal. These minimally invasive techniques confer less disruption of chest wall muscles and mechanics, potentially less damage to intercostal nerves, and are associated with decreased postoperative pain, opioid consumption, and reduced incidence of postoperative complications. Nevertheless, VATS still causes significant postoperative pain and carries similar risks of postoperative complications as thoracotomy (4-8).
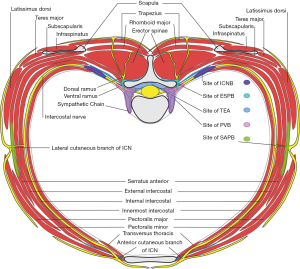
Multiple pathways are involved in transmission of pain signals in thoracic surgery. Innervation of the thorax (Figure 1) involves thoracic spinal nerves, with dorsal rami innervating the posterior thoracic wall and paraspinal muscles, and anterior rami becoming intercostal nerves which travel on the inferior rib surface between the innermost and internal intercostal muscles, branching into the lateral and anterior cutaneous nerves. The thoracic sympathetic chain runs adjacent to the spinal column in the paravertebral space and provides visceral innervation to the thorax and efferent sympathetic innervation. Other nerves relevant to the thorax include the vagus nerve, the phrenic nerve from C3-5 which innervates the diaphragm, and nerves of the brachial plexus including the dorsal scapular nerve to the rhomboids and levator scapulae, medial and lateral pectoral nerves to the pectoralis minor and major, long thoracic nerve to the serratus anterior, and thoracodorsal nerve to the latissimus dorsi (9). Severe pain could be from nociceptive, neuropathic, inflammatory, and ischemic sources, including incisional pain, damage to muscles, costovertebral joint disruption, intercostal nerve injury, and pleural disruption and inflammation. Referred ipsilateral shoulder pain (ISP) secondary to chest tubes and pleural/ diaphragmatic irritation can be present in up to 85% of patients (10).
Multimodal analgesia incorporates the use of non-opioid analgesics which have synergistic effects with opioids, providing superior analgesia and decreasing opioid requirements and side effects. This approach aims to maximize the physiologic and pharmacologic benefits of affecting multiple analgesic pathways while minimizing adverse effects and facilitating a rapid recovery to baseline (11). Consensus evidence-based guidelines on the management of postoperative pain encourage the use of multimodal analgesics as well as regional anesthesia techniques, including thoracic epidural analgesia (TEA), thoracic paravertebral blocks (PVB), and more recently novel fascial plane blocks. An effective multimodal analgesic strategy is essential to prevent the above negative effects and complications, and the optimal analgesic approach is influenced by dynamic factors including patient, surgical, and institutional considerations (12). Currently, there is no clear superior approach, especially with regards to regional anesthetic choices. Here we aim to review the available data for multimodal analgesia and regional anesthesia techniques for the management of acute postoperative pain for thoracic surgery, as well as the current challenges and future directions for further research in the field. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1740/rc).
Methods
The manuscript was prepared as a Narrative Review, with a goal of providing a broad overview of the evidence for various systemic analgesic and regional anesthesia approaches for treating pain related to thoracic surgery, rather than the more narrow and targeted focus of questions addressed in a systematic review. We searched PubMed and Google Scholar databases from inception to May 2021 using the search terms “thoracic surgery”, “thoracic surgery AND pain management”, “thoracic surgery AND analgesia”, “thoracic surgery AND regional anesthesia”, “thoracic surgery AND epidural”. We considered articles written in English and available to the reader, including prospective randomized trials, retrospective reports, review articles, expert opinion papers and society guidelines, and case reports and series. We excluded studies that were not clinically relevant to the scope of the review. Additional relevant articles suggested by peer reviewers after submission of the initial manuscript were also included when appropriate. Two authors (CH and XB) retrieved the full texts of all relevant articles, and additional references were obtained from references lists in the included articles where appropriately relevant (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | May 2021 |
| Databases and other sources searched | PubMed, Google Scholar |
| Search terms used | “thoracic surgery”, “thoracic surgery AND pain management”, “thoracic surgery AND analgesia”, “thoracic surgery AND regional anesthesia”, “thoracic surgery AND epidural” |
| Timeframe | Inception to May 2021 |
| Inclusion and exclusion criteria | Inclusion: articles written in English and available to reader, prospective randomized trials, retrospective reports, review articles, expert opinion papers, society guidelines, case reports and series. Exclusion: articles not clinically relevant to scope of review |
| Selection process | Two authors (CH and XB) retrieved full texts of relevant articles included in review |
| Any additional considerations, if applicable | Additional references obtained from reference lists in included articles, and at suggestion of peer reviewers after initial submission where appropriate |
Discussion
Multimodal/systemic analgesics
Acetaminophen
Acetaminophen is one of the most commonly used analgesics worldwide and has very few adverse effects. Although the exact analgesic mechanism of action remains unknown, it inhibits cyclooxygenase (COX) and may affect multiple central nervous system pathways involved in pain. It has little to no anti-inflammatory activity, does not affect platelet function, renal perfusion, or gastrointestinal (GI) tract mucosal integrity, and has an extremely safe profile when used in appropriate doses (13). The IV route provides a quicker onset to peak effect compared to oral (25 versus 45 minutes, respectively), however both have similar maximum analgesic activity and duration (13). A recent systematic review in a mixed surgical cohort suggests that both routes provide equivalent postoperative analgesia, with the oral formulation being less expensive; however, there are no studies specifically comparing different routes of administration in thoracic surgery (14).
Acetaminophen for thoracotomy
Acetaminophen has been shown to effectively reduce ipsilateral shoulder pain (ISP) when used perioperatively in conjunction with TEA in thoracotomy patients (10). Retrospective data in thoracic surgery patients at two academic centers suggest that IV acetaminophen is associated with reduced hospital and ICU length-of-stay (LOS) and time to extubation in thoracotomy patients (15).
Acetaminophen for VATS
Acetaminophen can significantly reduce postoperative pain in VATS patients (16). In a small randomized controlled trial (RCT) in VATS patients, IV acetaminophen was able to achieve analgesic effects close to ketorolac (17). Acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) have synergistic analgesic effects that result in much lower opioid requirements compared to either drug alone (18). Similar to thoracotomy, the same retrospective study assessing LOS, time to extubation, and ICU LOS proved acetaminophen’s benefits in VATS patients as well (15). Acetaminophen should be utilized in all patients without a strong contraindication given its efficacy and safety.
NSAIDs
NSAIDs inhibit the COX pathway to reduce the production of prostaglandins and improve pain control (19). Oral ibuprofen at a dose of 400 mg is almost as effective as 10 mg of intramuscular morphine (20), and ketorolac at a dose of 30 mg has similar analgesic effects to 10 mg of morphine as well (21,22). A meta-analysis demonstrated that preoperative celecoxib 200 mg was able to achieve an average reduction of 4 mg of morphine consumption and lead to lower pain scores and post-operative nausea and vomiting (PONV) (23). Non-specific NSAIDs such as ibuprofen and ketorolac inhibit COX-1 and COX-2 isoforms and confer possible risks of bleeding related to impaired platelet redundant function, acute kidney injury (AKI), and GI mucosal injury. Specific COX-2 inhibitors were developed last decade to have better GI tolerance and lower potential risks of bleeding, but carry similar risk of renal injury as non-selective NSAIDs (24,25). Severe adverse cardiovascular events forced several COX-2 inhibitors off market except for celecoxib.
NSAIDs for thoracotomy
The utility of NSAIDs in thoracic surgery has been known for decades. Indomethacin led to improved pain and reduced opioid consumption after thoracotomy (26). The same effects were observed with diclofenac without demonstrated risks of bleeding (27). In patients using morphine patient-controlled analgesia (PCA), ketorolac reduced postoperative opioid requirements after thoracotomy (28). Even in combination with TEA, the beneficial signals of NSAIDs can still be identified: ketorolac reduces pain and opioid consumption and may enhance pulmonary function after thoracotomy (29). Celecoxib was associated with lower pain scores at rest and with coughing and improved patient satisfaction after thoracotomy (30).
NSAIDs for VATS
Analgesic effects of NSAIDs can be observed in VATS as well. A small RCT demonstrated that diclofenac and ketorolac were equally effective in reducing opioid consumption after VATS without significant effects on bleeding (31). Interestingly, in a recent RCT, ketorolac did increase quantity of bleeding after VATS although none needed reoperation (32). Ketorolac and acetaminophen may provide similar analgesia after VATS (17), and in combination may provide comparable analgesia to morphine after VATS (32).
Based on the current literature, unless patients have contraindications including high bleeding risk, renal insufficiency, or GI bleeding, NSAIDs should be strongly considered as part of multimodal analgesia for both thoracotomy and VATS procedures (33).
N-methyl-D-aspartate (NMDA) receptor antagonists
Ketamine, a NMDA receptor antagonist, is a potent analgesic. Ketamine potentiates the analgesic effects of opioids, can improve analgesia and reduce opioid-consumption in the immediate postoperative period, may prevent acute opioid tolerance and central sensitization to nociceptive signaling, and reduce inflammation related to surgery (34-38). Interestingly, the analgesic effect of ketamine is more pronounced in certain types of operations including spine and abdominal surgery compared to others such as arthroplasty. Nevertheless, ketamine can lead to psychomimetic side effects such as hallucinations and nightmares. The prevention of delirium and complications of surgical treatment (PODCAST) study found that a single dose of ketamine in older patients led to increased incidence of hallucination and nightmares (39). Magnesium also has NMDA receptor antagonist properties and is used as a non-opioid analgesic with possible benefits including improved pain and reduced opioid requirements with few adverse effects (40-42).
NMDA receptor antagonists for thoracotomy
Ketamine has been demonstrated to effectively reduce pain scores and opioid consumption for acute thoracotomy pain either as a single bolus or mixed with morphine in PCA when TEA was not possible (43-45). The utility of ketamine when used as an adjunct to TEA is less clear. Suzuki et al. found that low dose continuous infusion of ketamine postoperatively could potentiate analgesic effects of TEA (37). However, another trial failed to find any difference in acute or chronic pain with ketamine infusions (46). Nevertheless, in the presence of a subpleural paravertebral catheter, continuous ketamine infusions may provide superior pain control (47). Magnesium may also reduce opioid consumption in thoracotomy in patients who do not receive TEA (48). There is also limited observational data that magnesium may have beneficial effects on both acute and chronic post-thoracotomy pain, however more data are needed to further investigate its effects (49).
NMDA receptor antagonists for VATS
There is limited data specifically describing the utility of ketamine for VATS. After multi-level PVB, the addition of a ketamine infusion was not found to have any beneficial effects in VATS patients (50). One small randomized trial showed that a combination fentanyl and ketamine PCA can provide similar analgesia to patient-controlled epidural analgesia (PCEA). Nevertheless, the study was confounded by intercostal nerve blocks (ICNB) in both groups as well as lack of control to assess opioid sparing effects of ketamine (51).
Given these data, ketamine may be a useful opioid-sparing adjunct in select patients, especially in those undergoing thoracotomies who are not candidates for TEA, but can be associated with adverse effects and should be used with caution, especially in older patients. Magnesium can also be considered as an adjunct with possible benefits and few adverse effects.
Gabapentinoids
Gabapentin and pregabalin inhibit voltage-gated calcium channels resulting in decreased excitatory neurotransmitter release with attenuated transmission of pain signals (52). There have been many studies evaluating the efficacy of gabapentin at reducing postoperative pain and opioid requirements, often with conflicting results (53,54). A systematic review from 2015 found an association between gabapentin and reduced postoperative pain and opioid consumption (55). Perioperative gabapentin seems to be associated with a shorter time to cessation of opioid therapy in a mixed surgical cohort (56). However, a systematic review of 281 trials which focused on clinically meaningful differences found no clinically significant effect on postoperative pain for pregabalin or gabapentin despite small but statistically significant reductions in pain scores (57). In addition, a large retrospective propensity score-matched study found that gabapentin was associated with respiratory depression, with a greater effect noted in older patients who had received intraoperative opioids (58), and both gabapentin and pregabalin may also cause increased sedation (53,55).
Gabapentinoids for thoracotomy
A small RCT by Sattari et al. found that, in the absence of TEA, preoperative single-dose pregabalin is able to reduce pain scores and opioid consumption over the first 24 hours, although the percent change in pain scores was small (59). Omran et al. observed that gabapentin administrated preoperatively and continued two days postoperatively was associated with decreased pain scores and opioid consumption in the presence of TEA (60). However, a larger prospective trial of patients undergoing thoracotomy found no benefit of gabapentin when used as an adjunct to TEA (61). These results were repeated in a prospective trial of pediatric patients undergoing thoracic surgery with TEA (62).
Gabapentinoids for VATS
There are limited data on the use of gabapentin specifically for VATS. Results from a mixed cohort study did not support the use of gabapentin in VATS; a single dose of pregabalin was found to lower pain scores but not opioid consumption in healthy young VATS patients (63). The efficacy of gabapentinoids in preventing chronic pain after thoracic surgery is still in debate (64,65).
Given the questionable clinically significant benefit and potential adverse effects of sedation, dizziness, and synergistic respiratory depression with opioids, gabapentinoids should not be routinely used in the acute setting for thoracic surgery (66).
Lidocaine
Inflammatory changes after surgery are associated with adverse outcomes including pain, cognitive dysfunction, arrhythmias, and AKI. IV lidocaine has been shown to reduce inflammation, with mixed data regarding the clinical significance on reduced postoperative pain and opioid requirements (67,68).
Lidocaine for thoracotomy
There is limited data on the use of IV lidocaine for thoracotomy. A small randomized trial of IV lidocaine infusion versus placebo for thoracic surgery which did not detail surgical approach showed reduced pain scores and lower opioid requirements in the first six hours in the PACU (69). However, a smaller study limited by sample size did not show any difference in postoperative pain or opioid consumption (70). Interestingly, pre-emptive lidocaine patches applied three days prior to surgery were able to reduce postoperative pain at rest and with coughing as well as opioid consumption (71).
Lidocaine for VATS
Unlike thoracotomy, several randomized studies did not find any differences in opioid requirements or pain scores up to 48 hours after VATS nor improvement of quality of recovery (72,73). There is currently an ongoing prospective trial to investigate IV lidocaine compared to paravertebral lidocaine for VATS on various outcomes including postoperative complications, inflammation, and pain (74).
Due to negative data in VATS patients and potential risk of local anesthetic systemic toxicity, IV lidocaine should not be considered as a part of a multimodal strategy unless patients are undergoing thoracotomy and regional anesthetics are not utilized.
Corticosteroids
Glucocorticoids including dexamethasone are often used perioperatively for PONV prophylaxis, and they also confer analgesic benefits likely by reducing inflammation. Multiple systematic reviews have demonstrated reduced postoperative pain scores, shorter PACU stays, and lower opioid requirements in patients who received intraoperative dexamethasone (75,76). There is no evidence to suggest an increased risk of infections or delayed wound healing, however hyperglycemia is a known side-effect of glucocorticoids (77). There is conflicting data on the optimal dose, with an initial systematic review showing analgesic effects at doses greater than 0.1 mg/kg (75), while more recent review did not confirm a dose-response relationship (76). Dexamethasone should be considered for all patients undergoing thoracic surgery unless strong contraindications exist. There is no available data describing the utility of corticosteroids specifically for thoracotomy or VATS.
Summary of multimodal/systemic analgesics (Table 2)
Table 2
| Drug | Benefits | Cautions | Thoracotomy | Thoracoscopy |
|---|---|---|---|---|
| Acetaminophen | Safe, synergistic with NSAIDs, effective for referred shoulder pain | Low-risk without significant adverse effects, caution if significant liver disease | Use unless contraindicated | Use unless contraindicated |
| NSAIDs | Opioid-sparing, synergistic with acetaminophen | Risk of bleeding, AKI, and GI mucosal damage | Use unless contraindicated | Use unless contraindicated |
| Ketamine | Opioid-sparing, synergistic with opioids, avoids respiratory depression | Hallucinations, nightmares | Consider in select patients (e.g., chronic pain on opioid therapy, contraindication to TEA) | Consider in select patients (e.g., chronic pain on opioid therapy, not candidate for other adjuncts) |
| Gabapentinoids | Possibly opioid-sparing, questionable clinical significance | Risk of sedation, respiratory depression with opioids | Avoid unless on prior to surgery | Avoid unless on prior to surgery |
| IV Lidocaine | Potential anti-inflammatory effect, pre-emptive topical effect | Risk of local anesthetic systemic toxicity | Consider if not using regional local anesthetics, but unclear benefit | Consider if not using regional local anesthetics, but unclear benefit |
| Dexamethasone | Opioid-sparing analgesic effects, PONV prophylaxis | Hyperglycemia | Use unless contraindicated | Use unless contraindicated |
| Opioids | Analgesia | Respiratory depression, sedation, constipation, tolerance, dependence | Use as needed (e.g., PCA, oral opioids with IV for breakthrough pain) | Use as needed (e.g., PCA, oral opioids with IV for breakthrough pain) |
NSAID, non-steroidal anti-inflammatory drug; AKI, acute kidney injury; GI, gastrointestinal; TEA, thoracic epidural analgesia; PONV, post-operative nausea and vomiting; PCA, patient-controlled analgesia; IV, intravenous.
Acetaminophen and NSAIDs can have synergistic effects and should be routinely utilized perioperatively unless contraindicated. Ketamine has its merits in patients with chronic pain or opioid tolerance as well as in patients undergoing thoracotomy who are unable to receive regional anesthesia, but caution is warranted in elderly patients given psychomimetic effects. Gabapentin may have some opioid-sparing effect, but can cause sedation and synergistic respiratory depression with opioids, and should not be routinely used in naïve patients. Although the utility of IV lidocaine is unclear in thoracic surgery, glucocorticoids including dexamethasone have multiple benefits including analgesia and should be used unless contraindicated.
Regional anesthesia
TEA
Epidural local anesthetic and opioid analgesics affect the spinal nerve roots and sympathetic chain, modulating afferent transmission of painful stimuli and efferent sympathetic transmission (Figure 1). Epidural analgesia has been postulated to have beneficial effects on immune and metabolic function, coagulation, and GI function (78). It has also been associated with improved outcomes including reduced mortality and fewer adverse events including dysrhythmias, deep venous thrombosis, respiratory depression, and atelectasis (79). Common adverse effects include hypotension from bilateral sympathetic block, urinary retention, and pruritis related to neuraxial opioids (78). The failure rate is variable and operator dependent, quoted up to 15% in certain centers (79). Given the excellent analgesia and beneficial effects on reducing pulmonary complications and morbidity, epidural analgesia has traditionally been advocated in thoracic operations (80).
TEA for thoracotomy
TEA has been the gold standard to manage post-thoracotomy pain. A large retrospective analysis of the Medicare claims database revealed a significantly lower odds of death at seven and 30 days after surgery in patients who underwent lung resection with epidural analgesia (81). A randomized trial of patients undergoing thoracotomy for esophageal cancer showed that TEA delays the increase in and shortens the elevation of cortisol levels in addition to reducing postoperative pain, hospital LOS, and intraoperative costs (82). There are many studies comparing the efficacy of different regional anesthesia techniques to TEA, which will be elaborated below.
TEA for VATS
Although the effectiveness of TEA for management of acute post-thoracotomy pain is well-demonstrated, its superiority over other analgesic approaches in VATS is less clear. Regional anesthesia techniques including PVBs, ICNB, and fascial plane blocks may provide effective analgesia with lower risk profiles as detailed below (33). Several randomized studies and retrospective reviews have shown no significant difference in pain, patient satisfaction, or change in FVC or FEV1 in patients who used PCA compared to TEA (51,83,84). In fact, a retrospective review linked TEA with prolonged hospital stay in VATS lobectomy patients (85).
Thoracic PVB
The paravertebral space runs alongside the vertebral column bilaterally. It is continuous with the intercostal space laterally and the epidural space medially, and contains the spinal nerve with its dorsal ramus as well as the sympathetic chain (Figure 1) (86). Paravertebral local anesthetic can spread multiple levels into the epidural and intercostal spaces, blocking the spinal nerve and sympathetics resulting in a segmental block and ipsilateral sympathectomy. Major risks or complications of PVB include pneumothorax, hypotension with bilateral PVBs, dural puncture, and possible risks associated with epidural injections including epidural abscess or hematoma (87). PVB has been long sought as an alternative to TEA in thoracic surgery.
PVB for thoracotomy
Numerous studies have compared PVB with TEA for thoracotomy pain, with an initial systematic review from 2006 showing no significant difference between TEA and continuous PVB on pain scores in the first 48 hours after thoracotomy, but fewer pulmonary complications and adverse effects including hypotension, urinary retention, and nausea/ vomiting with PVB (88). Subsequent systematic reviews concluded that continuous PVB may be as effective as TEA for post-thoracotomy pain with fewer complications (89-91). However, there is heterogeneity within and among studies including the types and concentrations of local anesthetics used for the different techniques, and differences in the use of neuraxial opioids (89), which also makes it difficult to delineate differences in static and dynamic pain scores for each technique. Although two recent RCTs favored TEA more for post-thoracotomy pain control, its side effects including hypotension and urinary retention cannot be ignored (92,93). Due to the heterogeneity of the studies and differences in local institutional expertise and experience, there is still a debate of the best way to control thoracotomy pain between TEA and PVB (94,95). Also, the differential impacts of each technique on the progression to chronic pain, hospital costs, postoperative respiratory function, and other clinically relevant outcomes remains to be explored further (89,90).
PVB for VATS
Given that VATS is less invasive and morbid than thoracotomy, PVB is more commonly utilized than TEA for VATS. A randomized study comparing single-shot PVB, paravertebral catheters, and TEA for VATS showed that although TEA was associated with better pain control at 24 and 48 hours, there were no differences in patient satisfaction, and single-shot PVB was as effective as the continuous PVB catheter approach (96). Preemptive PVB for VATS also results in significantly lower pain at rest and with coughing, less opioid consumption, and better preservation of respiratory function at four hours compared to ICNB (97). A systematic review suggested that PVB is effective at reducing pain scores within the first six hours after VATS and may also reduce postoperative analgesic requirements and LOS, though the limited and heterogenous data failed to show any significant difference on pain scores at 24 hours (98). Most recently, a randomized study proved that PVB was far superior to ICNB and erector spinae plane block (ESPB) in the first 24 hours in VATS patients (99). Overall, the data suggests that PVB provides excellent analgesia in VATS and continuous catheter techniques may provide non-inferior analgesia to TEA with fewer adverse effects.
ICNB
The ICNB involves injection of local anesthetic in the intercostal space inferior to the rib between the internal and innermost intercostal muscles (Figure 1) and can be performed percutaneously with landmark or ultrasound-guidance, or intraoperatively with direct or video visualization (100). Meta-analysis of ICNB versus PVB for thoracic and breast surgery showed that PVB was superior to ICNB with improved analgesia at one and 24 hours, lower opioid consumption, and potentially improved postoperative respiratory function (101). More recently, a large systematic review and meta-analysis demonstrated that ICNB has benefits including improved pain control and reduced opioid consumption in the first 24 hours after thoracic surgery compared to systemic therapy alone (102). There are relatively few studies directly comparing ICNB with TEA or other regional techniques for thoracic surgery, and the limited data has yielded mixed results
ICNB for thoracotomy
Luketich et al. conducted a RCT which demonstrated that in comparison to TEA, continuous ICNB with morphine PCA was able to achieve the same level of pain control for postoperative days one through five with no difference in postoperative pulmonary function, ICU admission, and hospital LOS (103). However, a single-shot ICNB with PCA in two separate randomized trials proved to be inferior to TEA with regards to rest and dynamic pain control and preservation of pulmonary function especially in the first 12–24 hours after thoracotomy (104,105). Although ICNB is superior to systemic analgesics after thoracotomy, PVB provides superior static and dynamic analgesia and opioid reduction (102). Due to high absorption of local anesthetics at the intercostal space, continuous infusion of ICNB requires closer monitoring for toxicity.
ICNB for VATS
ICNB is easy to perform and proves to decrease both intraoperative and postoperative opioid consumption (106). Although single-shot PVB has been favored over ICNB for better pain control (99), a small randomized trial comparing continuous PVB with multiple continuous ICNBs for VATS did not find any significant difference in pain in the first 24 hours postoperatively, with better analgesia at 48 hours in the PVB group; however this was a small study with the end of the ICNB catheters deposited along the sympathetic chain in a similar position to the PVB catheters, making it difficult to delineate differences between the two techniques (107).
Other variations of ICNB include cryoanalgesia, interpleural local anesthetics, and sub/extrapleural local anesthetic infusions. Cryoanalgesia involves freezing the intercostal nerve and damaging the myelin sheath, with theoretical regeneration and return to normal function over several months. This technique was used in the past prior to widespread use of TEA for thoracotomy, but it did not have significant benefit compared to IV analgesics and was not comparable to TEA (100). The subpleural block involves catheters with multiple side holes inserted in the extrapleural space adjacent to the sympathetic chain, however it does not offer advantages over PCA (108). Similarly, intrapleural analgesia is not beneficial and is not recommended after thoracic surgery (12,100). Given the simplicity but limited duration of single-shot ICNBs, there is interest in utilizing liposomal bupivacaine, which has a longer duration of action than bupivacaine due to its slow release over time, and is described separately below.
Fascial plane blocks
Over the past several years there has been growing interest in the development of novel ultrasound-guided fascial plane blocks, which involve the spread of large volumes of local anesthetics into fascial planes through which nerves penetrate or which communicate with other spaces that contain nerves of interest. The serratus anterior plane block (SAPB) and ESPB are the most relevant for thoracic surgery, and there is a growing body of evidence on their efficacy. These blocks are technically easier to perform and theoretically have lower risks of serious adverse events associated with TEA and PVB including epidural hematoma or abscess or pneumothorax, are less likely to cause sympathectomy or hypotension, and may provide adequate analgesia especially for VATS (109-112).
SAPB
The SAPB targets the lateral cutaneous branches of the intercostal nerves as they penetrate the serratus anterior muscle (SAM) as well as the long thoracic and thoracodorsal nerves which lie anterior the SAM, with possible diffusion into the intercostal space directly affecting the intercostal nerves (111). Performed at the level of the fourth or fifth rib in the midaxillary or posterior axillary line, the needle penetrates the latissimus dorsi muscle posteriorly or the pectoralis muscles anteriorly and enters the SAM which overlies the rib, and local anesthetic is deposited either superficial or deep to the SAM (Figure 1) (112,113). Injection of 20–40 mL of local anesthetic can spread up to seven levels with sensory changes in the T2-T9 distribution (112,114,115). Rare complications include pneumothorax, winged scapula from blocked long thoracic nerve, or vascular injury to the thoracodorsal artery. A recent systematic review of SAPB for thoracic surgery which included 8 trials (6 VATS, 2 with thoracotomy) showed evidence of reduced pain scores at multiple time points and reduced opioid consumption up to 24 hours (116).
SAPB for thoracotomy
An early retrospective analysis of SAPB for thoracotomy pain showed lower pain scores and lower morphine consumption in the first 24 hours after SAPB was introduced (117). In a small prospective study comparing TEA with SAPB for thoracotomy, there were no significant differences in pain scores between TEA and SAPB in the first 12 hours; though at multiple subsequent time points up to 24 hours TEA was associated with significantly lower pain score (118). Interestingly, a sub-group analysis of a meta-analysis data set failed to identify any beneficial effects of SAPB in thoracotomy patients (116). A subsequent study of 90 patients undergoing thoracotomy comparing PVB to SAPB showed no significant differences in pain scores in the immediate postoperative period, but at 12 and 24 hours PVB provided superior analgesia, suggesting that SAPB may provide good analgesia immediately after thoracotomy but the effect is not as durable as PVB (119).
SAPB for VATS
A prospective trial for SAPB for VATS comparing tramadol PCA to tramadol PCA plus SAPB showed lower pain scores and lower tramadol consumption postoperatively, however the trial included a small number of patients, and there was no placebo/sham control group (120). Compared to sham block, SAPB can improve postoperative pain scores and reduce fentanyl requirements after VATS (121), as well as improved composite quality of recovery scores for two days, lower pain scores up to six hours, and lower opioid consumption up to 24 hours, with no difference in pain scores at 24 and 48 hours (122). Multiple prospective trials investigating SAPB versus standard analgesia for thoracoscopic surgery have shown reduced pain scores, however the duration varies, with one study showing no difference after the first nine hours, and lower opioid consumption only within the first 30 minutes in the PACU (121,123). However, a small randomized trial failed to find any difference in pain scores or opioid consumption in patients who receive either SAPB or ICNB for VATS (124). Interestingly, a recent randomized non-inferiority trial of SAPB and PVB for VATS showed no significant difference in post-operative pain scores at two hour between the two approaches, but also no difference in pain scores for either compared to no block at 24 or 48 hours, and no difference in 24 hours post-operative opioid consumption (125).
Overall, the data suggests that SAPB may provide effective analgesia as part of a multimodal approach for thoracic surgery patients, especially in the immediate postoperative period, but the duration of analgesia may not extend beyond PACU or early postoperative courses. There are very few adverse effects to SAPB and this technique may confer additional benefits such as decreased PONV (116,123). As shown in Figure 1, SAPB covers the lateral cutaneous branch of the intercostal nerve and is unable to block pleural/visceral sensation conducted via sympathetic fibers, which may explain why PVB provides better analgesia for thoracotomy. Given that this is a relatively novel regional technique for thoracic surgery and there are limited data on its clinical impact compared to other proven techniques, more investigation will be necessary to delineate its efficacy and in which patients and procedures it may be most useful.
ESPB
The ESPB targets the facial plane between the erector spinae muscles and the posterior border of the transverse processes, blocking the dorsal rami of the spinal nerves, and potentially also spreading anteriorly into the adjacent paravertebral and epidural spaces to block the ventral rami and sympathetic chain (Figure 1). This block has been increasingly used for thoracic surgery over the past three to four years (112,113,126-129). A recent systematic review evaluating ESPB for breast and thoracic surgery found that ESPB improves pain and reduces 24-hour opioid consumption and PONV compared to no block in both subgroups. Although no significant differences between ESPB and PVB were observed in opioid consumption or pain scorers, the trend favored PVB (130).
ESPB for thoracotomy
A recent prospective trial investigating continuous infusion fascial plane blocks for thoracotomy showed that TEA provides superior analgesia after thoracotomy compared to SAPB up to 24 hours, however continuous ESPB resulted in similar pain scores as TEA; patients who received continuous ESPB had lower overall opioid consumption than those with continuous SAPB, suggesting that ESPB can provide effective analgesia and may be superior to SAPB after thoracotomy (131). For mini-thoracotomy, single-shot ESPB can result in lower static and dynamic pain scores, fewer requested additional analgesics, and improved patient satisfaction compared to multi-level intrapleural ICNB delivered by the surgeon at the end of the case, indicating that ESPB may be more effective than ICNB for thoracotomy (132).
ESPB for VATS
In VATS patients, ESPB resulted in lower PACU pain scores in the first six hours, lower opioid consumption, and shorter PACU stays compared to sham block (133). There are mixed data on the efficacy of ESPB compared to PVB. Although two non-inferiority studies failed to reveal any clinically significant differences in postoperative pain scores between PVB and ESPB for VATS (134,135), two other clinical trials demonstrated PVB provided better analgesia in VATS patients (9,99). Unlike in mini-thoracotomy, these two studies also found patients that had ESPB actually had higher dynamic pain score and higher opioid consumption compared to ICNB (9,99).
Several studies have recently compared ESPB to SAPB for VATS: in a prospective randomized trial, Gaballah et al. demonstrated reduced pain scores at multiple postoperative time points with ESPB and higher mean arterial pressures in SAPB, suggesting that ESPB may provide slightly improved analgesia but potentially some sympathectomy and decreased blood pressure from paravertebral/epidural spread (136). In a subsequent prospective trial, Finnerty et al. showed a higher composite quality of recovery score as well as longer time to first opioid use, lower static and dynamic pain scores, and lower in-hospital composite complication scores without significant effects on LOS or overall opioid consumption with ESPB compared to SAPB for minimally invasive thoracic surgery (137). One possible explanation for evidence supporting improved analgesia with ESPB compared to SAPB could the postulated spread to the paravertebral space with coverage of dorsal and ventral rami and sympathetic chain similar to a PVB, which may block noxious stimuli related to chest wall muscles, pleura, and thoracic viscera which is transmitted by muscular branches of the intercostal nerve, dorsal rami, and sympathetic chain. This is in contrast to the SAPB, which may only directly affect the lateral cutaneous branches of the intercostal nerves (Figure 1). However, there are some conflicting results on whether the ESPB reliably or actually spreads to the paravertebral and epidural spaces (138-140).
Liposomal bupivacaine (LB) for regional anesthesia
LB is a long-acting local anesthetic encased in a carrier matrix which slowly releases bupivacaine over time, with a duration of up to 96 hours, and it has been approved for local wound infiltration and interscalene brachial plexus blocks. Given its longer duration of action, there has been interest the last several years in incorporating LB for regional anesthesia in thoracic surgery (141,142). However, two recent meta-analyses of clinical trials cast doubt on the efficacy of LB (143,144).
LB for thoracotomy
An initial retrospective review by Rice et al. revealed that ICNB with LB produced comparable pain control and opioid consumption as TEA for up to three days postoperatively (145). Khalil et al. found that ICNB with LB provided even better pain control than TEA with decreased pain scores, pulmonary complications and hospital LOS (146). Although the findings suggested that LB could offer a safe, simple alternative to TEA for thoracotomy, the intrinsic pitfalls of retrospective studies could not be ignored, especially when comparing different treatment periods and surgical techniques. A RCT failed to yield any positive results of LB ICNB in thoracotomy (147). A separate RCT to compare LB ICNB with TEA was terminated due to insufficient enrollment numbers, though no difference was found on initial analysis (148).
LB for VATS
Efficacy of LB ICNB for VATS is controversial as well with mixed results. The retrospective study by Medina et al. favored LB to decrease pain scores and early discharge compared to TEA (149), while opposite results were presented by Sztain et al. (150). There are several retrospective studies comparing ICNB with bupivacaine or LB: three out of seven studies failed to show any significant benefits, while the others demonstrated benefits at different time points (151-157).
Although there is evidence that LB may be a safe analgesic option for thoracic surgery, the data are mixed with significant heterogeneity in study design and results, and clear recommendations on the use of LB for thoracic surgery are limited due to this.
Phrenic nerve block
ISP can occur in up to 85% of patients undergoing thoracic surgery, and is due to referred pain from diaphragmatic or pleural irritation transmitted via the phrenic nerve. Injection of local anesthetic at the phrenic fat pad was able to significantly lower shoulder pain in thoracotomy without change of blood gas postoperatively (158-160). Ultrasound-guided phrenic nerve blockade at the suprascapular or interscalene regions both significantly decreased shoulder pain in open lobectomy and pneumonectomy with no effect on postoperative pulmonary function tests (161,162). One concern for phrenic nerve blockade is diaphragmatic hemiparesis and possible perioperative pulmonary complications related to this, but surprisingly none of these studies were able to identify such complications even in the presence of continuous phrenic nerve blockade for three days (161,162). Although ISP can be refractory to pharmacological management and is not blocked by chest wall regional anesthesia approaches, the above studies identify a new avenue at controlling this common problem for thoracic surgery patients. There were intrinsic problems in study designs however, as patients were not blinded and overall patient satisfaction from upper extremity block was not mentioned. Caution has to be taken to extrapolate findings from thoracotomy to VATS patients as well especially regarding pulmonary complications.
Additives for regional blocks
There have been a number of additives investigated for prolonging or enhancing the analgesic effect of neuraxial and peripheral blocks. In addition to the routine use of epinephrine as an additive to local anesthetics for regional anesthesia, some of the most commonly used additives include dexamethasone and alpha-2 agonists including dexmedetomidine. Although both intravenous and perineural dexamethasone may both improve analgesia quality and duration of upper extremity peripheral nerve blocks, there is no conclusive data suggesting its effectiveness in other regional anesthesia techniques outside of its systemic benefits detailed above (163). Similarly, dexmedetomidine may prolong the duration of analgesia in upper limb nerve blocks; however, it also increases the risk of transient hypotension and bradycardia and may contribute to sedation, and its effectiveness in regional anesthesia techniques for thoracic surgery is not well-investigated (164). However, limited evidence suggests that magnesium and dexmedetomidine added to paravertebral bupivacaine may enhance the analgesic effect after thoracic surgery (165-167). Although such additives may prolong the effect of certain regional blocks, it is unclear if this is related to perineural or systemic effects, and more investigations are needed to determine if their routine use in thoracic surgery patients is beneficial.
Summary of regional anesthesia (Table 3)
Table 3
| Regional anesthesia | Nerves affected | Expected effect | Thoracotomy | Thoracoscopy |
|---|---|---|---|---|
| Thoracic epidural analgesia | Bilateral spinal nerves (includes dorsal ramus, ventral ramus/ intercostal nerve, and visceral fibers/sympathetic chain) | Multi-level bilateral segmental somatic and visceral block, sympathectomy | Consider unless contraindicated | Likely not necessary/ advantageous over other regional approaches |
| Paravertebral block | Ipsilateral spinal nerve (includes dorsal ramus, ventral ramus/intercostal nerve, and visceral fibers/sympathetic chain) | Multi-level unilateral segmental somatic and visceral | Consider continuous PVB | Strongly consider unless contraindicated |
| Intercostal nerve block | Intercostal nerve with lateral and anterior cutaneous branches, muscle and pleural branches | Single-level unilateral lateral and anterior somatic block | Consider if unable to use TEA or PVB | Consider, possibly in combination with other chest wall blocks |
| Serratus anterior plane block | Lateral cutaneous branch of intercostal nerve | Multi-level anterolateral somatic block | Consider if unable to use TEA or PVB | Consider, possibly in combination with other chest wall blocks |
| Erector spinae plane block | Dorsal ramus, potentially ventral ramus/intercostal nerve and visceral fibers/sympathetic chain | Multi-level unilateral posterior somatic block, potential segmental somatic and visceral block | Consider if unable to use TEA or PVB | Consider, possibly in combination with other chest wall blocks |
PVB, paravertebral block; TEA, thoracic epidural analgesia.
Regional and neuraxial anesthesia should be strongly considered in patients undergoing thoracic surgery. Thoracotomy patients should have either TEA or continuous PVB. For VATS, PVB targeting the origin of intercostal nerves and the sympathetic chain can attenuate pain signals from somatic and visceral sources, and have demonstrated advantages over other chest wall blocks. Fascial plane blocks and ICNB can be considered in patients with contraindications for PVB who are undergoing VATS, however the comparative efficacy or optimal block is still not clear. The data on the utility of LB for blocks for thoracic surgery is mixed, and future studies are needed to clarify its role in this field. Beyond LB, there is no real data comparing different types of local anesthetics, but utilizing long-acting agents such as bupivacaine or ropivacaine within their safe dose range offers optimal analgesia, especially for single-shot techniques.
Chronic pain after thoracic surgery
CPTPS involves pain persisting for greater than two months after thoracic surgery, and can follow thoracotomy or thoracoscopic approaches. The pain is often described as severe and burning likely related to neuropathic pain from damage to intercostal nerves. Approximately 40% of patients that have significant acute pain after thoracotomy may develop CPTPS, with risk factors including female gender, younger age, anxiety, and depression, as well as intensity of post-operative pain in the first three days (168-170). A Cochrane review of trials comparing local or regional anesthetics with conventional analgesic techniques for preventing chronic pain after surgery showed that regional anesthesia may help prevent chronic pain at six months after thoracotomy, with a similar signal for PVB for breast cancer surgery (171). A randomized prospective study of 300 patients undergoing thoracotomy found that the TEA group had significantly lower incidence of chronic pain compared to the ICNB group, while the PVB group showed no difference from the ICNB group (169). There is no conclusive data that ketamine, opioids, gabapentinoids, acetaminophen, or NSAIDs are effective preventive analgesics or can decrease the risk of developing chronic pain (57,80,172).
Conclusions and future directions
For the past decade, there has been a significant paradigm shift in pain management for thoracic surgery. With the advancement of minimally invasive surgery, the widespread use of ultrasound technology, a better understanding of anatomy, and the development of novel pharmacological strategies for perioperative pain management, it is incumbent on anesthesiologists and surgeons to leverage these resources to formulate individual pain management plans to enhance patient recovery. Also, preoperative discussions with patients focused on determining beliefs around pain, coping strategies, and expectations both of post-operative pain and the planned strategies to mitigate it, are essential for optimal patient care (170).
At the same time, attention needs to be paid to the deficiency of the current literature in determining the optimal perioperative pain management strategy for thoracic surgery patients. Significant heterogeneity in study design, small sample numbers in many studies, and a large amount of retrospective data with relatively fewer prospective studies make it difficult to ascertain the validity and generalizability of many conclusions. Strategies proven to effectively treat pain and improve outcomes for thoracotomy may not directly translate to thoracoscopic surgery, with TEA as an example. Outcome measurements such as opioid consumption and pain scores in isolation may not correlate with other clinically important outcomes such as morbidity, mortality, and a functional return to baseline. Also considering clinical significance to statistical significance is important when evaluating reported outcomes. A focus on such clinically important outcomes rather than only changes in pain scores or opioid consumption should be prioritized in study designs in this patient population.
Emerging new technology and pharmacologic agents provide ample opportunities to investigate and define the future of pain management for thoracic surgery. In addition to traditional head-to-head comparisons of the different regional anesthesia techniques described above, combinations of multiple approaches based on their anatomic targeting sites and pharmacokinetic profiles may offer interesting avenues of research; for instance, combining ICNB with SAPB or ESPB, or SAPB with ESPB. A more thorough understanding of the effects of timing of administration, such as pre-incisional to provide possible preventive analgesia, versus at the end of the procedure to maximize postoperative analgesia duration, could further guide customized analgesia plans for individual patients and procedures. Even for LB, it is quite possible that mixed results were due to initial low-level release of bupivacaine from depot that was insufficient to produce effective analgesia immediately after administration. Combination of LB with bupivacaine was able to produce significant analgesia by transversus abdominis plane (TAP) blocks in cesarean section patients, which may open a new avenue of utilizing LB in thoracic operations (173). Overall, many questions remain unanswered, and continued advancements in the field and ongoing research to address such questions will provide guidance for tailored pain management plans for each patient that will enhance their recovery after thoracic surgery.
Acknowledgments
We thank Dr. Hovig Chitilian and Dr. Kimberly Ting for their assistance in the preparation and review of this manuscript. We thank the reviewers of this manuscript for their helpful comments and suggestions to improve the quality and impact of this review.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1740/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1740/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1740/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sabanathan S, Eng J, Mearns AJ. Alterations in respiratory mechanics following thoracotomy. J R Coll Surg Edinb 1990;35:144-50. [PubMed]
- Ballantyne JC, Carr DB, deFerranti S, et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesth Analg 1998;86:598-612. [Crossref] [PubMed]
- Peng Z, Li H, Zhang C, et al. A retrospective study of chronic post-surgical pain following thoracic surgery: prevalence, risk factors, incidence of neuropathic component, and impact on qualify of life. PLoS One 2014;9:e90014. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
- Pu Q, Ma L, Mei J, et al. Video-assisted thoracoscopic surgery versus posterolateral thoracotomy lobectomy: A more patient-friendly approach on postoperative pain, pulmonary function and shoulder function. Thorac Cancer 2013;4:84-9. [Crossref] [PubMed]
- Brescia AA, Harrington CA, Mazurek AA, et al. Factors Associated With New Persistent Opioid Usage After Lung Resection. Ann Thorac Surg 2019;107:363-8. [Crossref] [PubMed]
- Chen N, Qiao Q, Chen R, et al. The effect of ultrasound-guided intercostal nerve block, single-injection erector spinae plane block and multiple-injection paravertebral block on postoperative analgesia in thoracoscopic surgery: A randomized, double-blinded, clinical trial. J Clin Anesth 2020;59:106-11. [Crossref] [PubMed]
- Mac TB, Girard F, Chouinard P, et al. Acetaminophen decreases early post-thoracotomy ipsilateral shoulder pain in patients with thoracic epidural analgesia: a double-blind placebo-controlled study. J Cardiothorac Vasc Anesth 2005;19:475-8. [Crossref] [PubMed]
- Wick EC, Grant MC, Wu CL. Postoperative Multimodal Analgesia Pain Management With Nonopioid Analgesics and Techniques: A Review. JAMA Surg 2017;152:691-7. [Crossref] [PubMed]
- Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016;17:131-57. [Crossref] [PubMed]
- Oscier CD, Milner QJ. Peri-operative use of paracetamol. Anaesthesia 2009;64:65-72. [Crossref] [PubMed]
- Mallama M, Valencia A, Rijs K, et al. A systematic review and trial sequential analysis of intravenous vs. oral peri-operative paracetamol. Anaesthesia 2021;76:270-6. [Crossref] [PubMed]
- Patel M, Jayakumar N, Bagheri K, et al. Use of intravenous acetaminophen to control pain and improve outcomes in thoracic surgery. Clinics in Surgery 2018;3:2105.
- Shikatani Y, Soh J, Shien K, et al. Effectiveness of scheduled intravenous acetaminophen in the postoperative pain management of video-assisted thoracic surgery. Surg Today 2021;51:589-94. [Crossref] [PubMed]
- Jahangiri Fard A, Farzanegan B, Khalili A, et al. Paracetamol Instead of Ketorolac in Post-Video-Assisted Thoracic Surgery Pain Management: A Randomized Trial. Anesth Pain Med 2016;6:e39175. [PubMed]
- Ong CK, Seymour RA, Lirk P, et al. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg 2010;110:1170-9. [Crossref] [PubMed]
- Martinez V, Beloeil H, Marret E, et al. Non-opioid analgesics in adults after major surgery: systematic review with network meta-analysis of randomized trials. Br J Anaesth 2017;118:22-31. [Crossref] [PubMed]
- Moore RA, Derry S, Aldington D, et al. Single dose oral analgesics for acute postoperative pain in adults - an overview of Cochrane reviews. Cochrane Database Syst Rev 2015;CD008659. [PubMed]
- Cepeda MS, Vargas L, Ortegon G, et al. Comparative analgesic efficacy of patient-controlled analgesia with ketorolac versus morphine after elective intraabdominal operations. Anesth Analg 1995;80:1150-3. [PubMed]
- Smith LA, Carroll D, Edwards JE, et al. Single-dose ketorolac and pethidine in acute postoperative pain: systematic review with meta-analysis. Br J Anaesth 2000;84:48-58. [Crossref] [PubMed]
- Khan JS, Margarido C, Devereaux PJ, et al. Preoperative celecoxib in noncardiac surgery: A systematic review and meta-analysis of randomised controlled trials. Eur J Anaesthesiol 2016;33:204-14. [Crossref] [PubMed]
- Leese PT, Hubbard RC, Karim A, et al. Effects of celecoxib, a novel cyclooxygenase-2 inhibitor, on platelet function in healthy adults: a randomized, controlled trial. J Clin Pharmacol 2000;40:124-32. [Crossref] [PubMed]
- Teerawattananon C, Tantayakom P, Suwanawiboon B, et al. Risk of perioperative bleeding related to highly selective cyclooxygenase-2 inhibitors: A systematic review and meta-analysis. Semin Arthritis Rheum 2017;46:520-8. [Crossref] [PubMed]
- Pavy T, Medley C, Murphy DF. Effect of indomethacin on pain relief after thoracotomy. Br J Anaesth 1990;65:624-7. [Crossref] [PubMed]
- Perttunen K, Kalso E, Heinonen J, et al. IV diclofenac in post-thoracotomy pain. Br J Anaesth 1992;68:474-80. [Crossref] [PubMed]
- Boussofara M, Mtaallah MH, Bracco D, et al. Co-analgesic effect of ketorolac after thoracic surgery. Tunis Med 2006;84:427-31. [PubMed]
- Singh H, Bossard RF, White PF, et al. Effects of ketorolac versus bupivacaine coadministration during patient-controlled hydromorphone epidural analgesia after thoracotomy procedures. Anesth Analg 1997;84:564-9. [PubMed]
- Senard M, Deflandre EP, Ledoux D, et al. Effect of celecoxib combined with thoracic epidural analgesia on pain after thoracotomy. Br J Anaesth 2010;105:196-200. [Crossref] [PubMed]
- Perttunen K, Nilsson E, Kalso E I.v.. diclofenac and ketorolac for pain after thoracoscopic surgery. Br J Anaesth 1999;82:221-7. [Crossref] [PubMed]
- Dastan F, Langari ZM, Salamzadeh J, et al. A comparative study of the analgesic effects of intravenous ketorolac, paracetamol, and morphine in patients undergoing video-assisted thoracoscopic surgery: A double-blind, active-controlled, randomized clinical trial. Ann Card Anaesth 2020;23:177-82. [Crossref] [PubMed]
- Kaplowitz J, Papadakos PJ. Acute pain management for video-assisted thoracoscopic surgery: an update. J Cardiothorac Vasc Anesth 2012;26:312-21. [Crossref] [PubMed]
- Sleigh J, Harvey M, Voss L, et al. Ketamine - more mechanisms of action than just NMDA blockade. Trends in Anaesthesia and Critical Care 2014;4:76-81. [Crossref]
- Guirimand F, Dupont X, Brasseur L, et al. The effects of ketamine on the temporal summation (wind-up) of the R(III) nociceptive flexion reflex and pain in humans. Anesth Analg 2000;90:408-14. [Crossref] [PubMed]
- Laskowski K, Stirling A, McKay WP, et al. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth 2011;58:911-23. [Crossref] [PubMed]
- Suzuki M, Haraguti S, Sugimoto K, et al. Low-dose intravenous ketamine potentiates epidural analgesia after thoracotomy. Anesthesiology 2006;105:111-9. [Crossref] [PubMed]
- Wang X, Lin C, Lan L, et al. Perioperative intravenous S-ketamine for acute postoperative pain in adults: A systematic review and meta-analysis. J Clin Anesth 2021;68:110071. [Crossref] [PubMed]
- Avidan MS, Maybrier HR, Abdallah AB, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 2017;390:267-75. [Crossref] [PubMed]
- Albrecht E, Kirkham KR, Liu SS, et al. Peri-operative intravenous administration of magnesium sulphate and postoperative pain: a meta-analysis. Anaesthesia 2013;68:79-90. [Crossref] [PubMed]
- De Oliveira GS Jr, Castro-Alves LJ, Khan JH, et al. Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 2013;119:178-90. [Crossref] [PubMed]
- Lysakowski C, Dumont L, Czarnetzki C, et al. Magnesium as an adjuvant to postoperative analgesia: a systematic review of randomized trials. Anesth Analg 2007;104:1532-9. table of contents. [Crossref] [PubMed]
- Michelet P, Guervilly C, Hélaine A, et al. Adding ketamine to morphine for patient-controlled analgesia after thoracic surgery: influence on morphine consumption, respiratory function, and nocturnal desaturation. Br J Anaesth 2007;99:396-403. [Crossref] [PubMed]
- Nesher N, Ekstein MP, Paz Y, et al. Morphine with adjuvant ketamine vs higher dose of morphine alone for immediate postthoracotomy analgesia. Chest 2009;136:245-52. [Crossref] [PubMed]
- Fiorelli A, Mazzella A, Passavanti B, et al. Is pre-emptive administration of ketamine a significant adjunction to intravenous morphine analgesia for controlling postoperative pain? A randomized, double-blind, placebo-controlled clinical trial. Interact Cardiovasc Thorac Surg 2015;21:284-90. [Crossref] [PubMed]
- Joseph C, Gaillat F, Duponq R, et al. Is there any benefit to adding intravenous ketamine to patient-controlled epidural analgesia after thoracic surgery? A randomized double-blind study. Eur J Cardiothorac Surg 2012;42:e58-65. [Crossref] [PubMed]
- Argiriadou H, Papagiannopoulou P, Foroulis CN, et al. Intraoperative infusion of S(+)-ketamine enhances post-thoracotomy pain control compared with perioperative parecoxib when used in conjunction with thoracic paravertebral ropivacaine infusion. J Cardiothorac Vasc Anesth 2011;25:455-61. [Crossref] [PubMed]
- Ozcan PE, Tugrul S, Senturk NM, et al. Role of magnesium sulfate in postoperative pain management for patients undergoing thoracotomy. J Cardiothorac Vasc Anesth 2007;21:827-31. [Crossref] [PubMed]
- Ghezel-Ahmadi V, Ghezel-Ahmadi D, Schirren J, et al. Perioperative systemic magnesium sulphate to minimize acute and chronic post-thoracotomy pain: a prospective observational study. J Thorac Dis 2019;11:418-26. [Crossref] [PubMed]
- Suksompong S, Chaikittisilpa N, Wanchiange S, et al. Low dose intraoperative ketamine infusion with multilevel paravertebral block for pain after video-assisted thoracic surgery: a randomized-controlled study. Ann Palliat Med 2021;10:7258-69. [Crossref] [PubMed]
- Tseng WC, Lin WL, Lai HC, et al. Fentanyl-based intravenous patient-controlled analgesia with low dose of ketamine is not inferior to thoracic epidural analgesia for acute post-thoracotomy pain following video-assisted thoracic surgery: A randomized controlled study. Medicine (Baltimore) 2019;98:e16403. [Crossref] [PubMed]
- Chincholkar M. Analgesic mechanisms of gabapentinoids and effects in experimental pain models: a narrative review. Br J Anaesth 2018;120:1315-34. [Crossref] [PubMed]
- Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth 2015;114:10-31. [Crossref] [PubMed]
- Fabritius ML, Geisler A, Petersen PL, et al. Gabapentin in procedure-specific postoperative pain management - preplanned subgroup analyses from a systematic review with meta-analyses and trial sequential analyses. BMC Anesthesiol 2017;17:85. [Crossref] [PubMed]
- Doleman B, Heinink TP, Read DJ, et al. A systematic review and meta-regression analysis of prophylactic gabapentin for postoperative pain. Anaesthesia 2015;70:1186-204. [Crossref] [PubMed]
- Hah J, Mackey SC, Schmidt P, et al. Effect of Perioperative Gabapentin on Postoperative Pain Resolution and Opioid Cessation in a Mixed Surgical Cohort: A Randomized Clinical Trial. JAMA Surg 2018;153:303-11. [Crossref] [PubMed]
- Verret M, Lauzier F, Zarychanski R, et al. Perioperative Use of Gabapentinoids for the Management of Postoperative Acute Pain: A Systematic Review and Meta-analysis. Anesthesiology 2020;133:265-79. [Crossref] [PubMed]
- Cavalcante AN, Sprung J, Schroeder DR, et al. Multimodal Analgesic Therapy With Gabapentin and Its Association With Postoperative Respiratory Depression. Anesth Analg 2017;125:141-6. [Crossref] [PubMed]
- Sattari H, Hashemian M, Lashkarizadeh MR, et al. Preoperative Oral Pregabalin Reduces Acute Pain after Thoracotomy. Open Access Maced J Med Sci 2018;6:1606-10. [Crossref] [PubMed]
- Omran AF, Mohamed AER. A randomized study of the effects of gabapentin versus placebo on post-thoracotomy pain and pulmonary function. Egyptian Journal of Anaesthesia 2005;21:277-81.
- Kinney MA, Mantilla CB, Carns PE, et al. Preoperative gabapentin for acute post-thoracotomy analgesia: a randomized, double-blinded, active placebo-controlled study. Pain Pract 2012;12:175-83. [Crossref] [PubMed]
- Tomaszek L, Fenikowski D, Maciejewski P, et al. Perioperative Gabapentin in Pediatric Thoracic Surgery Patients-Randomized, Placebo-Controlled, Phase 4 Trial. Pain Med 2020;21:1562-71. [Crossref] [PubMed]
- Kim JC, Byun S, Kim S, et al. Effect of preoperative pregabalin as an adjunct to a multimodal analgesic regimen in video-assisted thoracoscopic surgery: A randomized controlled trial. Medicine (Baltimore) 2017;96:e8644. [Crossref] [PubMed]
- Konstantatos AH, Howard W, Story D, et al. A randomised controlled trial of peri-operative pregabalin vs. placebo for video-assisted thoracoscopic surgery. Anaesthesia 2016;71:192-7. [Crossref] [PubMed]
- Homma T, Doki Y, Yamamoto Y, et al. Efficacy of 50 mg pregabalin for prevention of postoperative neuropathic pain after video-assisted thoracoscopic surgery and thoracotomy: a 3-month prospective randomized controlled trial. J Thorac Dis 2019;11:694-701. [Crossref] [PubMed]
- Zakkar M, Frazer S, Hunt I. Is there a role for gabapentin in preventing or treating pain following thoracic surgery? Interact Cardiovasc Thorac Surg 2013;17:716-9. [Crossref] [PubMed]
- Foo I, Macfarlane AJR, Srivastava D, et al. The use of intravenous lidocaine for postoperative pain and recovery: international consensus statement on efficacy and safety. Anaesthesia 2021;76:238-50. [Crossref] [PubMed]
- Weibel S, Jelting Y, Pace NL, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev 2018;6:CD009642. [Crossref] [PubMed]
- Cui W, Li Y, Li S, et al. Systemic administration of lidocaine reduces morphine requirements and postoperative pain of patients undergoing thoracic surgery after propofol-remifentanil-based anaesthesia. Eur J Anaesthesiol 2010;27:41-6. [Crossref] [PubMed]
- Herrera J, Vives M, Gasco I, et al. Use of intravenous lidocaine infusion during thoracic surgery: a prospective observational cohort. Journal of Cardiothoracic and Vascular Anesthesia 2019;33:S166. [Crossref]
- Fiorelli A, Pace C, Cascone R, et al. Preventive skin analgesia with lidocaine patch for management of post-thoracotomy pain: Results of a randomized, double blind, placebo controlled study. Thorac Cancer 2019;10:631-41. [Crossref] [PubMed]
- Slovack M, Taylor B, Bryce R, et al. Does intravenous lidocaine infusion during video-assisted thoracoscopic surgery reduce postoperative analgesia? A randomized controlled study. Can J Anaesth 2015;62:676-7. [Crossref] [PubMed]
- Yao Y, Jiang J, Lin W, et al. Efficacy of systemic lidocaine on postoperative quality of recovery and analgesia after video-assisted thoracic surgery: A randomized controlled trial. J Clin Anesth 2021;71:110223. [Crossref] [PubMed]
- De la Gala F, Piñeiro P, Reyes A, et al. Effect of intraoperative paravertebral or intravenous lidocaine versus control during lung resection surgery on postoperative complications: A randomized controlled trial. Trials 2019;20:622. [Crossref] [PubMed]
- De Oliveira GS Jr, Almeida MD, Benzon HT, et al. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 2011;115:575-88. [Crossref] [PubMed]
- Waldron NH, Jones CA, Gan TJ, et al. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth 2013;110:191-200. [Crossref] [PubMed]
- Corcoran TB, Myles PS, Forbes AB, et al. Dexamethasone and Surgical-Site Infection. N Engl J Med 2021;384:1731-41. [Crossref] [PubMed]
- Pöpping DM, Elia N, Marret E, et al. Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: a meta-analysis. Arch Surg 2008;143:990-9; discussion 1000. [Crossref] [PubMed]
- Pöpping DM, Elia N, Van Aken HK, et al. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta-analysis of randomized controlled trials. Ann Surg 2014;259:1056-67. [Crossref] [PubMed]
- Piccioni F, Droghetti A, Bertani A, et al. Recommendations from the Italian intersociety consensus on Perioperative Anesthesa Care in Thoracic surgery (PACTS) part 2: intraoperative and postoperative care. Perioper Med (Lond) 2020;9:31. [Crossref] [PubMed]
- Wu CL, Sapirstein A, Herbert R, et al. Effect of postoperative epidural analgesia on morbidity and mortality after lung resection in Medicare patients. J Clin Anesth 2006;18:515-20. [Crossref] [PubMed]
- Li Y, Dong H, Tan S, et al. Effects of thoracic epidural anesthesia/analgesia on the stress response, pain relief, hospital stay, and treatment costs of patients with esophageal carcinoma undergoing thoracic surgery: A single-center, randomized controlled trial. Medicine (Baltimore) 2019;98:e14362. [Crossref] [PubMed]
- Kim JA, Kim TH, Yang M, et al. Is intravenous patient controlled analgesia enough for pain control in patients who underwent thoracoscopy? J Korean Med Sci 2009;24:930-5. [Crossref] [PubMed]
- Yie JC, Yang JT, Wu CY, et al. Patient-controlled analgesia (PCA) following video-assisted thoracoscopic lobectomy: comparison of epidural PCA and intravenous PCA. Acta Anaesthesiol Taiwan 2012;50:92-5. [Crossref] [PubMed]
- Zeltsman M, Dozier J, Vaghjiani RG, et al. Decreasing use of epidural analgesia with increasing minimally invasive lobectomy: Impact on postoperative morbidity. Lung Cancer 2020;139:68-72. [Crossref] [PubMed]
- Karmakar MK. Thoracic paravertebral block. Anesthesiology 2001;95:771-80. [Crossref] [PubMed]
- Okitsu K, Iritakenishi T, Iwasaki M, et al. Risk of Hematoma in Patients With a Bleeding Risk Undergoing Cardiovascular Surgery With a Paravertebral Catheter. J Cardiothorac Vasc Anesth 2017;31:453-7. [Crossref] [PubMed]
- Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy--a systematic review and meta-analysis of randomized trials. Br J Anaesth 2006;96:418-26. [Crossref] [PubMed]
- Baidya DK, Khanna P, Maitra S. Analgesic efficacy and safety of thoracic paravertebral and epidural analgesia for thoracic surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2014;18:626-35. [Crossref] [PubMed]
- Ding X, Jin S, Niu X, et al. A comparison of the analgesia efficacy and side effects of paravertebral compared with epidural blockade for thoracotomy: an updated meta-analysis. PLoS One 2014;9:e96233. [Crossref] [PubMed]
- Yeung JH, Gates S, Naidu BV, et al. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev 2016;2:CD009121. [Crossref] [PubMed]
- Biswas S, Verma R, Bhatia VK, et al. Comparison between Thoracic Epidural Block and Thoracic Paravertebral Block for Post Thoracotomy Pain Relief. J Clin Diagn Res 2016;10:UC08-12. [Crossref] [PubMed]
- Tamura T, Mori S, Mori A, et al. A randomized controlled trial comparing paravertebral block via the surgical field with thoracic epidural block using ropivacaine for post-thoracotomy pain relief. J Anesth 2017;31:263-70. [Crossref] [PubMed]
- Teeter EG, Kumar PA. Pro: Thoracic Epidural Block Is Superior to Paravertebral Blocks for Open Thoracic Surgery. J Cardiothorac Vasc Anesth 2015;29:1717-9. [Crossref] [PubMed]
- Krakowski JC, Arora H. Con: Thoracic Epidural Block Is Not Superior to Paravertebral Blocks for Open Thoracic Surgery. J Cardiothorac Vasc Anesth 2015;29:1720-2. [Crossref] [PubMed]
- Yeap YL, Wolfe JW, Backfish-White KM, et al. Randomized Prospective Study Evaluating Single-Injection Paravertebral Block, Paravertebral Catheter, and Thoracic Epidural Catheter for Postoperative Regional Analgesia After Video-Assisted Thoracoscopic Surgery. J Cardiothorac Vasc Anesth 2020;34:1870-6. [Crossref] [PubMed]
- Matyal R, Montealegre-Gallegos M, Shnider M, et al. Preemptive ultrasound-guided paravertebral block and immediate postoperative lung function. Gen Thorac Cardiovasc Surg 2015;63:43-8. [Crossref] [PubMed]
- Hu Z, Liu D, Wang ZZ, et al. The efficacy of thoracic paravertebral block for thoracoscopic surgery: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2018;97:e13771. [Crossref] [PubMed]
- Turhan Ö, Sivrikoz N, Sungur Z, et al. Thoracic Paravertebral Block Achieves Better Pain Control Than Erector Spinae Plane Block and Intercostal Nerve Block in Thoracoscopic Surgery: A Randomized Study. J Cardiothorac Vasc Anesth 2021;35:2920-7. [Crossref] [PubMed]
- Detterbeck FC. Efficacy of methods of intercostal nerve blockade for pain relief after thoracotomy. Ann Thorac Surg 2005;80:1550-9. [Crossref] [PubMed]
- Huan S, Deng Y, Wang J, et al. Efficacy and safety of paravertebral block versus intercostal nerve block in thoracic surgery and breast surgery: A systematic review and meta-analysis. PLoS One 2020;15:e0237363. [Crossref] [PubMed]
- Guerra-Londono CE, Privorotskiy A, Cozowicz C, et al. Assessment of Intercostal Nerve Block Analgesia for Thoracic Surgery: A Systematic Review and Meta-analysis. JAMA Netw Open 2021;4:e2133394. [Crossref] [PubMed]
- Luketich JD, Land SR, Sullivan EA, et al. Thoracic epidural versus intercostal nerve catheter plus patient-controlled analgesia: a randomized study. Ann Thorac Surg 2005;79:1845-9; discussion 1849-50. [Crossref] [PubMed]
- Meierhenrich R, Hock D, Kühn S, et al. Analgesia and pulmonary function after lung surgery: is a single intercostal nerve block plus patient-controlled intravenous morphine as effective as patient-controlled epidural anaesthesia? A randomized non-inferiority clinical trial. Br J Anaesth 2011;106:580-9. [Crossref] [PubMed]
- Vilvanathan S, Kuppuswamy B, Sahajanandan R. A randomized control trial to compare thoracic epidural with intercostal block plus intravenous morphine infusion for postoperative analgesia in patients undergoing elective thoracotomy. Ann Card Anaesth 2020;23:127-33. [Crossref] [PubMed]
- Bousema JE, Dias EM, Hagen SM, et al. Subpleural multilevel intercostal continuous analgesia after thoracoscopic pulmonary resection: a pilot study. J Cardiothorac Surg 2019;14:179. [Crossref] [PubMed]
- Kadomatsu Y, Mori S, Ueno H, et al. Comparison of the analgesic effects of modified continuous intercostal block and paravertebral block under surgeon's direct vision after video-assisted thoracic surgery: a randomized clinical trial. Gen Thorac Cardiovasc Surg 2018;66:425-31. [Crossref] [PubMed]
- Jung J, Park SY, Haam S. Efficacy of subpleural continuous infusion of local anesthetics after thoracoscopic pulmonary resection for primary lung cancer compared to intravenous patient-controlled analgesia. J Thorac Dis 2016;8:1814-9. [Crossref] [PubMed]
- Steinthorsdottir KJ, Wildgaard L, Hansen HJ, et al. Regional analgesia for video-assisted thoracic surgery: a systematic review. Eur J Cardiothorac Surg 2014;45:959-66. [Crossref] [PubMed]
- Umari M, Carpanese V, Moro V, et al. Postoperative analgesia after pulmonary resection with a focus on video-assisted thoracoscopic surgery. Eur J Cardiothorac Surg 2018;53:932-8. [Crossref] [PubMed]
- Chin KJ, Versyck B, Pawa A. Ultrasound-guided fascial plane blocks of the chest wall: a state-of-the-art review. Anaesthesia 2021;76:110-26. [Crossref] [PubMed]
- Helander EM, Webb MP, Kendrick J, et al. PECS, serratus plane, erector spinae, and paravertebral blocks: A comprehensive review. Best Pract Res Clin Anaesthesiol 2019;33:573-81. [Crossref] [PubMed]
- Chin KJ, Pawa A, Forero M, et al. Ultrasound-Guided Fascial Plane Blocks of the Thorax: Pectoral I and II, Serratus Anterior Plane, and Erector Spinae Plane Blocks. Adv Anesth 2019;37:187-205. [Crossref] [PubMed]
- Biswas A, Castanov V, Li Z, et al. Serratus Plane Block: A Cadaveric Study to Evaluate Optimal Injectate Spread. Reg Anesth Pain Med 2018;43:854-8. [Crossref] [PubMed]
- Blanco R, Parras T, McDonnell JG, et al. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia 2013;68:1107-13. [Crossref] [PubMed]
- Liu X, Song T, Xu HY, et al. The serratus anterior plane block for analgesia after thoracic surgery: A meta-analysis of randomized controlled trails. Medicine (Baltimore) 2020;99:e20286. [Crossref] [PubMed]
- Ökmen K, Ökmen BM. The efficacy of serratus anterior plane block in analgesia for thoracotomy: a retrospective study. J Anesth 2017;31:579-85. [Crossref] [PubMed]
- Khalil AE, Abdallah NM, Bashandy GM, et al. Ultrasound-Guided Serratus Anterior Plane Block Versus Thoracic Epidural Analgesia for Thoracotomy Pain. J Cardiothorac Vasc Anesth 2017;31:152-8. [Crossref] [PubMed]
- Saad FS, El Baradie SY, Abdel Aliem MAW, et al. Ultrasound-guided serratus anterior plane block versus thoracic paravertebral block for perioperative analgesia in thoracotomy. Saudi J Anaesth 2018;12:565-70. [PubMed]
- Ökmen K, Metin Ökmen B. Evaluation of the effect of serratus anterior plane block for pain treatment after video-assisted thoracoscopic surgery. Anaesth Crit Care Pain Med 2018;37:349-53. [Crossref] [PubMed]
- Park MH, Kim JA, Ahn HJ, et al. A randomised trial of serratus anterior plane block for analgesia after thoracoscopic surgery. Anaesthesia 2018;73:1260-4. [Crossref] [PubMed]
- Kim DH, Oh YJ, Lee JG, et al. Efficacy of Ultrasound-Guided Serratus Plane Block on Postoperative Quality of Recovery and Analgesia After Video-Assisted Thoracic Surgery: A Randomized, Triple-Blind, Placebo-Controlled Study. Anesth Analg 2018;126:1353-61. [Crossref] [PubMed]
- Semyonov M, Fedorina E, Grinshpun J, et al. Ultrasound-guided serratus anterior plane block for analgesia after thoracic surgery. J Pain Res 2019;12:953-60. [Crossref] [PubMed]
- Lee J, Lee DH, Kim S. Serratus anterior plane block versus intercostal nerve block for postoperative analgesic effect after video-assisted thoracoscopic lobectomy: A randomized prospective study. Medicine (Baltimore) 2020;99:e22102. [Crossref] [PubMed]
- Qiu Y, Wu J, Huang Q, et al. Acute pain after serratus anterior plane or thoracic paravertebral blocks for video-assisted thoracoscopic surgery: A noninferiority randomised trial. Eur J Anaesthesiol 2021;38:S97-S105. [Crossref] [PubMed]
- Forero M, Adhikary SD, Lopez H, et al. The Erector Spinae Plane Block: A Novel Analgesic Technique in Thoracic Neuropathic Pain. Reg Anesth Pain Med 2016;41:621-7. [Crossref] [PubMed]
- Diwan S, Garud R, Nair A. Thoracic paravertebral and erector spinae plane block: A cadaveric study demonstrating different site of injections and similar destinations. Saudi J Anaesth 2019;13:399-401. [Crossref] [PubMed]
- Forero M, Rajarathinam M, Adhikary S, et al. Erector spinae plane (ESP) block in the management of post thoracotomy pain syndrome: A case series. Scand J Pain 2017;17:325-9. [Crossref] [PubMed]
- Tsui BCH, Fonseca A, Munshey F, et al. The erector spinae plane (ESP) block: A pooled review of 242 cases. J Clin Anesth 2019;53:29-34. [Crossref] [PubMed]
- Huang W, Wang W, Xie W, et al. Erector spinae plane block for postoperative analgesia in breast and thoracic surgery: A systematic review and meta-analysis. J Clin Anesth 2020;66:109900. [Crossref] [PubMed]
- Elsabeeny WY, Ibrahim MA, Shehab NN, et al. Serratus Anterior Plane Block and Erector Spinae Plane Block Versus Thoracic Epidural Analgesia for Perioperative Thoracotomy Pain Control: A Randomized Controlled Study. J Cardiothorac Vasc Anesth 2021;35:2928-36. [Crossref] [PubMed]
- Fiorelli S, Leopizzi G, Menna C, et al. Ultrasound-Guided Erector Spinae Plane Block Versus Intercostal Nerve Block for Post-Minithoracotomy Acute Pain Management: A Randomized Controlled Trial. J Cardiothorac Vasc Anesth 2020;34:2421-9. [Crossref] [PubMed]
- Shim JG, Ryu KH, Kim PO, et al. Evaluation of ultrasound-guided erector spinae plane block for postoperative management of video-assisted thoracoscopic surgery: a prospective, randomized, controlled clinical trial. J Thorac Dis 2020;12:4174-82. [Crossref] [PubMed]
- Taketa Y, Irisawa Y, Fujitani T. Comparison of ultrasound-guided erector spinae plane block and thoracic paravertebral block for postoperative analgesia after video-assisted thoracic surgery: a randomized controlled non-inferiority clinical trial. Reg Anesth Pain Med 2019; [Epub ahead of print]. [PubMed]
- Zhao H, Xin L, Feng Y. The effect of preoperative erector spinae plane vs. paravertebral blocks on patient-controlled oxycodone consumption after video-assisted thoracic surgery: A prospective randomized, blinded, non-inferiority study. J Clin Anesth 2020;62:109737. [Crossref] [PubMed]
- Gaballah KM, Soltan WA, Bahgat NM. Ultrasound-Guided Serratus Plane Block Versus Erector Spinae Block for Postoperative Analgesia After Video-Assisted Thoracoscopy: A Pilot Randomized Controlled Trial. J Cardiothorac Vasc Anesth 2019;33:1946-53. [Crossref] [PubMed]
- Finnerty DT, McMahon A, McNamara JR, et al. Comparing erector spinae plane block with serratus anterior plane block for minimally invasive thoracic surgery: a randomised clinical trial. Br J Anaesth 2020;125:802-10. [Crossref] [PubMed]
- Adhikary SD, Bernard S, Lopez H, et al. Erector Spinae Plane Block Versus Retrolaminar Block: A Magnetic Resonance Imaging and Anatomical Study. Reg Anesth Pain Med 2018;43:756-62. [Crossref] [PubMed]
- Ivanusic J, Konishi Y, Barrington MJ. A Cadaveric Study Investigating the Mechanism of Action of Erector Spinae Blockade. Reg Anesth Pain Med 2018;43:567-71. [Crossref] [PubMed]
- Yang HM, Choi YJ, Kwon HJ, et al. Comparison of injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: a cadaveric study. Anaesthesia 2018;73:1244-50. [Crossref] [PubMed]
- Rayaz H, Bravos ED, Gottschalk A. The role of liposomal bupivacaine in thoracic surgery. J Thorac Dis 2019;11:S1163-8. [Crossref] [PubMed]
- Martin LW, Mehran RJ. Intercostal nerve blockade for thoracic surgery with liposomal bupivacaine: the devil is in the details. J Thorac Dis 2019;11:S1202-5. [Crossref] [PubMed]
- Hussain N, Brull R, Sheehy B, et al. Perineural Liposomal Bupivacaine Is Not Superior to Nonliposomal Bupivacaine for Peripheral Nerve Block Analgesia. Anesthesiology 2021;134:147-64. [Crossref] [PubMed]
- Ilfeld BM, Eisenach JC, Gabriel RA. Clinical Effectiveness of Liposomal Bupivacaine Administered by Infiltration or Peripheral Nerve Block to Treat Postoperative Pain. Anesthesiology 2021;134:283-344. [Crossref] [PubMed]
- Rice DC, Cata JP, Mena GE, et al. Posterior Intercostal Nerve Block With Liposomal Bupivacaine: An Alternative to Thoracic Epidural Analgesia. Ann Thorac Surg 2015;99:1953-60. [Crossref] [PubMed]
- Khalil KG, Boutrous ML, Irani AD, et al. Operative Intercostal Nerve Blocks With Long-Acting Bupivacaine Liposome for Pain Control After Thoracotomy. Ann Thorac Surg 2015;100:2013-8. [Crossref] [PubMed]
-
Intercostal Nerve Block With Liposome Bupivacaine in Subjects Undergoing Posterolateral Thoracotomy -
Study of Exparel Versus Epidural for Pain Control After Thoracotomy - Medina M, Foiles SR, Francois M, et al. Comparison of cost and outcomes in patients receiving thoracic epidural versus liposomal bupivacaine for video-assisted thoracoscopic pulmonary resection. Am J Surg 2019;217:520-4. [Crossref] [PubMed]
- Sztain JF, Gabriel RA, Said ET. Thoracic Epidurals are Associated With Decreased Opioid Consumption Compared to Surgical Infiltration of Liposomal Bupivacaine Following Video-Assisted Thoracoscopic Surgery for Lobectomy: A Retrospective Cohort Analysis. J Cardiothorac Vasc Anesth 2019;33:694-8. [Crossref] [PubMed]
- Rincavage M, Hammond L, Reddy S, et al. Pain control using liposomal bupivacaine versus bupivacaine for robotic assisted thoracic surgery. Int J Clin Pharm 2019;41:258-63. [Crossref] [PubMed]
- Parascandola SA, Ibañez J, Keir G, et al. Liposomal bupivacaine versus bupivacaine/epinephrine after video-assisted thoracoscopic wedge resection†. Interact Cardiovasc Thorac Surg 2017;24:925-30. [Crossref] [PubMed]
- Kelley TM Jr, Bailey DW, Sparks P, et al. Intercostal Nerve Blockade with Exparel® Results in Lower Opioid Usage during the First 24 Hours after Video-Assisted Thorascopic Surgery. Am Surg 2018;84:1433-8. [Crossref] [PubMed]
- Dominguez DA, Ely S, Bach C, et al. Impact of intercostal nerve blocks using liposomal versus standard bupivacaine on length of stay in minimally invasive thoracic surgery patients. J Thorac Dis 2018;10:6873-9. [Crossref] [PubMed]
- Louis SG, King C, Baral P, et al. Liposomal Bupivacaine Enhances the Pain-Control Benefits of Uniportal Thoracoscopic Lobectomy. Ann Thorac Surg 2019;108:1514-8. [Crossref] [PubMed]
- Patel KM, van Helmond N, Kilzi GM, et al. Liposomal Bupivacaine Versus Bupivacaine for Intercostal Nerve Blocks in Thoracic Surgery: A Retrospective Analysis. Pain Physician 2020;23:E251-8. [Crossref] [PubMed]
- Pedoto A, Noel J, Park BJ, et al. Liposomal Bupivacaine Versus Bupivacaine Hydrochloride for Intercostal Nerve Blockade in Minimally Invasive Thoracic Surgery. J Cardiothorac Vasc Anesth 2021;35:1393-8. [Crossref] [PubMed]
- Scawn ND, Pennefather SH, Soorae A, et al. Ipsilateral shoulder pain after thoracotomy with epidural analgesia: the influence of phrenic nerve infiltration with lidocaine. Anesth Analg 2001;93:260-4, 1st contents page.
- Danelli G, Berti M, Casati A, et al. Ipsilateral shoulder pain after thoracotomy surgery: a prospective, randomized, double-blind, placebo-controlled evaluation of the efficacy of infiltrating the phrenic nerve with 0.2%wt/vol ropivacaine. Eur J Anaesthesiol 2007;24:596-601. [Crossref] [PubMed]
- Martinez-Barenys C, Busquets J, de Castro PE, et al. Randomized double-blind comparison of phrenic nerve infiltration and suprascapular nerve block for ipsilateral shoulder pain after thoracic surgery. Eur J Cardiothorac Surg 2011;40:106-12. [Crossref] [PubMed]
- Woo JH, Kim YJ, Kim KC, et al. The effect of interscalene block on ipsilateral shoulder pain and pulmonary function in patients undergoing lung lobectomy: A randomized controlled trial. Medicine (Baltimore) 2018;97:e11034. [Crossref] [PubMed]
- Blichfeldt-Eckhardt MR, Laursen CB, Berg H, et al. A randomised, controlled, double-blind trial of ultrasound-guided phrenic nerve block to prevent shoulder pain after thoracic surgery. Anaesthesia 2016;71:1441-8. [Crossref] [PubMed]
- Pehora C, Pearson AM, Kaushal A, et al. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst Rev 2017;11:CD011770. [Crossref] [PubMed]
- Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth 2017;118:167-81. [Crossref] [PubMed]
- Ammar AS, Mahmoud KM. Does the addition of magnesium to bupivacaine improve postoperative analgesia of ultrasound-guided thoracic paravertebral block in patients undergoing thoracic surgery? J Anesth 2014;28:58-63. [Crossref] [PubMed]
- Hassan ME, Mahran E. Evaluation of the role of dexmedetomidine in improvement of the analgesic profile of thoracic paravertebral block in thoracic surgeries: A randomised prospective clinical trial. Indian J Anaesth 2017;61:826-31. [Crossref] [PubMed]
- Xu J, Yang X, Hu X, et al. Multilevel Thoracic Paravertebral Block Using Ropivacaine With/Without Dexmedetomidine in Video-Assisted Thoracoscopic Surgery. J Cardiothorac Vasc Anesth 2018;32:318-24. [Crossref] [PubMed]
- Piccioni F, Segat M, Falini S, et al. Enhanced recovery pathways in thoracic surgery from Italian VATS Group: perioperative analgesia protocols. J Thorac Dis 2018;10:S555-63. [Crossref] [PubMed]
- Khoronenko V, Baskakov D, Leone M, et al. Influence of Regional Anesthesia on the Rate of Chronic Postthoracotomy Pain Syndrome in Lung Cancer Patients. Ann Thorac Cardiovasc Surg 2018;24:180-6. [Crossref] [PubMed]
- Brown LM, Kratz A, Verba S, et al. Pain and Opioid Use After Thoracic Surgery: Where We Are and Where We Need To Go. Ann Thorac Surg 2020;109:1638-45. [Crossref] [PubMed]
- Andreae MH, Andreae DA. Local anaesthetics and regional anaesthesia for preventing chronic pain after surgery. Cochrane Database Syst Rev 2012;10:CD007105. [Crossref] [PubMed]
- Grosen K, Drewes AM, Højsgaard A, et al. Perioperative gabapentin for the prevention of persistent pain after thoracotomy: a randomized controlled trial. Eur J Cardiothorac Surg 2014;46:76-85. [Crossref] [PubMed]
- Nedeljkovic SS, Kett A, Vallejo MC, et al. Transversus Abdominis Plane Block With Liposomal Bupivacaine for Pain After Cesarean Delivery in a Multicenter, Randomized, Double-Blind, Controlled Trial. Anesth Analg 2020;131:1830-9. [Crossref] [PubMed]


